Chitosan/Hydroxyapatite Scaffolds with P28 as a Promising Osteoinductive Scaffold for Bone Healing Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Chitosan/Hydroxyapatite Scaffolds with P28 or BMP-2 (CS/HAp, CS/HAp/P28 and CS/HAp/BMP-2) Using a UV Photocrosslinking
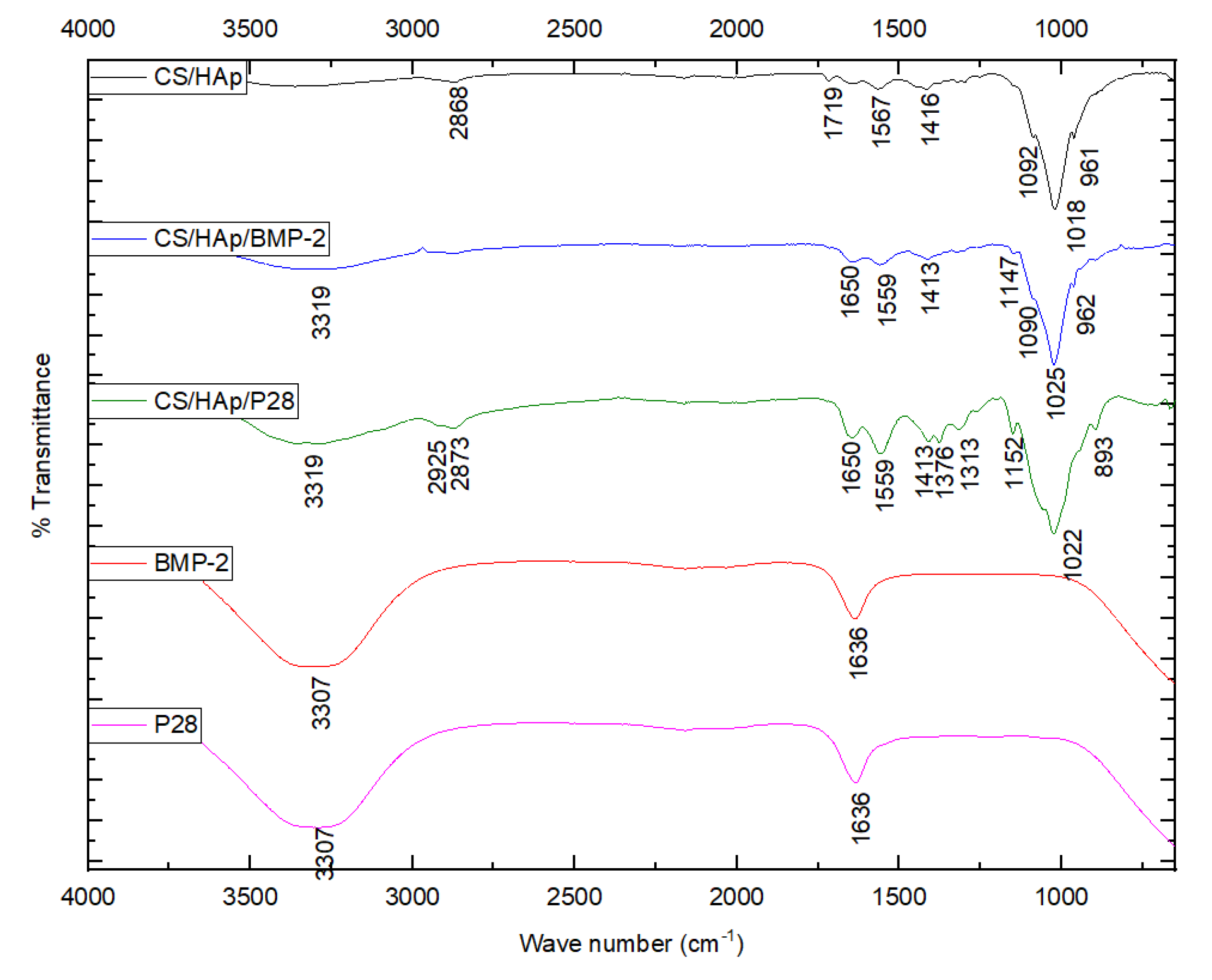
2.2. Fourier-Transform Infrared Spectroscopy
2.3. In Vitro Release Kinetics of BMP-2 and P28
- (i)
- Analyte concentration (µg/mL) = Concentration from equation × dilution factor.
- (ii)
- Mass of analyte (µg) = Analyte concentration × volume of samples.
- (iii)
- Percentage of analyte (%) = Mass of analyte ÷ Initial mass of P28 × 100.
2.4. Alkaline Phosphatase Assay
- B = ρNP concentration from the equation (µmol)
- ∆T = reaction time (min)
- V = Original sample volume added into the reaction well (mL)
- D = Sample dilution factor
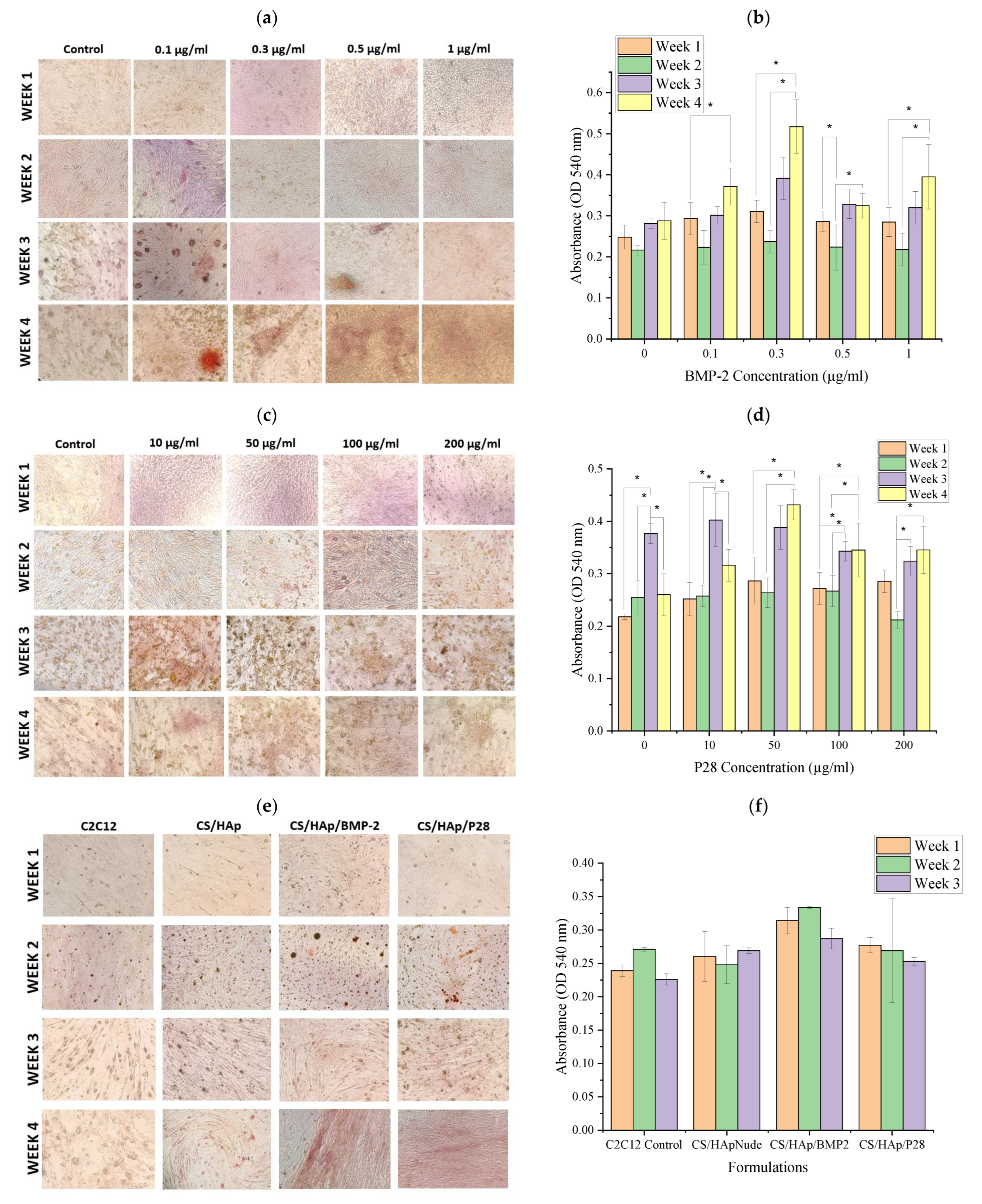
2.5. Alizarin Red Staining and Cetylpyridinium Chloride Assay
2.6. Femoral Defect Induction and Implantation
2.6.1. Post-Operative Monitoring
- Weight loss > 20% of the mean weight of rats;
- Severe lameness;
- Diarrhoea/blood in faecal material;
- Circling phenomenon;
- Severe necrosis at the implantation site;
- Persistent self-induced trauma five days after analgesic treatment as well as local and general treatment;
- Abnormal behaviour even in the presence of appropriate treatment (e.g., sign of pain even under analgesia).
