In-Vitro Cell Response to Strontium/Magnesium-Doped Calcium Phosphate Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Instruments
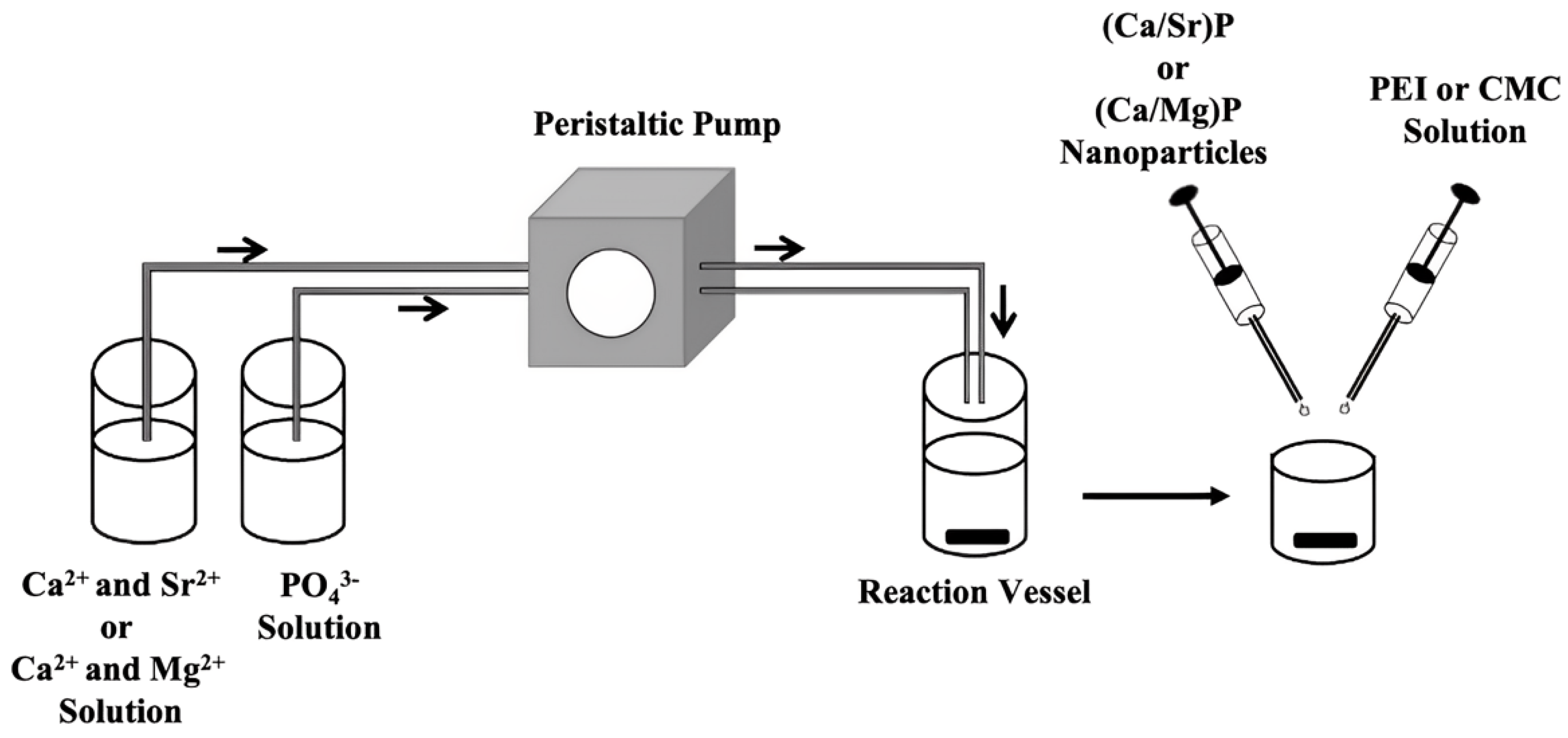
2.3. Synthesis of Strontium-Doped Calcium Phosphate Nanoparticles
2.4. Synthesis of Magnesium-Doped Calcium Phosphate Nanoparticles
2.5. Cell Culture Studies
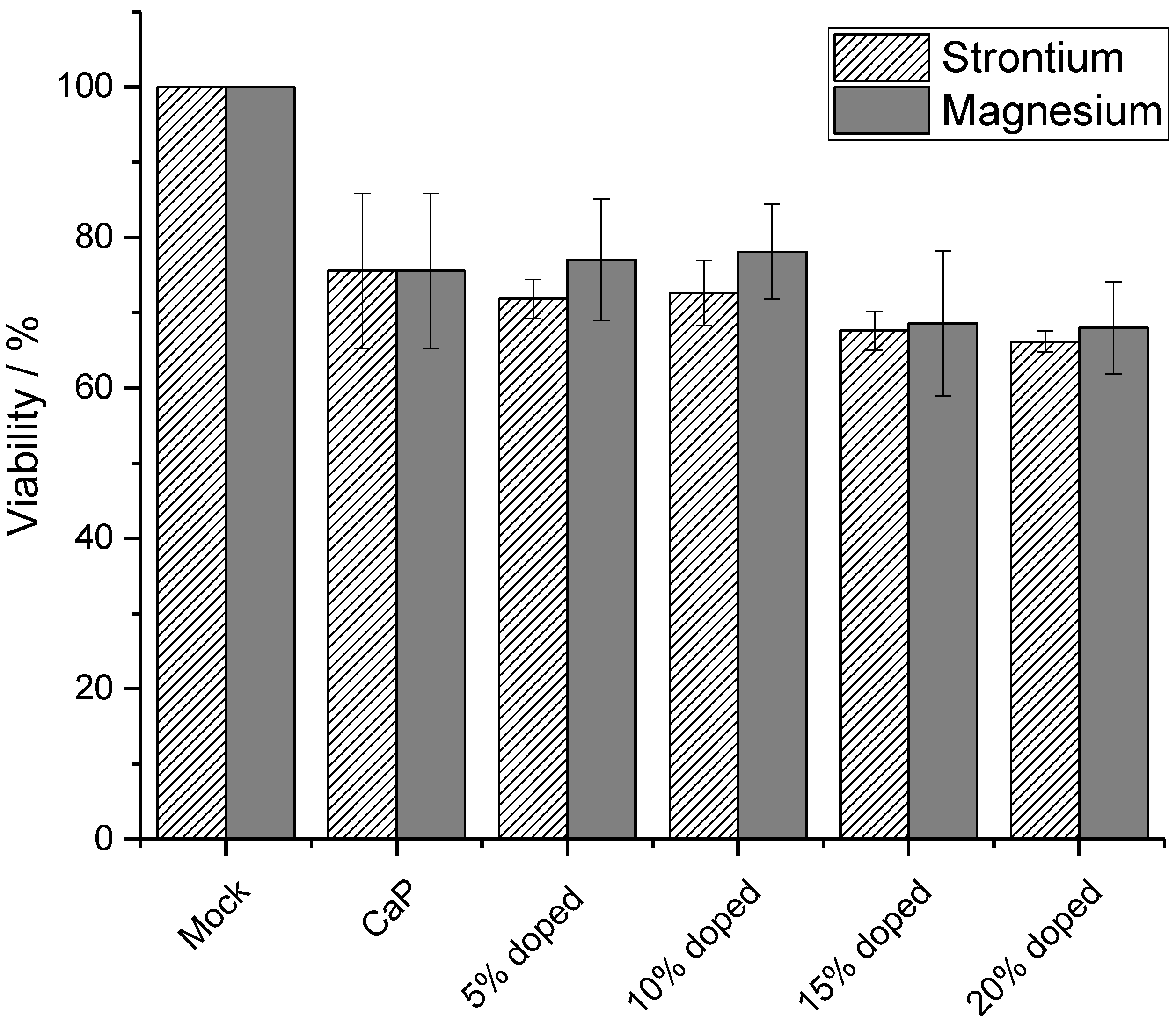
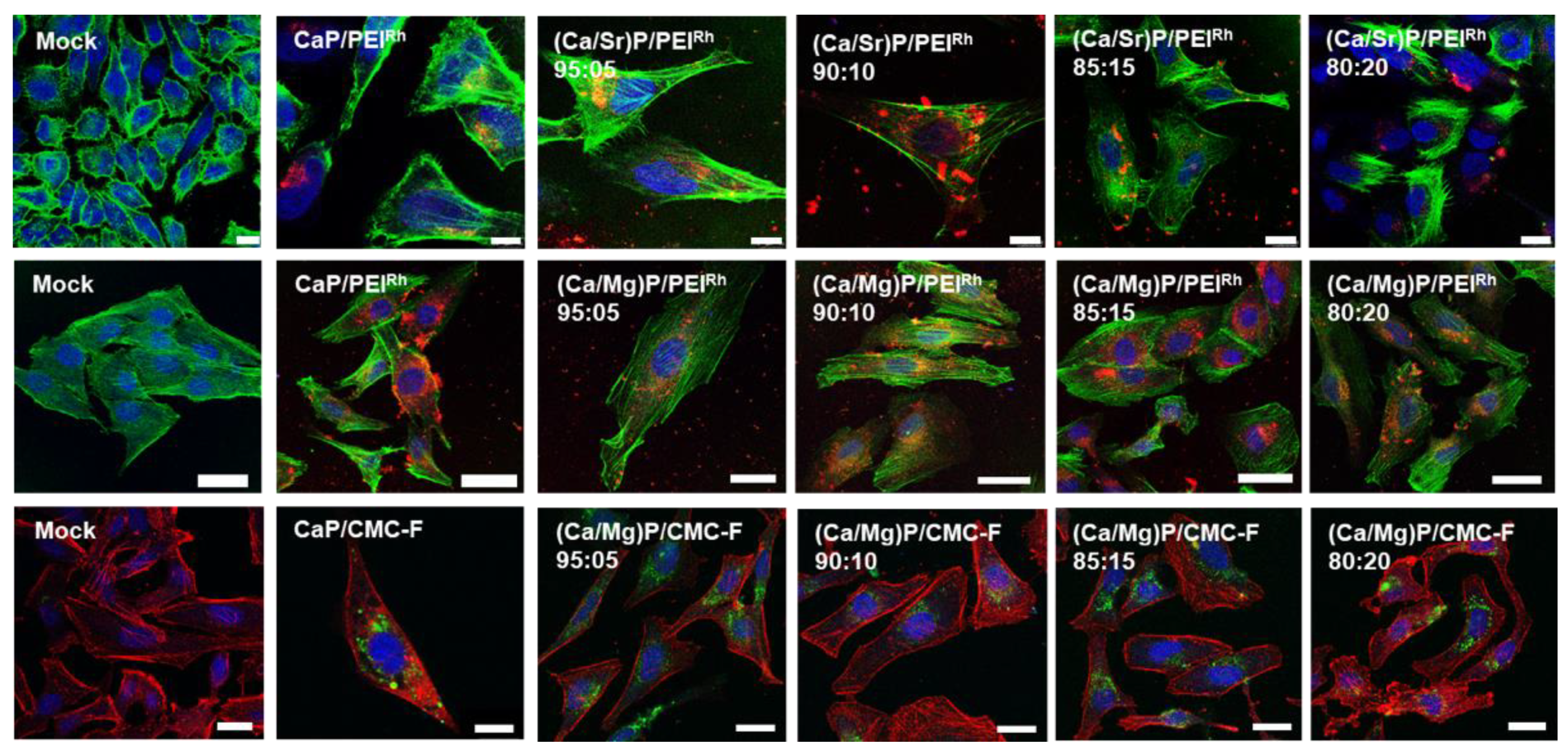
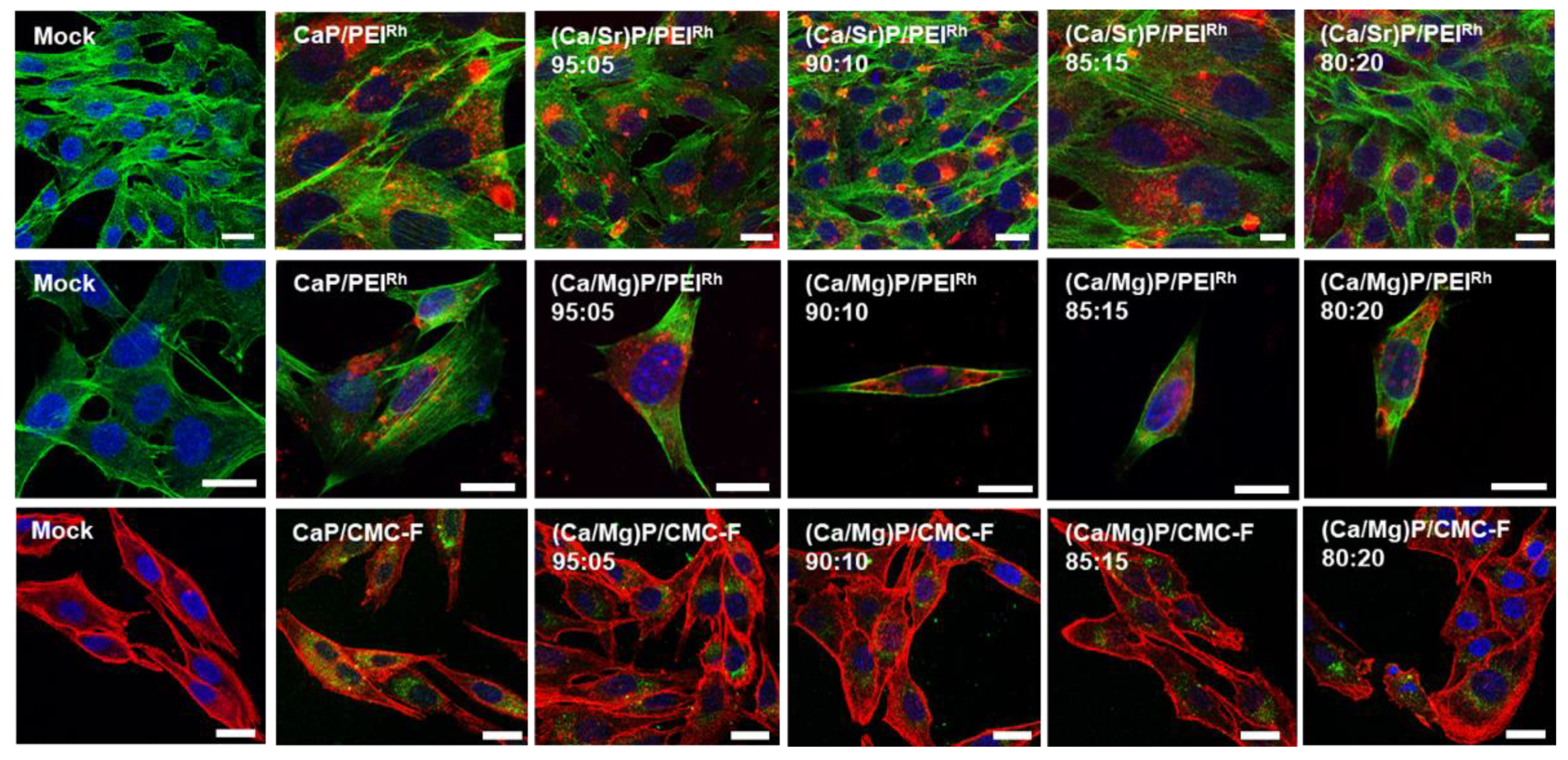
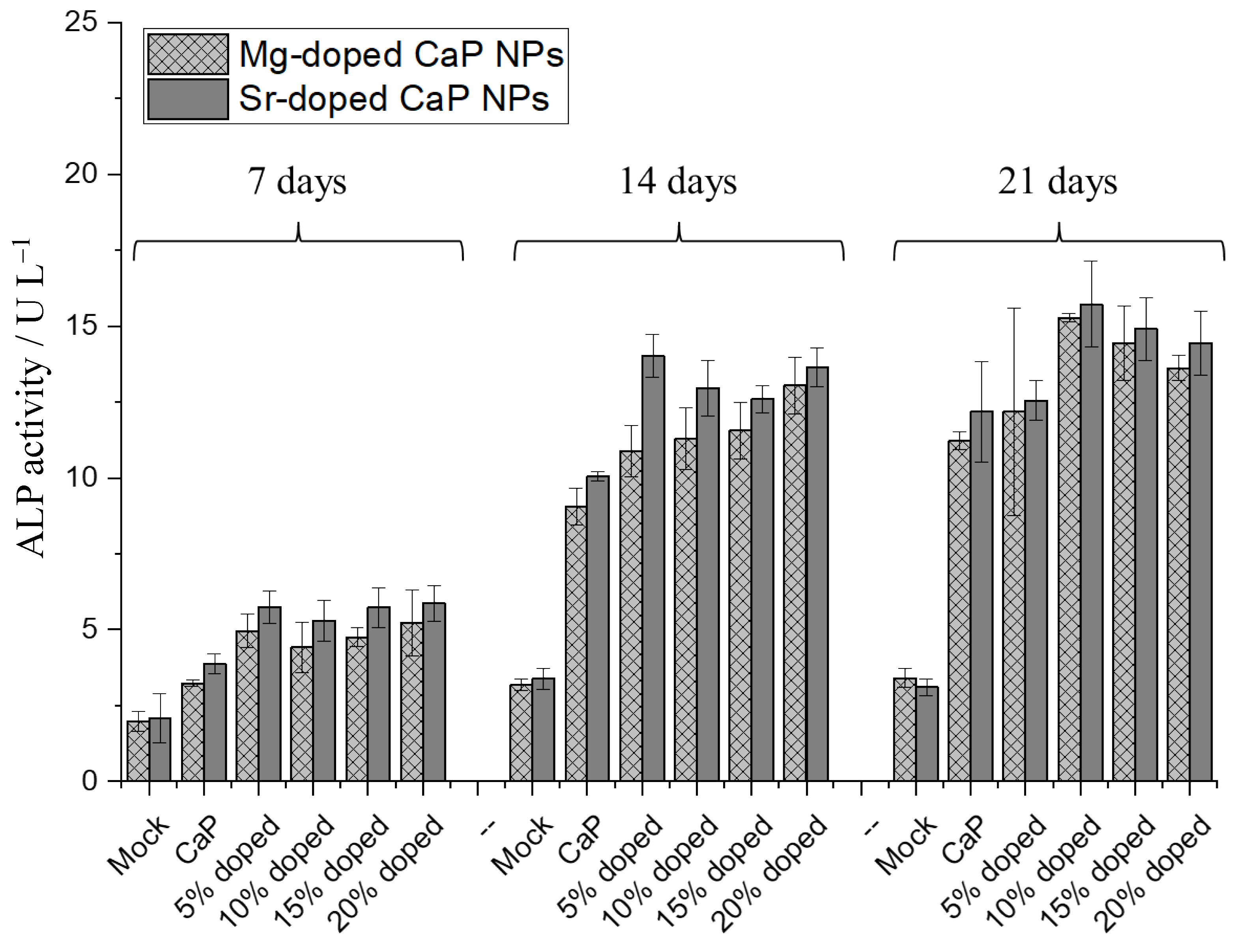
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dorozhkin, S.V. Functionalized calcium orthophosphates (CaPO4) and their biomedical applications. J. Mater. Chem. B 2019, 7, 7471–7489. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.Y.; Yu, H.J.; Chen, C.Z. Biological properties of calcium phosphate biomaterials for bone repair: A review. RSC Adv. 2018, 8, 2015–2033. [Google Scholar] [CrossRef]
- Arcos, D.; Boccaccini, A.R.; Bohner, M.; Diez-Perez, A.; Epple, M.; Gomez-Barrena, E.; Herrera, A.; Planell, J.A.; Rodriguez-Manas, L.; Vallet-Regi, M. The relevance of biomaterials to the prevention and treatment of osteoporosis. Acta Biomater. 2014, 10, 1793–1805. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, S.M.; Sharif Zein, S.H.; Othman, M.R.; Yang, F.; Jansen, J.A. Nanophase hydroxyapatite as a biomaterial in advanced hard tissue engineering: A review. Tissue Eng. B 2013, 19, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Epple, M. Review of potential health risks associated with nanoscopic calcium phosphate. Acta Biomater. 2018, 77, 1–14. [Google Scholar] [CrossRef]
- Sokolova, V.; Epple, M. Biological and medical applications of calcium phosphate nanoparticles. Chem. Eur. J. 2021, 27, 7471–7488. [Google Scholar] [CrossRef]
- Habraken, W.; Habibovic, P.; Epple, M.; Bohner, M. Calcium phosphates in biomedical applications: Materials for the future? Mater. Today 2016, 19, 69–87. [Google Scholar] [CrossRef]
- Mostaghaci, B.; Loretz, B.; Lehr, C.M. Calcium phosphate system for gene delivery: Historical background and emerging opportunities. Curr. Pharm. Des. 2016, 22, 1529–1533. [Google Scholar] [CrossRef]
- Liu, C.; He, H. Developments and Applications of Calcium Phosphate Bone Cements; Springer: Berlin, Germany, 2018. [Google Scholar]
- Demirel, M.; Canakci, D.; Aydin, M. Morphological and antibacterial effects of silver, magnesium, silicon and strontium modified calcium phosphate bone cements prepared by the sol–gel method. Adv. Appl. Ceram. 2020, 119, 324–433. [Google Scholar] [CrossRef]
- Tenkumo, T.; Vanegas Sáenz, J.R.; Nakamura, K.; Shimizu, Y.; Sokolova, V.; Epple, M.; Kamano, Y.; Egusa, H.; Sugaya, T.; Sasaki, K. Prolonged release of bone morphogenetic protein-2 in vivo by gene transfection with DNA-functionalized calcium phosphate nanoparticle-loaded collagen scaffolds. Mater. Sci. Eng. C 2018, 92, 172–183. [Google Scholar] [CrossRef]
- Schlickewei, C.; Klatte, T.O.; Wildermuth, Y.; Laaff, G.; Rueger, J.M.; Ruesing, J.; Chernousova, S.; Lehmann, W.; Epple, M. A bioactive nano-calcium phosphate paste for in-situ transfection of BMP-7 and VEGF-A in a rabbit critical-size bone defect: Results of an in vivo study. J. Mater. Sci. Mater. Med. 2019, 30, 15. [Google Scholar] [CrossRef] [PubMed]
- Tenkumo, T.; Rojas-Sánchez, L.; Vanegas Sáenz, J.R.; Ogawa, T.; Miyashita, M.; Yoda, N.; Prymak, O.; Sokolova, V.; Sasaki, K.; Epple, M. Reduction of inflammation in a chronic periodontitis model in rats by TNF-α gene silencing with a topically applied siRNA-loaded calcium phosphate paste. Acta Biomater. 2020, 105, 263–279. [Google Scholar] [CrossRef] [PubMed]
- Van Norman, G.A. Update to drugs, devices, and the FDA: How recent legislative changes have impacted approval of new therapies. JACC Basic Transl. Sci. 2020, 5, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Van Norman, G.A. Drugs and devices: Comparison of European and U.S. approval processes. JACC Basic Transl. Sci. 2016, 1, 399–412. [Google Scholar] [CrossRef]
- Van Norman, G.A. Drugs, Devices, and the FDA: Part 2: An overview of approval processes: FDA approval of medical devices. JACC Basic Transl. Sci. 2016, 1, 277–287. [Google Scholar] [CrossRef]
- Ramalhinho, A.C.; Castelo-Branco, M. Preparation of an academic clinical trial. Methods Mol. Biol. 2021, 2197, 317–330. [Google Scholar]
- Enax, J.; Meyer, F.; Schulze zur Wiesche, E.; Epple, M. On the application of calcium phosphate micro- and nanoparticles as food additive. Nanomaterials 2022, 12, 4075. [Google Scholar] [CrossRef]
- Graziani, V.; Fosca, M.; Egorov, A.A.; Zobkov, Y.V.; Fedotov, A.Y.; Baranchikov, A.E.; Ortenzi, M.; Caminiti, R.; Komlev, V.S.; Rau, J.V. Zinc-releasing calcium phosphate cements for bone substitute materials. Ceram. Int. 2016, 42, 17310–17316. [Google Scholar] [CrossRef]
- Khan, A.F.; Saleem, M.; Afzal, A.; Ali, A.; Khan, A.; Khan, A.R. Bioactive behavior of silicon substituted calcium phosphate based bioceramics for bone regeneration. Mater. Sci. Eng. C 2014, 35, 245–252. [Google Scholar] [CrossRef]
- Yang, G.; Liu, J.; Li, F.; Pan, Z.; Ni, X.; Shen, Y.; Xu, H.; Huang, Q. Bioactive calcium sulfate/magnesium phosphate cement for bone substitute applications. Mater. Sci. Eng. C 2014, 35, 70–76. [Google Scholar] [CrossRef]
- Habibovic, P.; Juhl, M.V.; Clyens, S.; Martinetti, R.; Dolcini, L.; Theilgaard, N.; Van Blitterswijk, C.A. Comparison of two carbonated apatite ceramics in vivo. Acta Biomater. 2010, 6, 2219–2226. [Google Scholar] [CrossRef] [PubMed]
- Khairoun, I.; Magne, D.; Gauthier, O.; Bouler, J.M.; Aguado, E.; Daculsi, G.; Weiss, P. In vitro characterization and in vivo properties of a carbonated apatite bone cement. J. Biomed. Mater. Res. 2002, 60, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Thian, E.S.; Huang, J.; Best, S.M.; Barber, Z.H.; Bonfield, W. Silicon-substituted hydroxyapatite: The next generation of bioactive coatings. Mater. Sci. Eng. C 2007, 27, 251–256. [Google Scholar] [CrossRef]