2.6.2. Fluorescent Bone Labelling for Dynamic Bone Formation
2.7. Micro-CT Analysis of Femoral Condyle Defects
2.8. Histological Processing, Staining and Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Scaffold Characterisation through a Fourier-Transform Infrared Spectroscopy
3.2. In Vitro Kinetic Release of P28
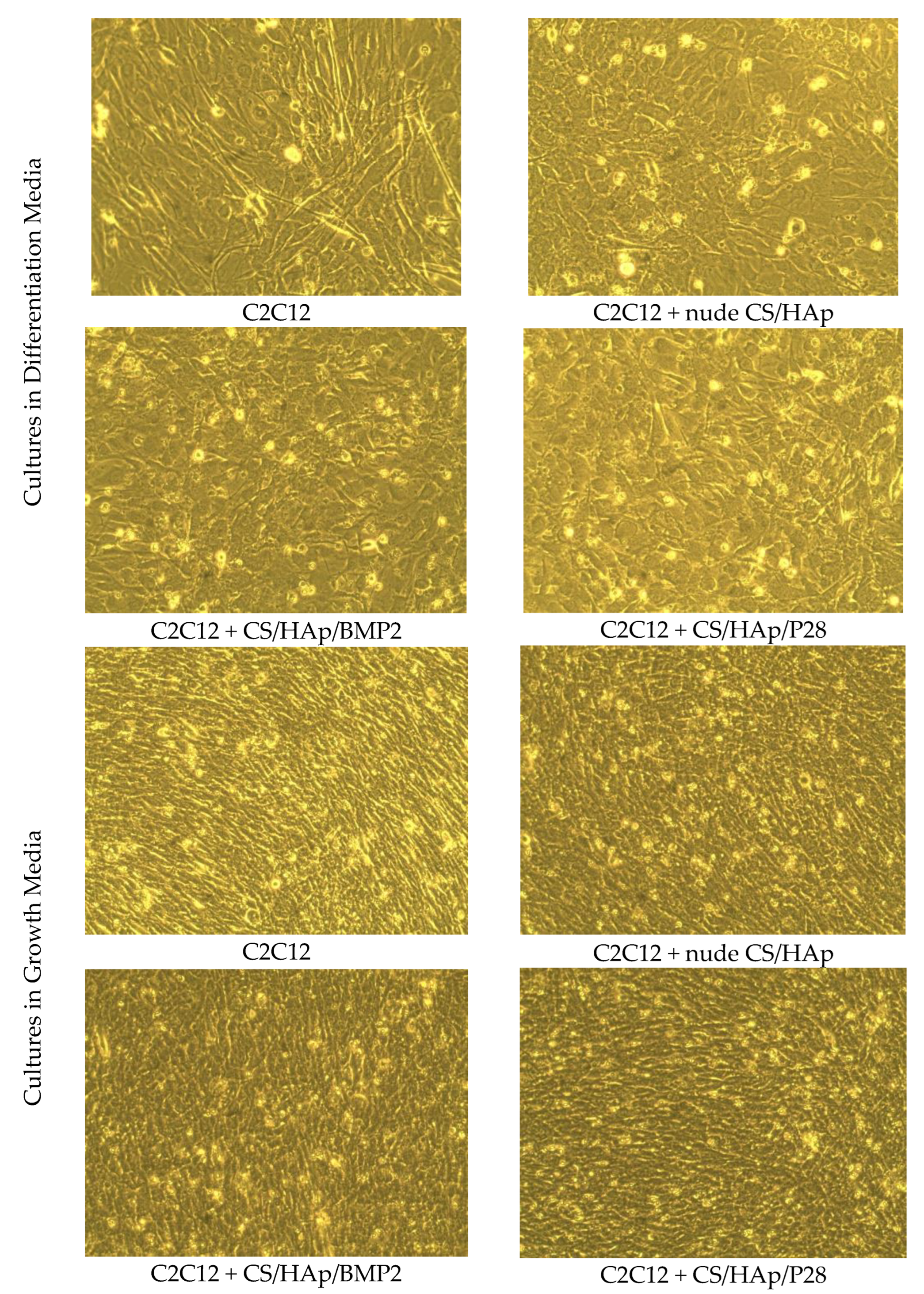
3.3. C2C12 Mineralisation through Culture with P28 Peptide and BMP-2 Loaded Scaffolds Using Alkaline Phosphatase
3.4. C2C12 Mineralisation through Culture with P28 Peptide and BMP-2 Loaded Scaffolds Using Alizarin Red Staining
3.5. Post-Operative Evaluations of the Animals
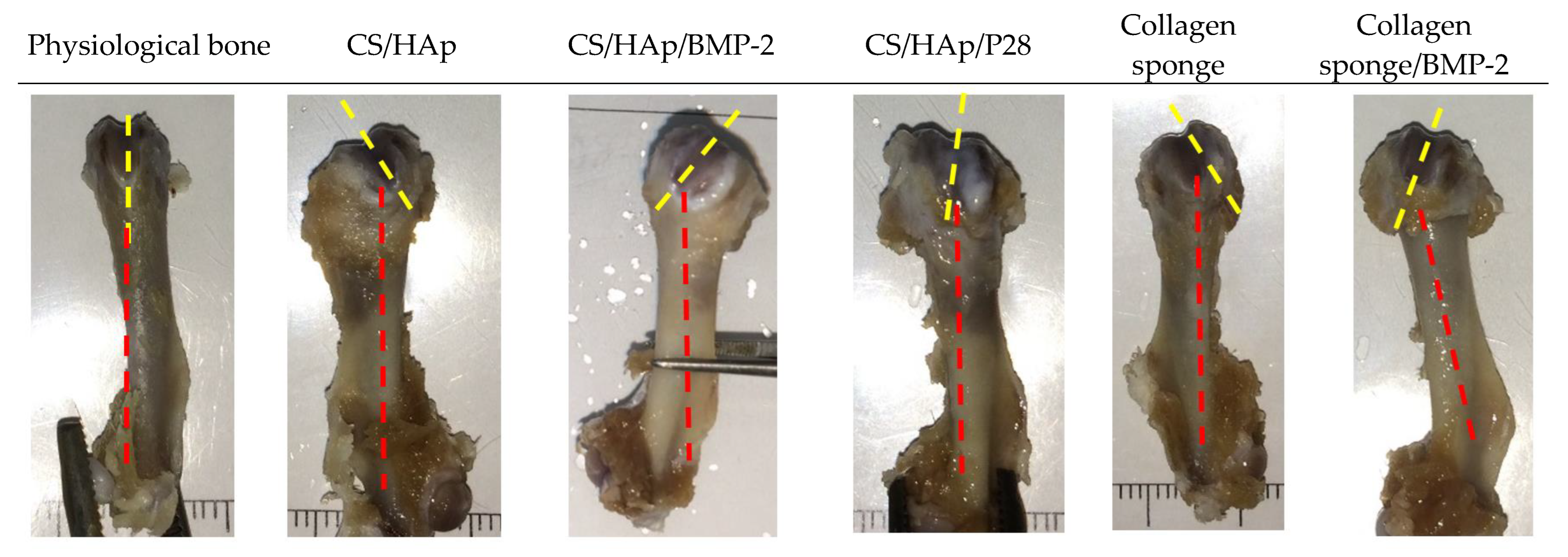
3.6. Macroscopic Evaluation
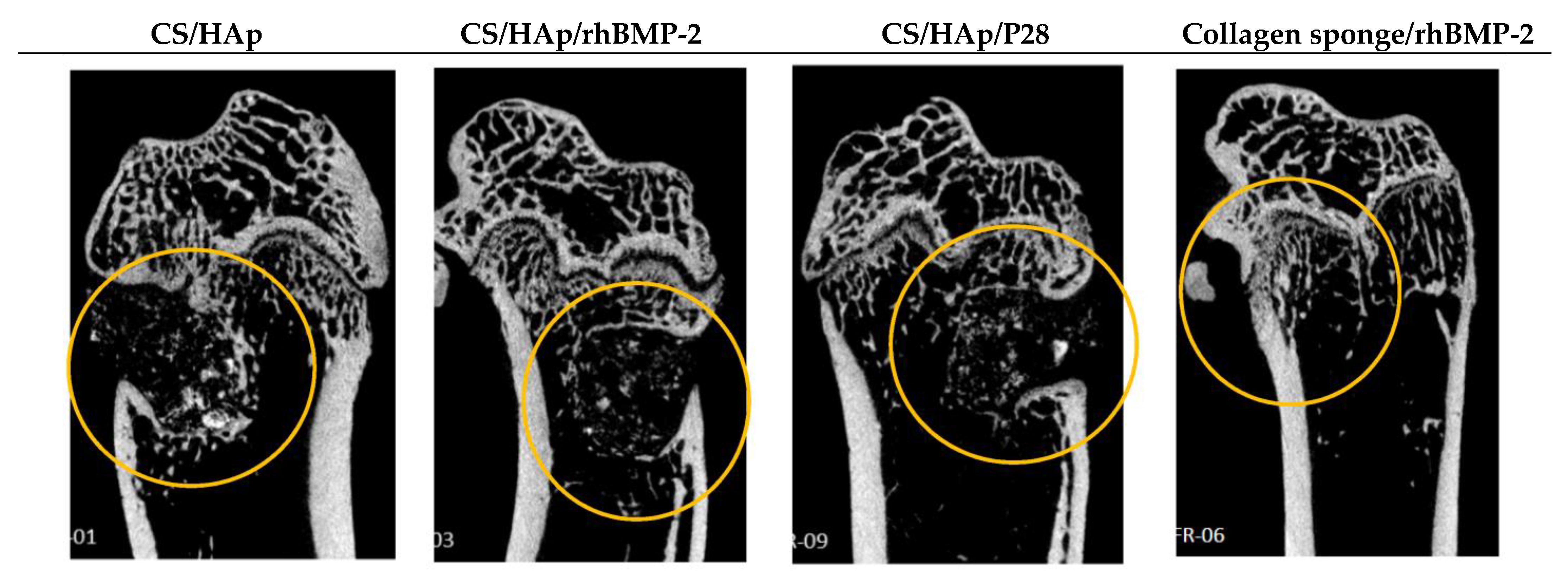
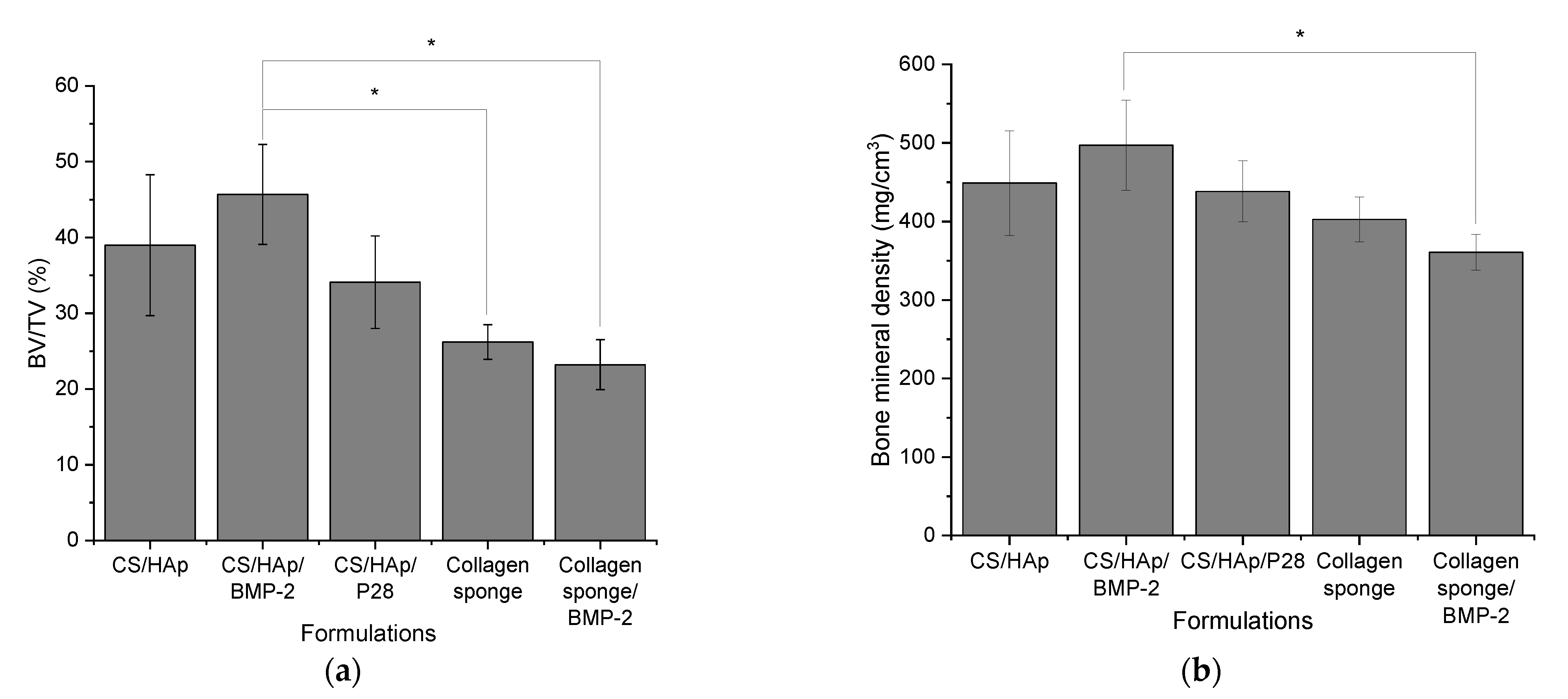
3.7. Micro-CT Evaluation of the Femoral Defects Treated with the Scaffolds
Quantitative Analysis of Micro-Computed Tomography Scanning
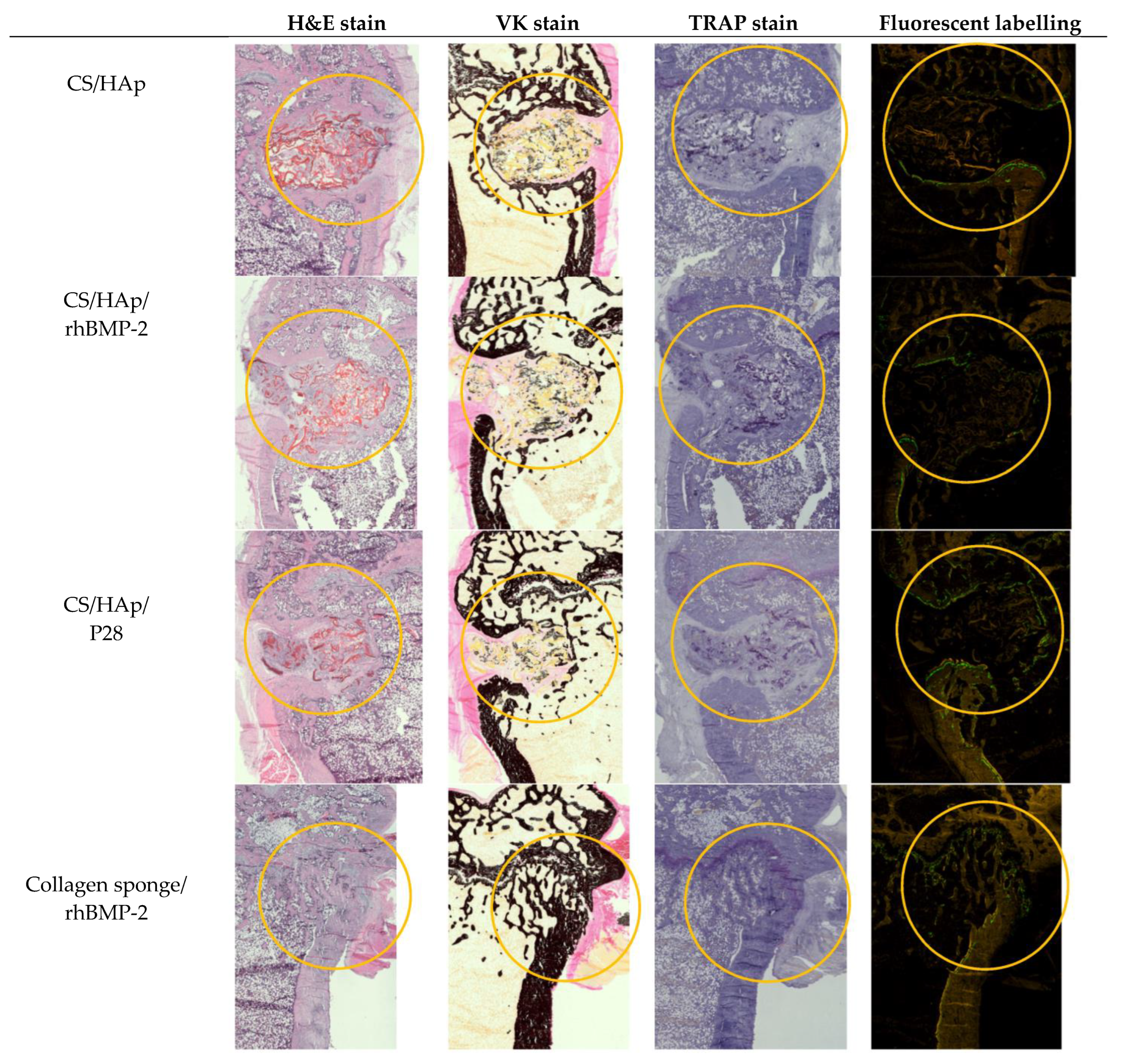
3.8. Histological Assessment of the Femoral Defects Treated with the Scaffolds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, T.; Qu, Y.; Cui, W.; Yang, L.; Ji, Y.; Yu, W.; Navinduth, R.; Shao, Z.; Yang, H.; Guo, X. Evaluation of osteogenic inductivity of a novel BMP2-mimicking peptide P28 and P28-containing bone composite. J. Biomed. Mater. Res. Part A 2017, 106, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Liu, Q.; Yang, L.; Wang, K.; Sun, T.; Ji, Y.; Liu, L.; Yu, W.; Qu, Y.; Wang, J. Sustained Delivery of BMP-2-Related Peptide from the True Bone Ceramics/Hollow Mesoporous Silica Nanoparticles Scaffold for Bone Tissue Regeneration. ACS Biomater. Sci. Eng. 2018, 4, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Meng, C.; Su, W.; Liu, M.; Yao, S.; Ding, Q.; Yu, K.; Xiong, Z.; Chen, K.; Guo, X.; Bo, L.; et al. Controlled delivery of bone morphogenic protein-2-related peptide from mineralised extracellular matrix-based scaffold induces bone regeneration. Mater. Sci. Eng. C 2021, 126, 112182. [Google Scholar] [CrossRef] [PubMed]
- Sparks, D.S.; Saifzadeh, S.; Savi, F.M.; Dlaska, C.E.; Berner, A.; Henkel, J.; Reichert, J.C.; Wullschleger, M.; Ren, J.; Cipitria, A.; et al. A preclinical large-animal model for the assessment of critical-size load-bearing bone defect reconstruction. Nat. Protoc. 2020, 15, 877–924. [Google Scholar] [CrossRef]
- Klein, A.; Baranowski, A.; Ritz, U.; Mack, C.; Götz, H.; Langendorf, E.; Al-Nawas, B.; Rommens, P.M.; Hofmann, A. Effect of bone sialoprotein coating on progression of bone formation in a femoral defect model in rats. Eur. J. Trauma Emerg. Surg. 2019, 46, 277–286. [Google Scholar] [CrossRef]
- Jahan, K.; Manickam, G.; Tabrizian, M.; Murshed, M. In vitro and in vivo investigation of osteogenic properties of self-contained phosphate-releasing injectable purine-crosslinked chitosan-hydroxyapatite constructs. Sci. Rep. 2020, 10, 11603. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, Y.; Han, J.; Mo, J.; Dong, P.; Zhuo, Y.; Feng, Y. Biocompatiable silk fibroin/carboxymethyl chitosan/strontium substituted hydroxyapatite/cellulose nanocrystal composite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2019, 136, 1247–1257. [Google Scholar] [CrossRef]
- Henkel, J. Bone Tissue Engineering in Two Preclinical Ovine Animal Models. Ph.D. Thesis, Queensland University of Technology, Brisbane, Australia, 2017. [Google Scholar]
- Taraballi, F.; Bauza, G.; McCulloch, P.; Harris, J.; Tasciotti, E. Concise Review: Biomimetic Functionalization of Biomaterials to Stimulate the Endogenous Healing Process of Cartilage and Bone Tissue. Tissue Eng. Regen. Med. 2017, 6, 2186–2196. [Google Scholar] [CrossRef] [Green Version]
- Burke, G.; Cao, Z.; Devine, D.M.; Major, I. Preparation of biodegradable polyethylene glycol dimethacrylate hydrogels via thiol-ene chemistry. Polymers 2019, 11, 1339. [Google Scholar] [CrossRef] [Green Version]
- Fournet, M.E.B.; Azaman, F.A.; Gunbay, S.; Chen, Y.Y.; Devine, D.M. Orthopedic 3D Printing in Orthopedic Medicine. In Polymer-Based Additive Manufaturing: Biomedical Application; Devine, D.M., Ed.; Springer: Cham, Switzerland, 2019; pp. 121–142. [Google Scholar]
- Makadia, H.K.; Steven, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2012, 3, 1377–1397. [Google Scholar] [CrossRef]
- Chen, L.; Liu, J.; Guan, M.; Zhou, T.; Duan, X.; Xiang, Z. Growth factor and its polymer scaffold-based delivery system for cartilage tissue engineering. Int. J. Nanomed. 2020, 15, 6097–6111. [Google Scholar] [CrossRef]
- Azaman, F.A.; Zhou, K.; Blanes-Martínez, M.M.; Fournet, M.B.; Devine, D.M. Bioresorbable Chitosan-Based Bone Regeneration Scaffold Using Various Bioceramics and the Alteration of Photoinitiator Concentration in an Extended UV Photocrosslinking Reaction. Gels 2022, 8, 696. [Google Scholar] [CrossRef]
- Sukpaita, T.; Chirachanchai, S.; Suwattanachai, P.; Everts, V.; Pimkhaokham, A.; Ampornaramveth, R.S. In vivo bone regeneration induced by a Scaffold of chitosan/dicarboxylic acid seeded with human periodontal ligament cells. Int. J. Mol. Sci. 2019, 20, 4883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maji, K.; Dasgupta, S.; Pramanik, K.; Bissoyi, A. Preparation and Evaluation of Gelatin-Chitosan-Nanobioglass 3D Porous Scaffold for Bone Tissue Engineering. Int. J. Biomater. 2016, 2016, 9825659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, F.C.; Tsao, C.T.; Lin, A.; Zhang, M.; Levengood, S.; Zhang, M. PEG-Chitosan Hydrogel with Tunable Stiffness for Study of Drug Response of Breast Cancer Cells. Polymers 2016, 8, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fourie, J.; Taute, F.; du Preez, L.; de Beer, D. Chitosan Composite Biomaterials for Bone Tissue Engineering—A Review. Regen. Eng. Transl. Med. 2022, 8, 1–21. [Google Scholar] [CrossRef]
- Marques, C.; Som, C.; Schmutz, M.; Borges, O.; Borchard, G. How the Lack of Chitosan Characterization Precludes Implementation of the Safe-by-Design Concept. Front. Bioeng. Biotechnol. 2020, 8, 165. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Kumar, A. Chitosan as a biomedical material: Properties and applications. In Biopolymers: Structure, Performance and Applications; Mishra, A.K., Hussain, C.M., Mishra, S.B., Eds.; Nova Science Publishers, Inc.: New York, NY, USA, 2017; pp. 139–154. [Google Scholar]
- Venkatesan, J.; Kim, S.K. Chitosan composites for bone tissue engineering—An overview. Mar. Drugs 2010, 8, 2252–2266. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; King, M.W. Biodegradable Polymers as the Pivotal Player in the Design of Tissue Engineering Scaffolds. Adv. Healthc. Mater. 2020, 9, 1901358. [Google Scholar] [CrossRef]
- Jhala, D.; Rather, H.A.; Vasita, R. Extracellular matrix mimicking polycaprolactone-chitosan nanofibers promote stemness maintenance of mesenchymal stem cells via spheroid formation. Biomed. Mater. 2020, 15, 035011. [Google Scholar] [CrossRef]
- Nicolas, J.; Magli, S.; Rabbachin, L.; Sampaolesi, S.; Nicotra, F.; Russo, L. 3D Extracellular Matrix Mimics: Fundamental Concepts and Role of Materials Chemistry to Influence Stem Cell Fate. Biomacromolecules 2020, 21, 1968–1994. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Barroso, L.G.; Azaman, F.A.; Pogue, R.; Devine, D.; Fournet, M.B. Monitoring In Vitro Extracellular Matrix Protein Conformations in the Presence of Biomimetic Bone-Regeneration Scaffolds Using Functionalized Gold-Edge-Coated Triangular Silver Nanoparticles. Nanomaterials 2022, 13, 57. [Google Scholar] [CrossRef] [PubMed]
- Maachou, H.; Bal, K.E.; Bal, Y.; Chagnes, A.; Cote, G.; Alliouche, D. Characterization and in vitro bioactivity of chitosan/hydroxyapatite composite membrane prepared by freeze-gelation method. Trends Biomater. Artif. Organs 2008, 22, 15–24. [Google Scholar]
- Li, B.; Wang, J.; Moustafa, M.E.; Yang, H. Ecofriendly Method to Dissolve Chitosan in Plain Water. ACS Biomater. Sci. Eng. 2019, 5, 6355–6360. [Google Scholar] [CrossRef]
- Zheng, X.; Yin, Y.; Jiang, W.; Xing, L.; Pu, J. Low-mass chitosan. BioResources 2015, 10, 2338–2349. [Google Scholar]