- Bohner, M. Silicon-substituted calcium phosphates—A critical view. Biomaterials 2009, 30, 6403–6406. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.R. Review of bioactive glass: From Hench to hybrids. Acta Biomater. 2013, 9, 4457–4486. [Google Scholar] [CrossRef]
- Ivanova, A.A.; Surmenev, R.A.; Surmeneva, M.A.; Mukhametkaliyev, T.; Loza, K.; Prymak, O.; Epple, M. Hybrid biocomposite with a tunable antibacterial activity and bioactivity based on RF magnetron sputter deposited coating and silver nanoparticles. Appl. Surf. Sci 2015, 329, 212–218. [Google Scholar] [CrossRef]
- Ewald, A.; Hösel, D.; Patel, S.; Grover, L.M.; Barralet, J.E.; Gbureck, U. Silver-doped calcium phosphate cements with antimicrobial activity. Acta Biomater. 2011, 7, 4064–4070. [Google Scholar] [CrossRef]
- Shimabukuro, M.; Hayashi, K.; Kishida, R.; Tsuchiya, A.; Ishikawa, K. Surface functionalization with copper endows carbonate apatite honeycomb scaffold with antibacterial, proangiogenic, and pro-osteogenic activities. Biomater Adv. 2022, 135, 212751. [Google Scholar] [CrossRef]
- Ghosh, R.; Swart, O.; Westgate, S.; Miller, B.L.; Yates, M.Z. Antibacterial copper-hydroxyapatite composite coatings via electrochemical synthesis. Langmuir 2019, 35, 5957–5966. [Google Scholar] [CrossRef]
- Vukomanovic, M.; Gazvoda, L.; Anicic, N.; Rubert, M.; Suvorov, D.; Müller, R.; Hofmann, S. Multi-doped apatite: Strontium, magnesium, gallium and zinc ions synergistically affect osteogenic stimulation in human mesenchymal cells important for bone tissue engineering. Biomater Adv. 2022, 140, 213051. [Google Scholar] [CrossRef]
- Wang, X.; Ito, A.; Sogo, Y.; Li, X.; Oyane, A. Zinc-containing apatite layers on external fixation rods promoting cell activity. Acta Biomater. 2010, 6, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Iafisco, M.; Ruffini, A.; Adamiano, A.; Sprio, S.; Tampieri, A. Biomimetic magnesium–carbonate-apatite nanocrystals endowed with strontium ions as anti-osteoporotic trigger. Mater. Sci. Eng. C 2014, 35, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Maguire, M.E.; Cowan, J.A. Magnesium chemistry and biochemistry. Biometals 2002, 15, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Nabiyouni, M.; Ren, Y.; Bhaduri, S.B. Magnesium substitution in the structure of orthopedic nanoparticles: A comparison between amorphous magnesium phosphates, calcium magnesium phosphates, and hydroxyapatites. Mater. Sci. Eng. C 2015, 52, 11–17. [Google Scholar] [CrossRef]
- He, F.; Luc, T.; Fang, X.; Li, Y.; Zuo, F.; Deng, X.; Ye, J. Effects of strontium amount on the mechanical strength and cell-biological performance of magnesium-strontium phosphate bioceramics for bone regeneration. Mater. Sci. Eng. C 2020, 112, 110892. [Google Scholar] [CrossRef]