- Reves, B.T.; Jennings, J.A.; Bumgardner, J.D.; Haggard, W.O. Osteoinductivity assessment of BMP-2 loaded composite chitosan-nano-hydroxyapatite scaffolds in a rat muscle pouch. Materials 2011, 4, 1360–1374. [Google Scholar] [CrossRef] [Green Version]
- Bjelić, D.; Finšgar, M. The role of growth factors in bioactive coatings. Pharmaceutics 2021, 13, 1083. [Google Scholar] [CrossRef]
- El Bialy, I.; Jiskoot, W.; Reza Nejadnik, M. Formulation, Delivery and Stability of Bone Morphogenetic Proteins for Effective Bone Regeneration. Pharm. Res. 2017, 34, 1152–1170. [Google Scholar] [CrossRef] [Green Version]
- Devine, D.M.; Hoctor, E.; Hayes, J.S.; Sheehan, E.; Evans, C.H. Extended release of proteins following encapsulation in hydroxya- patite/chitosan composite scaffolds for bone tissue engineering applications. Mater. Sci. Eng. C 2018, 84, 281–289. [Google Scholar] [CrossRef]
- Bullock, G.; Atkinson, J.; Gentile, P.; Hatton, P.; Miller, C. Osteogenic peptides and attachment methods determine tissue regeneration in modified bone graft substitutes. J. Funct. Biomater. 2021, 12, 22. [Google Scholar] [CrossRef]
- Alves, A.; Wancket, L.; Metz, A. Current considerations in medical device pathology. In Biocompatibility and Performance of Medical Devices, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2020; pp. 491–543. [Google Scholar]
- Rosenberg, M.; Shilo, D.; Galperin, L.; Capucha, T.; Tarabieh, K.; Rachmiel, A.; Segal, E. Bone morphogenic protein 2-loaded porous silicon carriers for osteoinductive implants. Pharmaceutics 2019, 11, 602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, G.; Zhang, L.; Che, L.; Li, H.; Liu, Y.; Fang, J. Revisiting bone morphogenetic protein-2 knuckle epitope and redesigning the epitope-derived peptides. J. Pept. Sci. 2021, 27, e3309. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Jia, G.; Chi, H.; Jiao, Z.; Sun, Y. Integrated In Silico-In Vitro Identification and Optimization of Bone Morphogenic Protein-2 Armpit Epitope as Its Antagonist Binding Site. Protein J. 2020, 39, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Sun, G.; Qu, Y.; Xiong, Y.; Sun, T.; Ji, Y.; Yang, L.; Shao, Z.; Ma, J.; Zhang, S.; et al. Repair of rat calvarial defects using Si-doped hydroxyapatite scaffolds loaded with a bone morphogenetic protein-2-related peptide. J. Orthop. Res. 2016, 34, 1874–1882. [Google Scholar] [CrossRef] [Green Version]
- Bain, J.L.; Bonvallet, P.P.; Abou-Arraj, R.V.; Schupbach, P.; Reddy, M.S.; Bellis, S.L. Enhancement of the Regenerative Potential of Anorganic Bovine Bone Graft Utilising a Polyglutamate-Modified BMP2 Peptide with Improved Binding to Calcium-Containing Materials. Tissue Eng. Part A 2015, 17–18, 2426–2436. [Google Scholar] [CrossRef] [Green Version]
- De Gorter, D.J.J.; Van Dinther, M.; Ten Dijke, P. Measurement of Constitutive Activity of BMP Type I Receptors, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2010; Volume 484. [Google Scholar]
- Hidaka, Y.; Chiba-ohkuma, R.; Karakida, T.; Onuma, K. Combined Effect of Midazolam and Bone Morphogenetic Protein-2 for Differentiation Induction from C2C12 Myoblast Cells to Osteoblasts. Pharmaceutics 2020, 12, 218. [Google Scholar] [CrossRef] [Green Version]
- Blackwood, K.A.; Bock, N.; Dargaville, T.R.; Ann Woodruff, M. Scaffolds for growth factor delivery as applied to bone tissue engineering. Int. J. Polym. Sci. 2012, 2012, 174942. [Google Scholar] [CrossRef] [Green Version]
- Katagiri, T.; Yamaguchi, A.; Komaki, M.; Abe, E.; Takahashi, N.; Ikeda, T.; Rosen, V.; Wozney, J.M.; Fujisawa-Sehara, A.; Suda, T. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J. Cell Biol. 1994, 127, 1755–1766. [Google Scholar] [CrossRef] [Green Version]
- Kelly, F. Evaluation of Modified Flow-Through Pulsed UV Technology for Bacterial Inactivation with Comparison to a Standard Continuous-Flow Low Pressure UV System. Ph.D. Thesis, National University of Ireland Galway, Galway, Ireland, 2019. [Google Scholar]
- Becerra, J.; Rodriguez, M.; Leal, D.; Noris-Suarez, K.; Gonzalez, G. Chitosan-collagen-hydroxyapatite membranes for tissue engineering. J. Mater. Sci. Mater. Med. 2022, 33, 18. [Google Scholar] [CrossRef]
- Mohiuddin, O.A.; Campbell, B.; Poche, J.N.; Ma, M.; Rogers, E.; Gaupp, D.; Harrison, M.A.A.; Bunnell, B.A.; Hayes, D.J.; Gimble, J.M. Decellularized Adipose Tissue Hydrogel Promotes Bone Regeneration in Critical-Sized Mouse Femoral Defect Model. Front. Bioeng. Biotechnol. 2019, 7, 211. [Google Scholar] [CrossRef] [Green Version]
- van Gaalen, S.M.; Kruyt, M.C.; Geuze, R.E.; de Bruijn, J.D.; Alblas, J.; Dhert, W.J.A. Use of fluorochrome labels in in vivo bone tissue engineering research. Tissue Eng. Part B. Rev. 2010, 16, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Porter, A.; Irwin, R.; Miller, J.; Horan, D.J.; Robling, A.G.; McCabe, L.R. Quick and inexpensive paraffin-embedding method for dynamic bone formation analyses. Sci. Rep. 2017, 7, 42505. [Google Scholar] [CrossRef]
- Liao, Y.; Li, H.; Shu, R.; Chen, H.; Zhao, L.; Song, Z.; Zhou, W. Mesoporous Hydroxyapatite/Chitosan Loaded with Recombinant- Human Amelogenin Could Enhance Antibacterial Effect and Promote Periodontal Regeneration. Front. Cell. Infect. Microbiol. 2020, 10, 180. [Google Scholar] [CrossRef] [PubMed]
- Fern, H.W.; Salimi, M.N. Hydroxyapatite nanoparticles produced by direct precipitation method: Optimisation and characterisation studies. AIP Conf. Proc. 2021, 2339, 020215. [Google Scholar]
- Nazeer, M.A.; Yilgör, E.; Yilgör, I. Intercalated chitosan/hydroxyapatite nanocomposites: Promising materials for bone tissue engineering applications. Carbohydr. Polym. 2017, 175, 38–46. [Google Scholar] [CrossRef]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V.; Stan, G.E.; Buton, N. Synthesis, characterisation, and antimicrobial activity of magnesium- doped hydroxyapatite suspensions. Nanomaterials 2019, 9, 1295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shemshad, S.; Kamali, S.; Khavandi, A.; Azari, S. Synthesis, characterisation and in-vitro behavior of natural chitosan- hydroxyapatite-diopside nanocomposite scaffold for bone tissue engineering. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 516–526. [Google Scholar] [CrossRef]
- Halloran, D.; Vrathasha, V.; Durbano, H.W.; Nohe, A. Bone morphogenetic protein-2 conjugated to quantum dot®s is biologically functional. Nanomaterials 2020, 10, 1208. [Google Scholar] [CrossRef]
- Anesi, A.; Di Bartolomeo, M.; Pellacani, A.; Ferretti, M.; Cavani, F.; Salvatori, R.; Nocini, R.; Palumbo, C.; Chiarini, L. Bone Healing Evaluation Following Different Osteotomic Techniques in Animal Models: A Suitable Method for Clinical Insights. Appl. Sci. 2020, 10, 7165. [Google Scholar] [CrossRef]
- Sun, T.; Liu, M.; Yao, S.; Ji, Y.; Shi, L.; Tang, K.; Xiong, Z.; Yang, F.; Chen, K.; Guo, X. Guided osteoporotic bone regeneration with composite scaffolds of mineralised ECM/heparin membrane loaded with BMP2-related peptide. Int. J. Nanomed. 2018, 13, 791–804. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Z.; Cui, W.; Sun, T.; Teng, Y.; Qu, Y.; Yang, L.; Zhou, J.; Chen, K.; Yao, S.; Shao, Z.; et al. Sustained delivery of PlGF-2123-144-fused BMP2-related peptide P28 from small intestinal submucosa/polylactic acid scaffold material for bone tissue regeneration. RSC Adv. 2020, 10, 7289–7300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Xiong, Z.; Liu, M.; Yang, L.; Yao, S.; Chen, K.; Yu, K.; Qu, Y.; Sun, T.; Guo, X. Creation of bony microenvironment with extracellular matrix doped-bioactive ceramics to enhance osteoblast behavior and delivery of aspartic acid-modified bmp-2 peptides. Int. J. Nanomed. 2020, 15, 8465–8478. [Google Scholar] [CrossRef]