- Verberckmoes, S.C.; De Broe, M.E.; D’Haese, P.C. Dose-dependent effects of strontium on osteoblast function and mineralization. Kidney Int. 2003, 64, 534–543. [Google Scholar] [CrossRef]
- Schrooten, I.; Behets, G.J.; Cabrera, W.E.; Vercauteren, S.R.; Lamberts, L.V.; Verberckmoes, S.C.; Bervoets, A.J.; Dams, G.; Goodman, W.G.; De Broe, M.E.; et al. Dose-dependent effects of strontium on bone of chronic renal failure rats. Kidney Int. 2003, 63, 927–935. [Google Scholar] [CrossRef]
- Marx, D.; Yazdi, A.R.; Papini, M.; Towler, M. A review of the latest insights into the mechanism of action of strontium in bone. Bone Rep. 2020, 12, 100273. [Google Scholar] [CrossRef]
- Caverzasio, J. Strontium ranelate promotes osteoblastic cell replication through at least two different mechanisms. Bone 2008, 42, 1131–1136. [Google Scholar] [CrossRef]
- Fardellone, P.; Roux, C.; Fechtenbaum, J.; Kolta, S.; Kruse, H.P.; Sawicki, A.; Hoszowski, K.; Padrino, J.; Sorensen, O.; Reginster, J.Y.; et al. Strontium ranelate reduces the risk of vertebral fractures in osteoporotic postmenopausal women whatever the baseline vertebral fracture status. Bone 2005, 36, 403. [Google Scholar]
- Pilmane, M.; Salma-Ancane, K.; Loca, D.; Locs, J.; Berzina-Cimdina, L. Strontium and strontium ranelate: Historical review of some of their functions. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 1222–1230. [Google Scholar] [CrossRef] [PubMed]
- Scalera, F.; Palazzo, B.; Barca, A.; Gervaso, F. Sintering of magnesium-strontium doped hydroxyapatite nanocrystals: Towards the production of 3D biomimetic bone scaffolds. J. Biomed. Mater. Res. A 2020, 108, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Khalifehzadeh, R.; Arami, H. DNA-templated strontium-doped calcium phosphate nanoparticles for gene delivery in bone cells. ACS Biomater. Sci. Eng. 2019, 5, 3201–3211. [Google Scholar] [CrossRef] [PubMed]
- Panzavolta, S.; Torricelli, P.; Casolari, S.; Parrilli, A.; Fini, M.; Bigi, A. Strontium-substituted hydroxyapatite-gelatin biomimetic scaffolds modulate bone cell response. Macromol. Biosci. 2018, 18, e1800096. [Google Scholar] [CrossRef]
- Lode, A.; Heiss, C.; Knapp, G.; Thomas, J.; Nies, B.; Gelinsky, M.; Schumacher, M. Strontium-modified premixed calcium phosphate cements for the therapy of osteoporotic bone defects. Acta Biomater. 2018, 65, 475–485. [Google Scholar] [CrossRef]
- Kruppke, B.; Heinemann, C.; Gebert, A.; Rohnke, M.; Weiß, M.; Henß, A.; Wiesmann, H.P.; Hanke, T. Strontium substitution of gelatin modified calcium hydrogen phosphates as porous hard tissue substitutes. J. Biomed. Mater. Res. A 2021, 109, 722–732. [Google Scholar] [CrossRef]
- Kruppke, B.; Ray, S.; Alt, V.; Rohnke, M.; Kern, C.; Kampschulte, M.; Heinemann, C.; Budak, M.; Adam, J.; Döhner, N.; et al. Gelatin-modified calcium/strontium hydrogen phosphates stimulate bone regeneration in osteoblast/osteoclast co-culture and in osteoporotic rat femur defects—In vitro to in vivo translation. Molecules 2020, 25, 5103. [Google Scholar] [CrossRef]