- Honda, T.; Yamamoto, H.; Ishii, A.; Inui, M. PDZRN3 Negatively Regulates BMP-2–induced Osteoblast Differentiation through Inhibition of Wnt Signaling. Mol. Biol. Cell 2010, 21, 3269–3277. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Suzuki, Y.; Ogata, S.I.; Ohtsuki, C.; Tanihara, M. Activation of osteo-progenitor cells by a novel synthetic peptide derived from the bone morphogenetic protein-2 knuckle epitope. Biochim. Biophys. Acta Proteins Proteom. 2003, 1651, 60–67. [Google Scholar] [CrossRef]
- Gudivada, V.N.; Huang, C.J.; Luo, Y.H.; Dong, G.C. A cyclic bmp-2 peptide upregulates bmp-2 protein-induced cell signaling in myogenic cells. Polymers 2021, 13, 2549. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhou, J.; Li, Y.; Zhou, Y.; Cui, Y.; Yang, G.; Hong, Y. Rap1A regulates osteoblastic differentiation via the ERK and p38 mediated signaling. PLoS ONE 2015, 10, e0143777. [Google Scholar] [CrossRef]
- Metzger, S. Tunable and Cell-Responsive 3D poly(ethylene glycol) Microenvironments for the Development of Tissue Models. Ph.D. Thesis, University of Zurich, Zurich, Switzerland, 2016. [Google Scholar]
- Sondag, G.R.; Salihoglu, S.; Lababidi, S.L.; Crowder, D.C.; Moussa, F.M.; Abdelmagid, S.M.; Safadi, F.F. Osteoactivin induces transdifferentiation of C2C12 myoblasts into osteoblasts. J. Cell. Physiol. 2014, 229, 955–966. [Google Scholar] [CrossRef]
- Kang, Q.; Sun, M.H.; Cheng, H.; Peng, Y.; Montag, A.G.; Deyrup, A.T.; Jiang, W.; Luu, H.H.; Luo, J.; Szatkowski, J.P.; et al. Characterisation of the distinct orthotopic bone-forming activity of 14 BMPs using recombinant adenovirus-mediated gene delivery. Gene Ther. 2004, 11, 1312–1320. [Google Scholar] [CrossRef] [Green Version]
- Akiyama, S.; Katagiri, T.; Namiki, M.; Yamaji, N.; Yamamoto, N.; Miyama, K.; Shibuya, H.; Ueno, N.; Wozney, J.M.; Suda, T. Constitutively active BMP type I receptors transduce BMP-2 signals without the ligand in C2C12 myoblasts. Exp. Cell Res. 1997, 235, 362–369. [Google Scholar] [CrossRef]
- Rauch, C.; Brunet, A.C.; Deleule, J.; Farge, E. C2C12 myoblast/osteoblast transdifferentiation steps enhanced by epigenetic inhibition of BMP2 endocytosis C2C12 myoblast/osteoblast transdifferentiation steps enhanced by epigenetic inhibition of BMP2 endocytosis. Am. J. Physiol. Cell Physiol. 2002, 283, 235–243. [Google Scholar] [CrossRef] [Green Version]
- Lin, D.P.L.; Carnagarin, R.; Dharmarajan, A.; Dass, C.R. Transdifferentiation of myoblasts into osteoblasts—Possible use for bone therapy. J. Pharm. Pharmacol. 2017, 69, 1661–1671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gleeson, J.P.; Plunkett, N.A.; O’Brien, F.J. Addition of hydroxyapatite improves stiffness, interconnectivity and osteogenic potential of a highly porous collagen-based scaffold for bone tissue regeneration. Eur. Cells Mater. 2010, 20, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Wang, D.; Zeng, R.; Wang, J. Synergistic effects of fibroblast growth factor-2 and bone morphogenetic protein-2 on bone induction. Mol. Med. Rep. 2017, 16, 4483–4492. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.H.; Liao, M.H.; Lin, Y.L.; Lai, C.H.; Lin, P.I.; Chen, R.M. Improving effects of chitosan nanofiber scaffolds on osteoblast proliferation and maturation. Int. J. Nanomed. 2014, 9, 4293–4304. [Google Scholar]
- Shalumon, K.T.; Sheu, C.; Fong, Y.T.; Liao, H.T.; Chen, J.P. Microsphere-based hierarchically juxtapositioned biphasic scaffolds prepared frompoly(lactic-co-glycolic acid) and nanohydroxyapatite for osteochondral tissue engineering. Polymers 2016, 8, 429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kisiel, M. Bone Enhancement with BMP-2 for Safe Clinical Translation. Ph.D. Thesis, Uppsala University, Uppsala, Sweden, 2013. [Google Scholar]
- Bouyer, M.; Guillot, R.; Lavaud, J.; Plettinx, C.; Olivier, C.; Curry, V.; Boutonnat, J.; Coll, J.L.; Peyrin, F.; Josserand, V.; et al. Surface delivery of tunable doses of BMP-2 from an adaptable polymeric scaffold induces volumetric bone regeneration. Biomaterials 2016, 104, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Wufuer, M.; Kim, I.; Choi, T.H.; Kim, B.J.; Jung, H.G.; Jeon, B.; Lee, G.; Jeon, O.H.; Chang, H.; et al. Sequential dual-drug delivery of BMP-2 and alendronate from hydroxyapatite-collagen scaffolds for enhanced bone regeneration. Sci. Rep. 2021, 11, 746. [Google Scholar] [CrossRef]