- Wagner, A.S.; Schumacher, M.; Rohnke, M.; Glenske, K.; Gelinsky, M.; Arnhold, S.; Mazurek, S.; Wenisch, S. Incorporation of silicon into strontium modified calcium phosphate bone cements promotes osteoclastogenesis of human peripheral mononuclear blood cells. Biomed. Mater. 2019, 14, 025004. [Google Scholar] [CrossRef]
- Kern, C.; Quade, M.; Ray, S.; Thomas, J.; Schumacher, M.; Gemming, T.; Gelinsky, M.; Alt, V.; Rohnke, M. Investigation of strontium transport and strontium quantification in cortical rat bone by time-of-flight secondary ion mass spectrometry. J. R. Soc. Interface 2019, 16, 20180638. [Google Scholar] [CrossRef]
- Detsch, R.; Hagmeyer, D.; Neumann, M.; Schaefer, S.; Vortkamp, A.; Wuelling, M.; Ziegler, G.; Epple, M. The resorption of nanocrystalline calcium phosphates by osteoclast-like cells. Acta Biomater. 2010, 6, 3223–3233. [Google Scholar] [CrossRef]
- Kollenda, S.A.; Klose, J.; Knuschke, T.; Sokolova, V.; Schmitz, J.; Staniszewska, M.; Costa, P.F.; Herrmann, K.; Westendorf, A.M.; Fendler, W.P.; et al. In vivo biodistribution of calcium phosphate nanoparticles after intravascular, intramuscular, intratumoral, and soft tissue administration in mice investigated by small animal PET/CT. Acta Biomater. 2020, 109, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, V.; Rojas-Sanchez, L.; Bialas, N.; Schulze, N.; Epple, M. Calcium phosphate nanoparticle-mediated transfection in 2D and 3D mono- and co-culture cell models. Acta Biomater. 2019, 84, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Kozlova, D.; Chernousova, S.; Knuschke, T.; Buer, J.; Westendorf, A.M.; Epple, M. Cell targeting by antibody-functionalized calcium phosphate nanoparticles. J. Mater. Chem. 2012, 22, 396–404. [Google Scholar] [CrossRef]
- Pelaz, B.; Alexiou, C.; Alvarez-Puebla, R.A.; Alves, F.; Andrews, A.M.; Ashraf, S.; Balogh, L.P.; Ballerini, L.; Bestetti, A.; Brendel, C.; et al. Diverse applications of nanomedicine. ACS Nano 2017, 11, 2313–2381. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Kim, J.; Herrera, M.; Mukherjee, A.; Kabanov, A.V.; Sahay, G. Brief update on endocytosis of nanomedicines. Adv. Drug Deliv. Rev. 2019, 144, 90–111. [Google Scholar] [CrossRef]
- Foroozandeh, P.; Aziz, A.A. Insight into cellular uptake and intracellular trafficking of nanoparticles. Nanoscale Res. Lett. 2018, 13, 339. [Google Scholar] [CrossRef]
- Zhang, S.; Gao, H.; Bao, G. Physical principles of nanoparticle cellular endocytosis. ACS Nano 2015, 9, 8655–8671. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Nanosized and nanocrystalline calcium orthophosphates. Acta Biomater. 2010, 6, 715–734. [Google Scholar] [CrossRef]
- Dorozhkin, S.V.; Epple, M. Biological and medical significance of calcium phosphates. Angew. Chem. Int. Ed. 2002, 41, 3130–3146. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Amorphous calcium (ortho)phosphates. Acta Biomater. 2010, 6, 4457–4475. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Synthetic amorphous calcium phosphates (ACPs): Preparation, structure, properties, and biomedical applications. Biomater. Sci. 2021, 9, 7748–7798. [Google Scholar] [PubMed]
- Koutsoukos, P.G.; Nancollas, G.H. Influence of strontium ion on the crystallization of hydroxyapatite from aqueous solution. J. Phys. Chem. 1981, 85, 2403–2408. [Google Scholar] [CrossRef]
- Bigi, A.; Falini, G.; Foresti, E.; Ripamonti, A.; Gazzano, M.; Roveri, N. Magnesium influence on hydroxyapatite crystallization. J. Inorg. Biochem. 1993, 49, 69–78. [Google Scholar] [CrossRef]
- Paul, A.; Eun, C.J.; Song, J.M. Cytotoxicity mechanism of non-viral carriers polyethylenimine and poly-l-lysine using real time high-content cellular assay. Polymer 2014, 55, 5178–5188. [Google Scholar] [CrossRef]
- Parhamifar, L.; Larsen, A.K.; Hunter, A.C.; Andresen, T.L.; Moghimi, S.M. Polycation cytotoxicity: A delicate matter for nucleic acid therapy-focus on polyethylenimine. Soft Matter 2010, 6, 4001–4009. [Google Scholar] [CrossRef]
- Neumann, S.; Kovtun, A.; Dietzel, I.D.; Epple, M.; Heumann, R. The use of size-defined DNA-functionalized calcium phosphate nanoparticles to minimise intracellular calcium disturbance during transfection. Biomaterials 2009, 30, 6794–6802. [Google Scholar] [CrossRef]
- Ewence, A.E.; Bootman, M.; Roderick, H.L.; Skepper, J.N.; McCarthy, G.; Epple, M.; Neumann, M.; Shanahan, C.M.; Proudfoot, D. Calcium phosphate crystals induce cell death in human vascular smooth muscle cells—A potential mechanism in atherosclerotic plaque destabilization. Circ. Res. 2008, 103, e28–e32. [Google Scholar] [CrossRef]
- Miclau, T.; Edin, M.L.; Lester, G.E.; Lindsey, R.W.; Dahners, L.E. Bone toxicity of locally applied aminoglycosides. J. Orthpaed. Trauma 1995, 9, 401–406. [Google Scholar] [CrossRef]
- Varanasi, V.G.; Saiz, E.; Loomer, P.M.; Ancheta, B.; Uritani, N.; Ho, S.P.; Tomsia, A.P.; Marshall, S.J.; Marshall, G.W. Enhanced osteocalcin expression by osteoblast-like cells (MC3T3-E1) exposed to bioactive coating glass (SiO2-CaO-P2O5-MgO-K2O-Na2O system) ions. Acta Biomater. 2009, 5, 3536–3547. [Google Scholar] [CrossRef]
- Sokolova, V.; Kozlova, D.; Knuschke, T.; Buer, J.; Westendorf, A.M.; Epple, M. Mechanism of the uptake of cationic and anionic calcium phosphate nanoparticles by cells. Acta Biomater. 2013, 9, 7527–7535. [Google Scholar] [CrossRef]
- Rotan, O.; Severin, K.N.; Pöpsel, S.; Peetsch, A.; Merdanovic, M.; Ehrmann, M.; Epple, M. Uptake of the proteins HTRA1 and HTRA2 by cells mediated by calcium phosphate nanoparticles. Beilstein J. Nanotechnol. 2017, 8, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Kollenda, S.; Kopp, M.; Wens, J.; Koch, J.; Schulze, N.; Papadopoulos, C.; Pöhler, R.; Meyer, H.; Epple, M. A pH-sensitive fluorescent protein sensor to follow the pathway of calcium phosphate nanoparticles into cells. Acta Biomater. 2020, 111, 406–417. [Google Scholar] [CrossRef] [PubMed]