- Hettiaratchi, M.H.; Krishnan, L.; Rouse, T.; Chou, C.; McDevitt, T.C.; Guldberg, R.E. Heparin-mediated delivery of bone morphogenetic protein-2 improves spatial localisation of bone regeneration. Sci. Adv. 2020, 6, eaay1240. [Google Scholar] [CrossRef] [Green Version]
- Mumcuoglu, D.; Fahmy-Garcia, S.; Ridwan, Y.; Nickel, J.; Farrell, E.; Kluijtmans, S.G.J.M.; van Osch, G.J.V.M. Injectable BMP-2 delivery system based on collagen-derived microspheres and alginate induced bone formation in a time-and dose-dependent manner. Eur. Cells Mater. 2018, 35, 242–254. [Google Scholar] [CrossRef]
- Durham, E.L.; Kishinchand, R.; Grey, Z.J.; Cray, J.J. rhBMP2 alone does not induce macrophage polarisation towards an increased inflammatory response. Mol. Immunol. 2020, 117, 94–100. [Google Scholar] [CrossRef]
- Alghamdi, H.S.; Van Den Beucken, J.J.J.P.; Jansen, J.A. Osteoporotic rat models for evaluation of osseointegration of bone implants. Tissue Eng. Part C Methods 2014, 20, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Luckanagul, J.A.; Metavarayuth, K.; Feng, S.; Maneesaay, P.; Clark, A.Y.; Yang, X.; García, A.J.; Wang, Q. Tobacco Mosaic Virus Functionalized Alginate Hydrogel Scaffolds for Bone Regeneration in Rats with Cranial Defect. ACS Biomater. Sci. Eng. 2016, 2, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.P.; Sinusas, A.J.; Horvath, T.L.; Collins, J.G.; Harding, M.J. Correlation between body weight changes and postoperative pain in rats treated with meloxicam or buprenorphine. Lab Anim. 2009, 38, 87–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, E.A. Enhancing Osseointegration of Orthopaedic Implants with Titania Nanotube Surfaces. Ph.D. Thesis, Michigan Technological University, Houghton, MI, USA, 2016. [Google Scholar]
- Zhao, Z.; Yang, D.; Ma, X.; Zhao, H.; Nie, C.; Si, Z. Successful repair of a critical-sized bone defect in the rat femur with a newly developed external fixator. Tohoku J. Exp. Med. 2009, 219, 115–120. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Liu, Q.; Liu, J.Q.; Wang, J.; Yang, H.X.; Xu, X.J.; Xie, M.J.; Liu, X.D.; Yu, S.B.; Zhang, M.; et al. Molecular changes in peripheral blood involving osteoarthritic joint remodelling. J. Oral Rehabil. 2019, 46, 820–827. [Google Scholar] [CrossRef]
- Zhang, J.; Liao, L.; Zhu, J.; Wan, X.; Xie, M.; Zhang, H.; Zhang, M.; Lu, L.; Yang, H.; Jing, D.; et al. Osteochondral Interface Stiffening in Mandibular Condylar Osteoarthritis. J. Dent. Res. 2018, 97, 563–570. [Google Scholar] [CrossRef]
- Bejar, J.; Peled, E.; Boss, J.H. Vasculature deprivation-Induced osteonecrosis of the rat femoral head as a model for therapeutic trials. Theor. Biol. Med. Model. 2005, 2, 24. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Zhou, R.; Chen, B.; Chen, J. Nanohydroxyapatite/cellulose nanocrystals/silk fibroin ternary scaffolds for rat calvarial defect regeneration. RSC Adv. 2016, 6, 35684–35691. [Google Scholar] [CrossRef]
- Lyles, M.B.; Hu, J.C.; Varanasi, V.G.; Hollinger, J.O.; Athanasiou, K.A. Bone tissue engineering. Regen. Eng. Musculoskelet. Tissues Interfaces 2015, 1, 97–134. [Google Scholar]
- Durham, E.L.; Nicole Howie, R.; Hall, S.R.; Larson, N.; Oakes, B.; Houck, R.; Grey, Z.; Steed, M.; LaRue, A.C.; Muise-Helmericks, R.; et al. Optimising bone wound healing using BMP2 with absorbable collagen sponge and Talymed nanofiber scaffold. J. Transl. Med. 2018, 16, 321. [Google Scholar] [CrossRef]
- Oliveira, É.R.; Nie, L.; Podstawczyk, D.; Allahbakhsh, A.; Ratnayake, J.; Brasil, D.L.; Shavandi, A. Advances in growth factor delivery for bone tissue engineering. Int. J. Mol. Sci. 2021, 22, 903. [Google Scholar] [CrossRef] [PubMed]
- Maglio, M.; Salamanna, F.; Brogini, S.; Borsari, V.; Pagani, S.; Nicoli Aldini, N.; Giavaresi, G.; Fini, M. Histological, Histomorphometrical, and Biomechanical Studies of Bone-Implanted Medical Devices: Hard Resin Embedding. Biomed Res. Int. 2020, 2020, 1804630. [Google Scholar] [CrossRef] [Green Version]
- Ansari, M. Bone tissue regeneration: Biology, strategies and interface studies. Prog. Biomater. 2019, 8, 223–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Stocum, D.L. Fracture Healing. In Basic and Applied Bone Biology; Burr, D.B., Allen, M.R., Eds.; Elsevier Science Publishing Co., Inc.: Amsterdam, The Netherlands, 2014; pp. 205–223. [Google Scholar]
- Gruber, H.E.; Ingram, J.A. Basic Staining and Histochemical Techniques and Immunohistochemical Localizations Using Bone Sections. In Handbook of Histology Methods for Bone and Cartilage, 1st ed.; An, Y.H., Martin, K.L., Eds.; Humana Press: Totowa, NJ, USA, 2003; pp. 281–286. [Google Scholar]
- de Azevedo e Sousa Munhoz, M.; Torres Pomini, K.; de Guzzi Plepis, A.M.; da Conceic¸ão Amaro Martins, V.; Machado, E.G.; de Moraes, R. Elastin-derived scaffolding associated or not with bone morphogenetic protein (BMP) or hydroxyapatite (HA) in the repair process of metaphyseal bone defects. PLoS ONE 2020, 15, e0231112. [Google Scholar]
- Bin Sulaiman, S.; Keong, T.K.; Cheng, C.H.; Bin Saim, A.; Hj Idrus, R.B. Tricalcium phosphate/hydroxyapatite (TCP-HA) bone scaffold as potential candidate for the formation of tissue engineered bone. Indian J. Med. Res. 2013, 137, 1093–1101. [Google Scholar]
- Chen, X.; Zhao, Y.; Geng, S.; Miron, R.J.; Zhang, Q.; Wu, C.; Zhang, Y. In vivo experimental study on bone regeneration in critical bone defects using PIB nanogels/boron-containing mesoporous bioactive glass composite scaffold. Int. J. Nanomed. 2015, 10, 839–846. [Google Scholar]
- e Silva, E.P.; Huang, B.; Helaehil, J.V.; Nalesso, P.R.L.; Bagne, L.; de Oliveira, M.A.; Albiazetti, G.C.C.; Aldalbahi, A.; El-Newehy, M.; Santamaria, M.; et al. In vivo study of conductive 3D printed PCL/MWCNTs scaffolds with electrical stimulation for bone tissue engineering. Bio-Design Manuf. 2021, 4, 190–202. [Google Scholar] [CrossRef]
- Erben, R.G. Bone-Labeling Techniques. In Handbook of Histology Methods for Bone and Cartilage, 1st ed.; An, Y.H., Martin, K.L., Eds.; Humana Press: Totowa, NJ, USA, 2003; pp. 99–117. [Google Scholar]
- Shim, M.-J. Bone Changes in Femoral Bone of Mice Using Calcein Labeling. Korean J. Clin. Lab. Sci. 2016, 48, 114–117. [Google Scholar] [CrossRef]
- Goldschlager, T.; Abdelkader, A.; Kerr, J.; Boundy, I.; Jenkin, G. Undecalcified bone preparation for histology, histomorphometry and fluorochrome analysis. J. Vis. Exp. 2010, 35, e1707. [Google Scholar]
- Xiao, C.; Zhou, H.; Liu, G.; Zhang, P.; Fu, Y.; Hou, H.; Tang, T.; Fan, X. Bone marrow stromal cells with a combined expression of BMP-2 and VEGF-165 enhanced bone regeneration. Biomed. Mater. 2011, 6, 015013. [Google Scholar] [CrossRef]

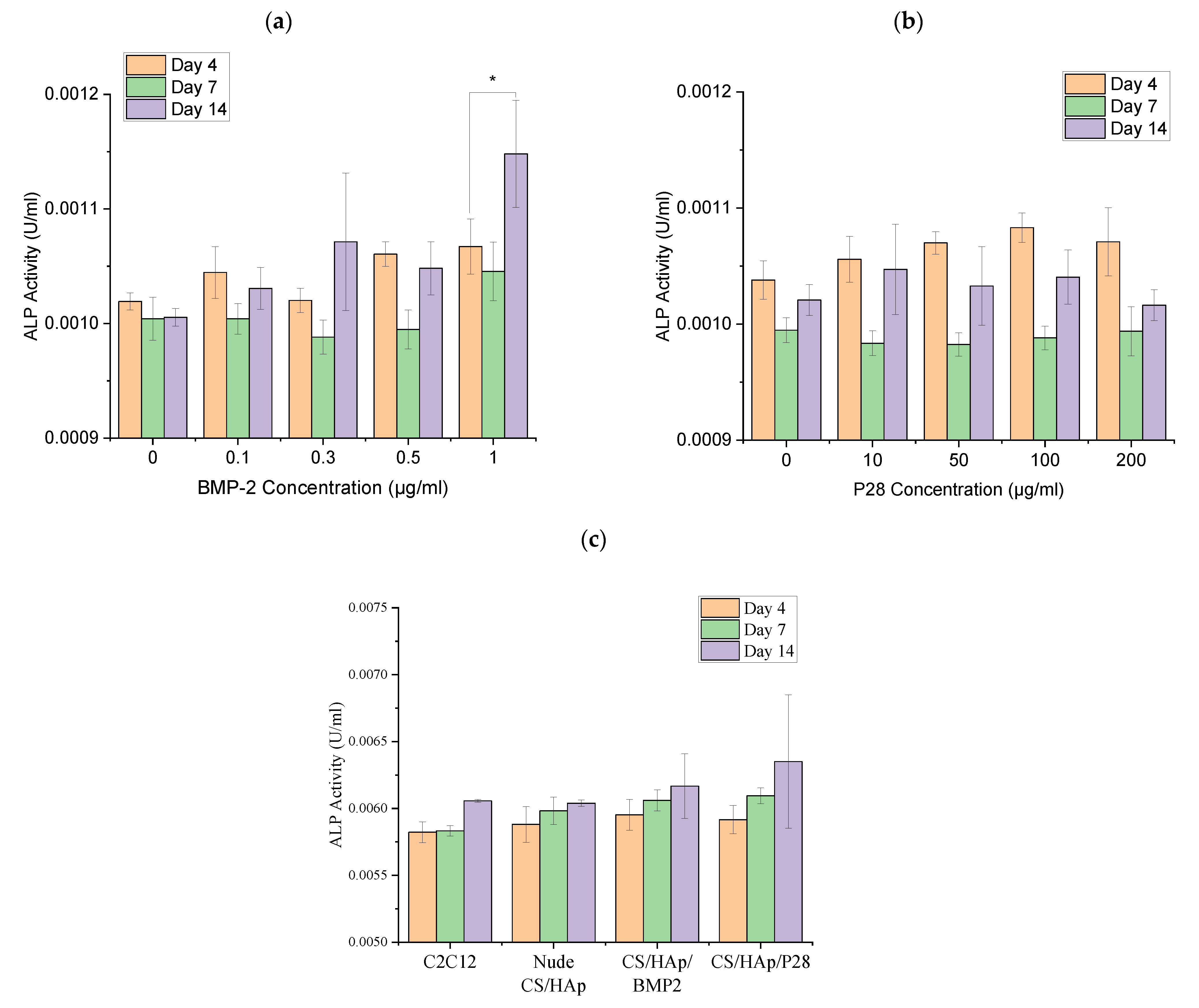

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azaman, F.A.; Daubiné, F.; Lebatard, A.; Brennan Fournet, M.E.; Devine, D.M. Chitosan/Hydroxyapatite Scaffolds with P28 as a Promising Osteoinductive Scaffold for Bone Healing Applications. Micro 2023, 3, 118-142. https://doi.org/10.3390/micro3010010
Azaman FA, Daubiné F, Lebatard A, Brennan Fournet ME, Devine DM. Chitosan/Hydroxyapatite Scaffolds with P28 as a Promising Osteoinductive Scaffold for Bone Healing Applications. Micro. 2023; 3(1):118-142. https://doi.org/10.3390/micro3010010
Chicago/Turabian StyleAzaman, Farah Alwani, Florence Daubiné, Amélie Lebatard, Margaret E. Brennan Fournet, and Declan M. Devine. 2023. "Chitosan/Hydroxyapatite Scaffolds with P28 as a Promising Osteoinductive Scaffold for Bone Healing Applications" Micro 3, no. 1: 118-142. https://doi.org/10.3390/micro3010010
APA StyleAzaman, F. A., Daubiné, F., Lebatard, A., Brennan Fournet, M. E., & Devine, D. M. (2023). Chitosan/Hydroxyapatite Scaffolds with P28 as a Promising Osteoinductive Scaffold for Bone Healing Applications. Micro, 3(1), 118-142. https://doi.org/10.3390/micro3010010