- Beddoes, C.M.; Case, C.P.; Briscoe, W.H. Understanding nanoparticle cellular entry: A physicochemical perspective. Adv. Coll. Interface Sci. 2015, 218, 48–68. [Google Scholar] [CrossRef]
- Canton, I.; Battaglia, G. Endocytosis at the nanoscale. Chem. Soc. Rev. 2012, 41, 2718–2739. [Google Scholar] [CrossRef] [PubMed]
- Feliu, N.; Sun, X.; Alvarez Puebla, R.A.; Parak, W.J. Quantitative particle–cell interaction: Some basic physicochemical pitfalls. Langmuir 2017, 33, 6639–6646. [Google Scholar] [CrossRef] [PubMed]
- Nazarenus, M.; Zhang, Q.; Soliman, M.G.; del Pino, P.; Pelaz, B.; Carregal-Romero, S.; Rejman, J.; Rothen-Rutishauser, B.; Clift, M.J.D.; Zellner, R.; et al. In vitro interaction of colloidal nanoparticles with mammalian cells: What have we learned thus far? Beilstein J. Nanotechnol. 2014, 5, 1477–1490. [Google Scholar] [CrossRef]
- Wennberg, C.; Hessle, L.; Lundberg, P.; Mauro, S.; Narisawa, S.; Lerner, U.H.; Millan, J.L. Functional characterization of osteoblasts and osteoclasts from alkaline phosphatase knockout mice. J. Bone Miner. Res. 2000, 15, 1879–1888. [Google Scholar] [CrossRef]
- Hessle, L.; Johnson, K.A.; Anderson, H.C.; Narisawa, S.; Sali, A.; Goding, J.W.; Terkeltaub, R.; Millan, J.L. Tissue-nonspecific alkaline phosphatase and plasma cell membrane glycoprotein-1 are central antagonistic regulators of bone mineralization. Proc. Natl. Acad. Sci. USA 2002, 99, 9445–9449. [Google Scholar] [CrossRef]
- Ko, C.L.; Chen, J.C.; Tien, Y.C.; Hung, C.C.; Wang, J.C.; Chen, W.C. Osteoregenerative capacities of dicalcium phosphate-rich calcium phosphate bone cement. J. Biomed. Mater. Res. A 2015, 103, 203–210. [Google Scholar] [CrossRef]
- Tsikourkitoudi, V.; Karlsson, J.; Merkl, P.; Loh, E.; Henriques-Normark, B.; Sotiriou, G.A. Flame-made calcium phosphate nanoparticles with high drug loading for delivery of biologics. Molecules 2020, 25, E1747. [Google Scholar] [CrossRef]
- Rojas-Sanchez, L.; Loza, K.; Epple, M. Synthesis and intracellular tracing surface-functionalized calcium phosphate nanoparticles by super-resolution microscopy (STORM). Materialia 2020, 12, 100773. [Google Scholar] [CrossRef]
- Khalifehzadeh, R.; Arami, H. Biodegradable calcium phosphate nanoparticles for cancer therapy. Adv. Coll. Interface Sci. 2020, 279, 102157. [Google Scholar] [CrossRef] [PubMed]
- Uskoković, V.; Tang, S.; Nikolić, M.G.; Marković, S.; Wu, V.M. Calcium phosphate nanoparticles as intrinsic inorganic antimicrobials: In search of the key particle property. Biointerphases 2019, 14, 031001. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.; Lode, A.; Helth, A.; Gelinsky, M. A novel strontium(II)-modified calcium phosphate bone cement stimulates human-bone-marrow-derived mesenchymal stem cell proliferation and osteogenic differentiation in vitro. Acta Biomater. 2013, 9, 9547–9557. [Google Scholar] [CrossRef] [PubMed]
- Pina, S.; Vieira, S.I.; Rego, P.; Torres, P.M.C.; da Cruz e Silva, O.A.B.; da Cruz e Silva, E.F.; Ferreira, J.M.F. Biological responses of brushite-forming Zn-and ZnSr-substituted β-Tricalcium phosphate bone cements. Eur. Cells Mater. 2010, 20, 162–177. [Google Scholar] [CrossRef]
- Pina, S.; Torres, P.M.; Goetz-Neunhoeffer, F.; Neubauer, J.; Ferreira, J.M.F. Newly developed Sr-substituted α-TCP bone cements. Acta Biomater. 2010, 6, 928–935. [Google Scholar] [CrossRef]
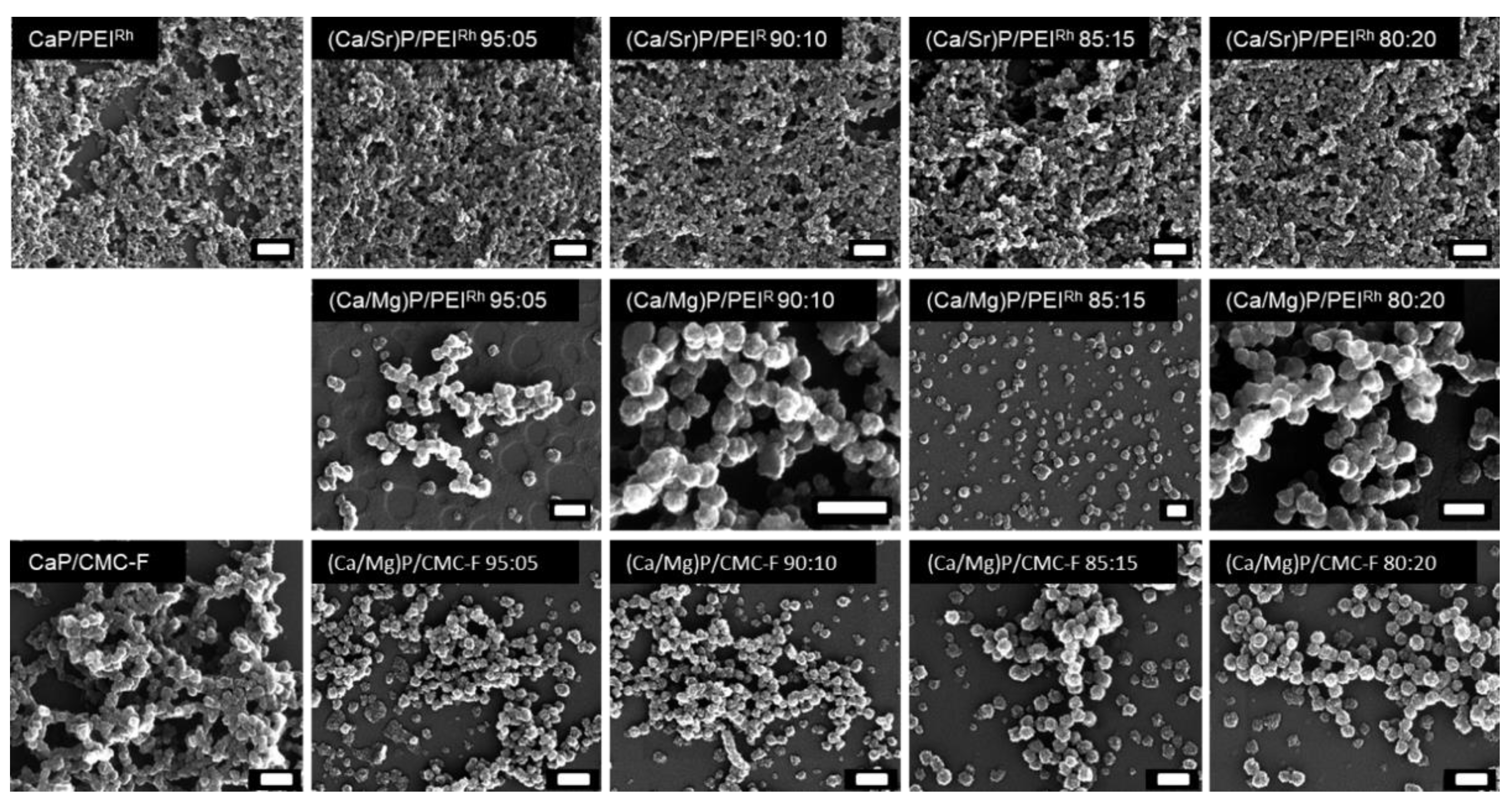
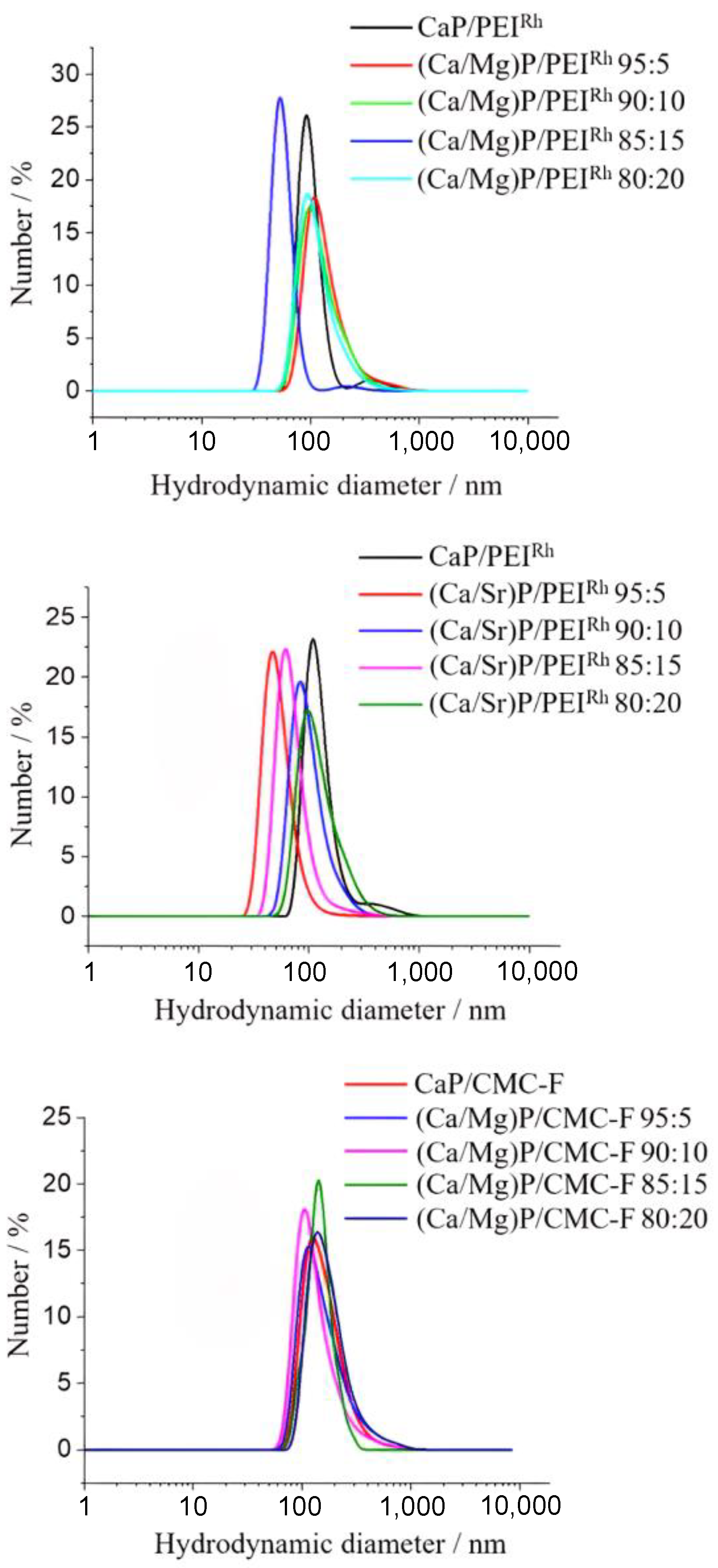
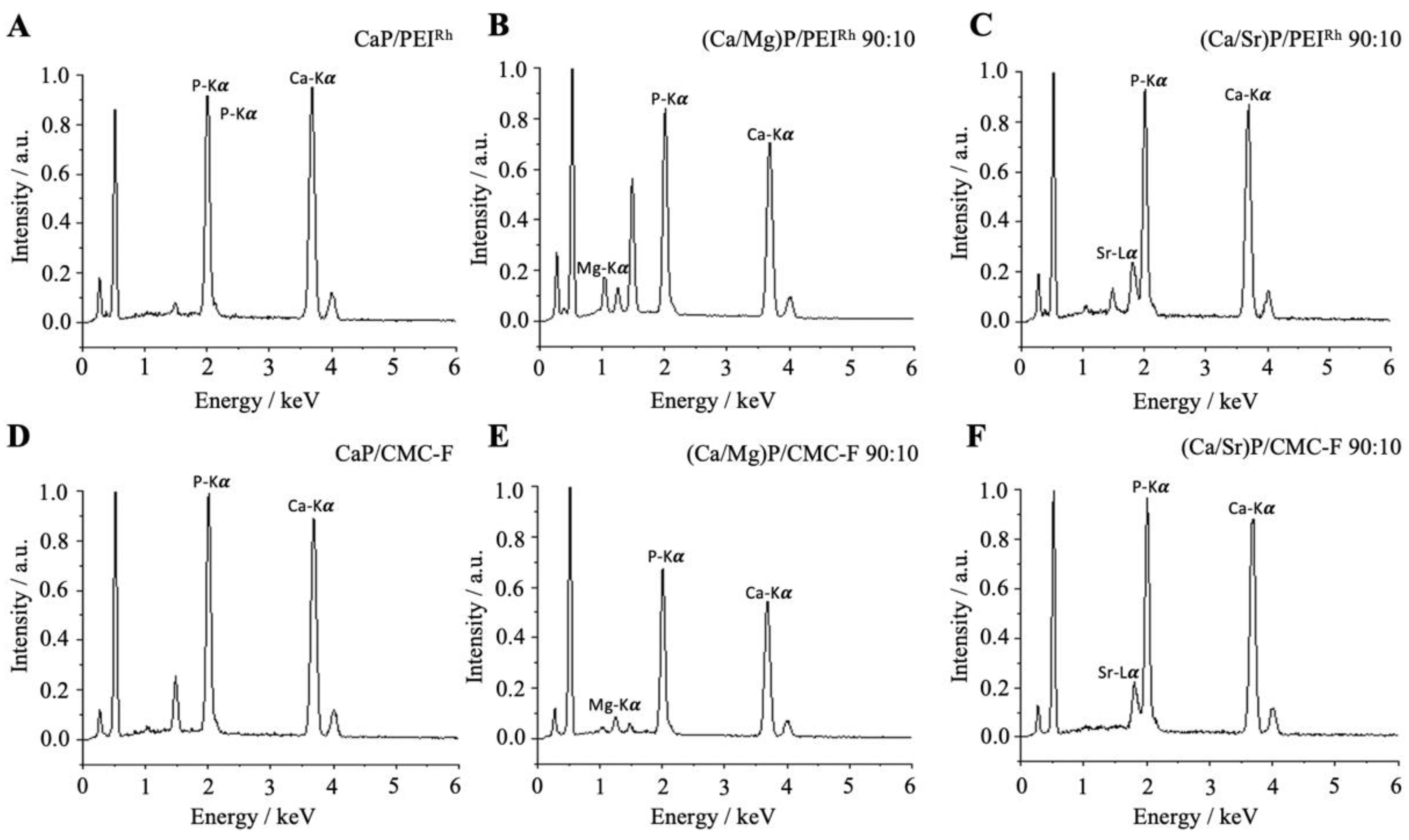
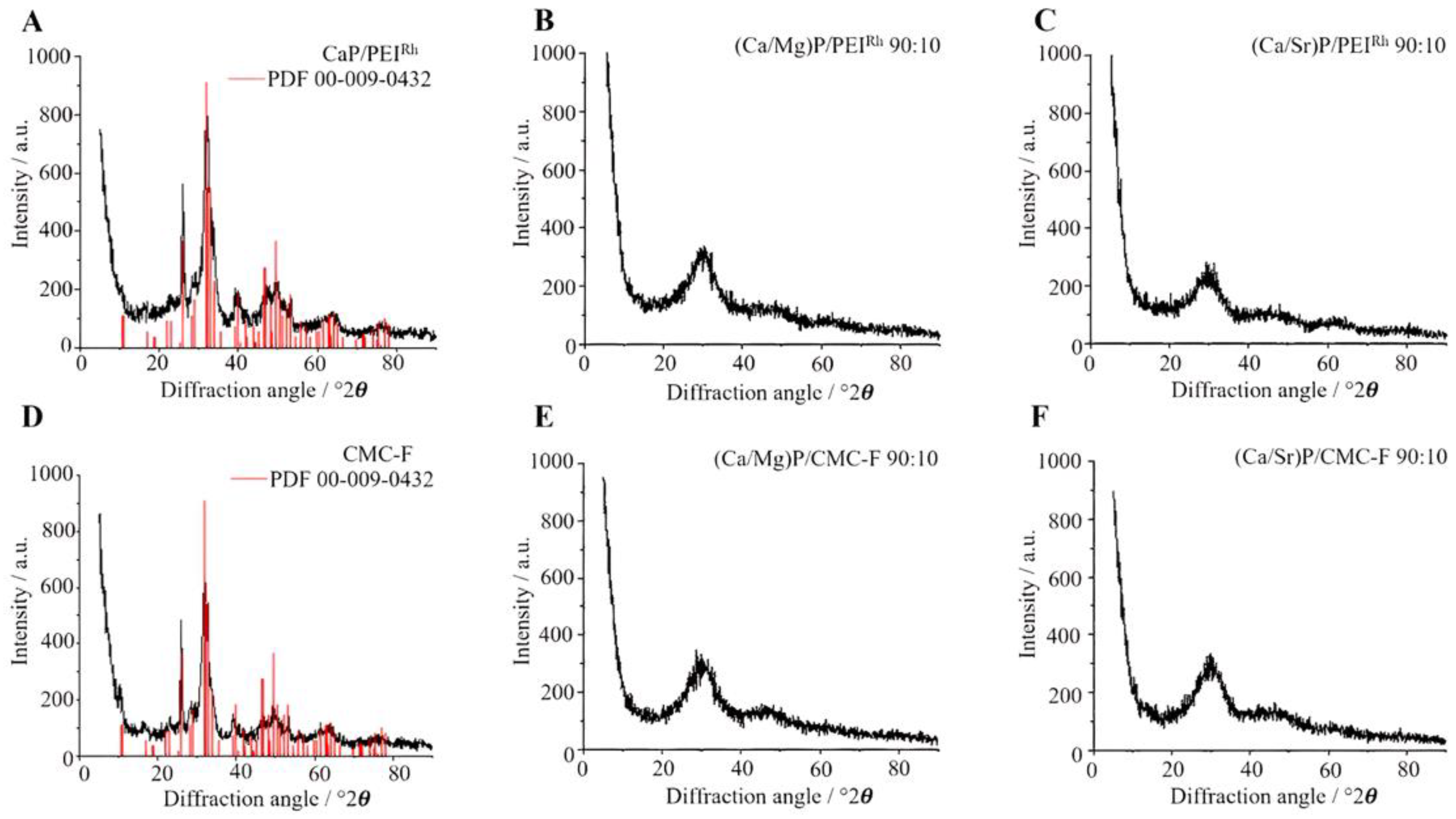
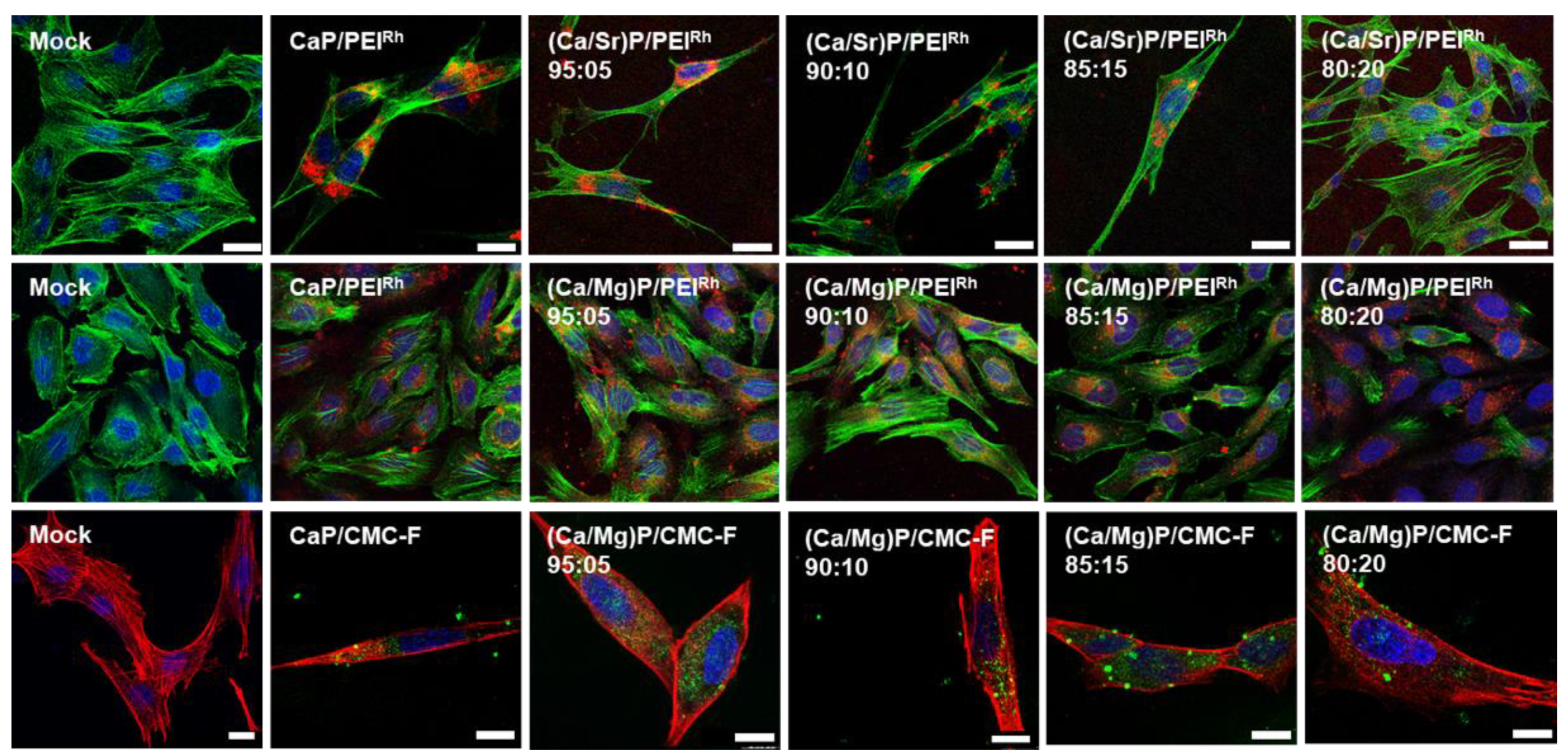
| Sample | Hydrodynamic Diameter (DLS)/nm | Zeta Potential/mV | Solid Core Diameter (SEM)/nm | Molar Ratio Ca:Mg/Sr by AAS/mol% | Molar Ratio Ca:Mg/Sr by EDX/mol% |
|---|---|---|---|---|---|
| CaP/PEIRh | 105 ± 28 | +11 ± 3 | 50 ± 12 | 100:0 | 100:0 |
| (Ca/Sr)P/PEIRh 95:5 | 80 ± 15 | +9 ± 2 | 39 ± 07 | 94:6 | 95:5 |
| (Ca/Sr)P/PEIRh 90:10 | 100 ± 29 | +8 ± 3 | 36 ± 05 | 92:8 | 91:9 |
| (Ca/Sr)P/PEIRh 85:15 | 90 ± 19 | +9 ± 4 | 40 ± 08 | 84:16 | 84:16 |
| (Ca/Sr)P/PEIRh 80:20 | 102 ± 37 | +11 ± 4 | 42 ± 06 | 81:19 | 82:18 |
| (Ca/Mg)P/PEIRh 95:5 | 105 ± 36 | +5 ± 3 | 60 ± 18 | 93:7 | 93:7 |
| (Ca/Mg)P/PEIRh 90:10 | 95 ± 37 | +8 ± 4 | 60 ± 15 | 89:11 | 89:11 |
| (Ca/Mg)P/PEIRh 85:15 | 95 ± 12 | +8 ± 3 | 65 ± 20 | 84:16 | 83:17 |
| (Ca/Mg)P/PEIRh 80:20 | 90 ± 32 | +15 ± 4 | 75 ± 16 | 81:19 | 82:18 |
| CaP/CMC-F | 125 ± 53 | −17 ± 5 | 70 ± 11 | 100:0 | 100:0 |
| (Ca/Mg)P/CMC-F 95:5 | 115 ± 48 | −13 ± 3 | 65 ± 10 | 92:8 | 97:3 |
| (Ca/Mg)P/CMC-F 90:10 | 105 ± 35 | −19 ± 4 | 60 ± 10 | 93:7 | 90:10 |
| (Ca/Mg)P/CMC-F 85:15 | 140 ± 33 | −20 ± 5 | 75 ± 13 | 89:11 | 83:17 |
| (Ca/Mg)P/CMC-F 80:20 | 140 ± 54 | −18 ± 7 | 75 ± 14 | 86:14 | 79:21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostka, K.; Hosseini, S.; Epple, M. In-Vitro Cell Response to Strontium/Magnesium-Doped Calcium Phosphate Nanoparticles. Micro 2023, 3, 156-171. https://doi.org/10.3390/micro3010012
Kostka K, Hosseini S, Epple M. In-Vitro Cell Response to Strontium/Magnesium-Doped Calcium Phosphate Nanoparticles. Micro. 2023; 3(1):156-171. https://doi.org/10.3390/micro3010012
Chicago/Turabian StyleKostka, Kathrin, Shabnam Hosseini, and Matthias Epple. 2023. "In-Vitro Cell Response to Strontium/Magnesium-Doped Calcium Phosphate Nanoparticles" Micro 3, no. 1: 156-171. https://doi.org/10.3390/micro3010012
APA StyleKostka, K., Hosseini, S., & Epple, M. (2023). In-Vitro Cell Response to Strontium/Magnesium-Doped Calcium Phosphate Nanoparticles. Micro, 3(1), 156-171. https://doi.org/10.3390/micro3010012






