Optimization of Planar Interdigitated Microelectrode Array for Enhanced Sensor Responses
Abstract
:1. Introduction
2. Materials and Methods
2.1. ACET Flow
2.2. ACET Enhanced Reaction
2.3. Binding Efficiency Parameters
- Dimensionless Normalized Binding: Dimensionless normalized binding is an indicator of how much of the probe in the reaction channel is being utilized. Dimensionless normalized binding is measured by taking the ratio of the reaction curve at an applied voltage with respect to the active site concentration [25].
- Enhancement Factor: The enhancement factor in the context of biosensors is defined as the ratio of the initial slope of the binding reaction curve when at an applied voltage to the initial slope of the same reaction curve without applying any voltage. This factor quantifies the effect of the applied AC voltage on the binding kinetics of the analyte and ligand on the biosensor’s surface. The enhancement factor provides valuable insights into how the AC voltage influences the association and dissociation rates of the analyte–ligand interaction. A higher enhancement factor indicates that the AC voltage positively impacts the binding kinetics, leading to a more significant change in the biosensor’s response and, consequently, an improved sensitivity in detecting the target analyte [22].
- Detection Time: The detection time in the context of biosensors refers to the time it takes for the biosensor’s response to reach a certain threshold value, specifically when the surface concentration of the analyte–ligand complex reaches 95% of its maximum value. This parameter is essential as it quantifies the time required for the biosensor to detect and reach a significant level of binding between the analyte and ligand on its surface. A shorter detection time indicates that the biosensor can rapidly and efficiently detect the target analyte, making it more suitable for real-time monitoring and point-of-care testing scenarios [24].
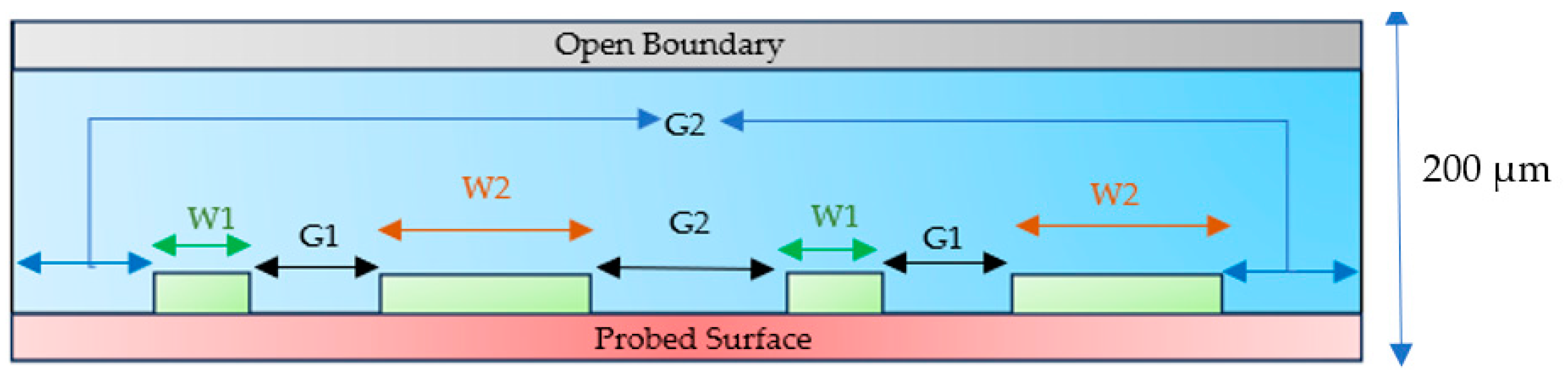
2.4. Device Structure
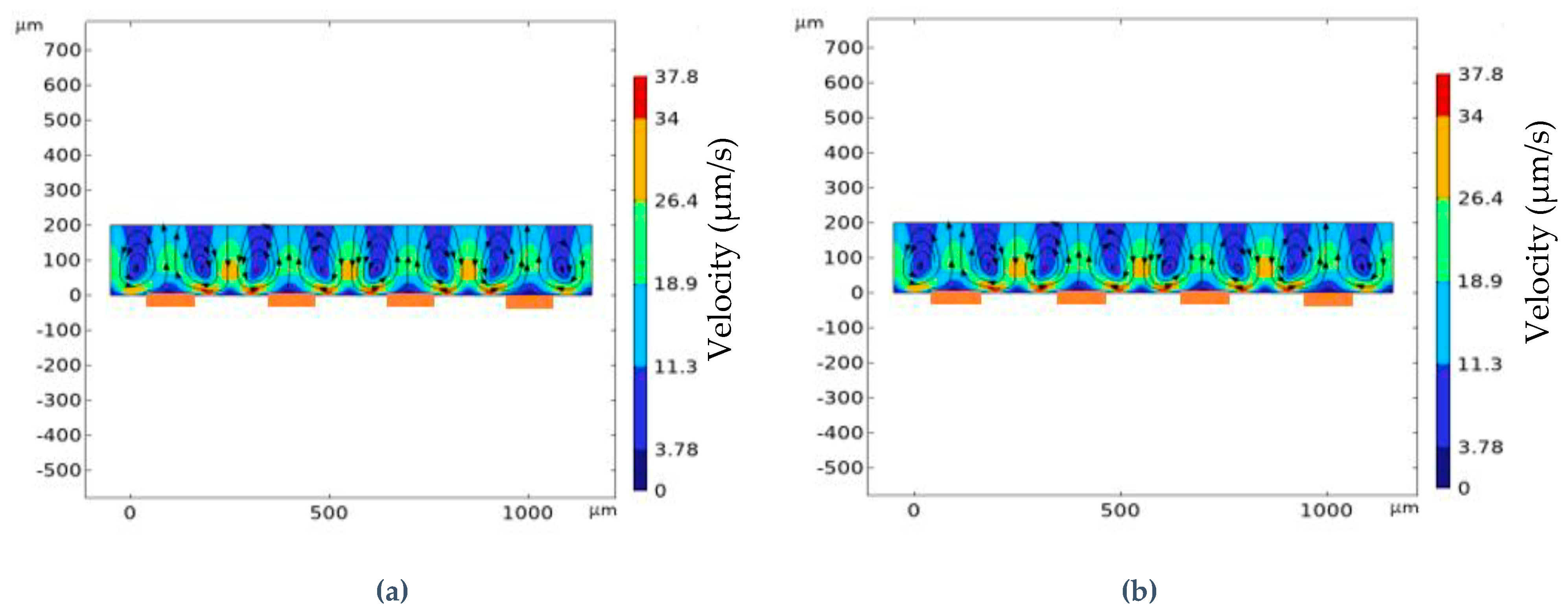
2.5. Simulation Verification
3. Results
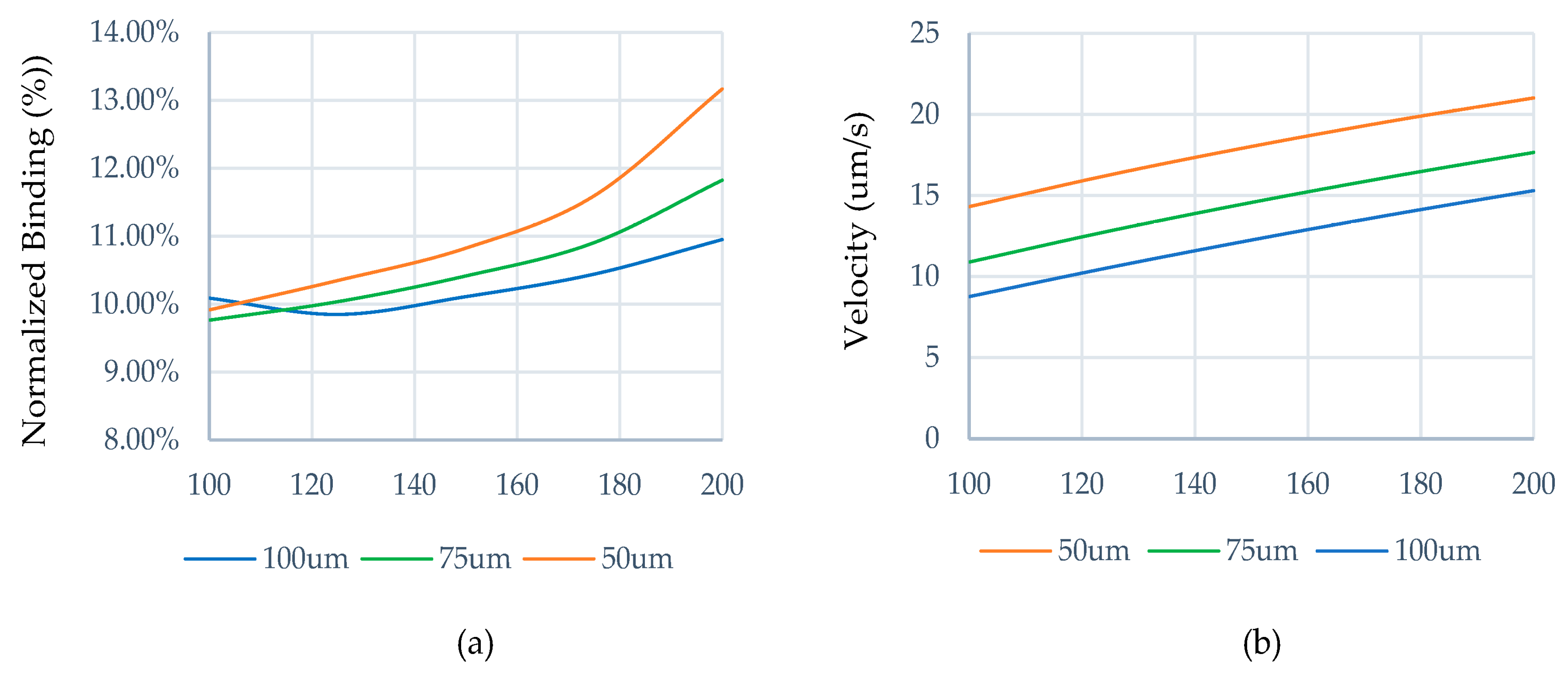
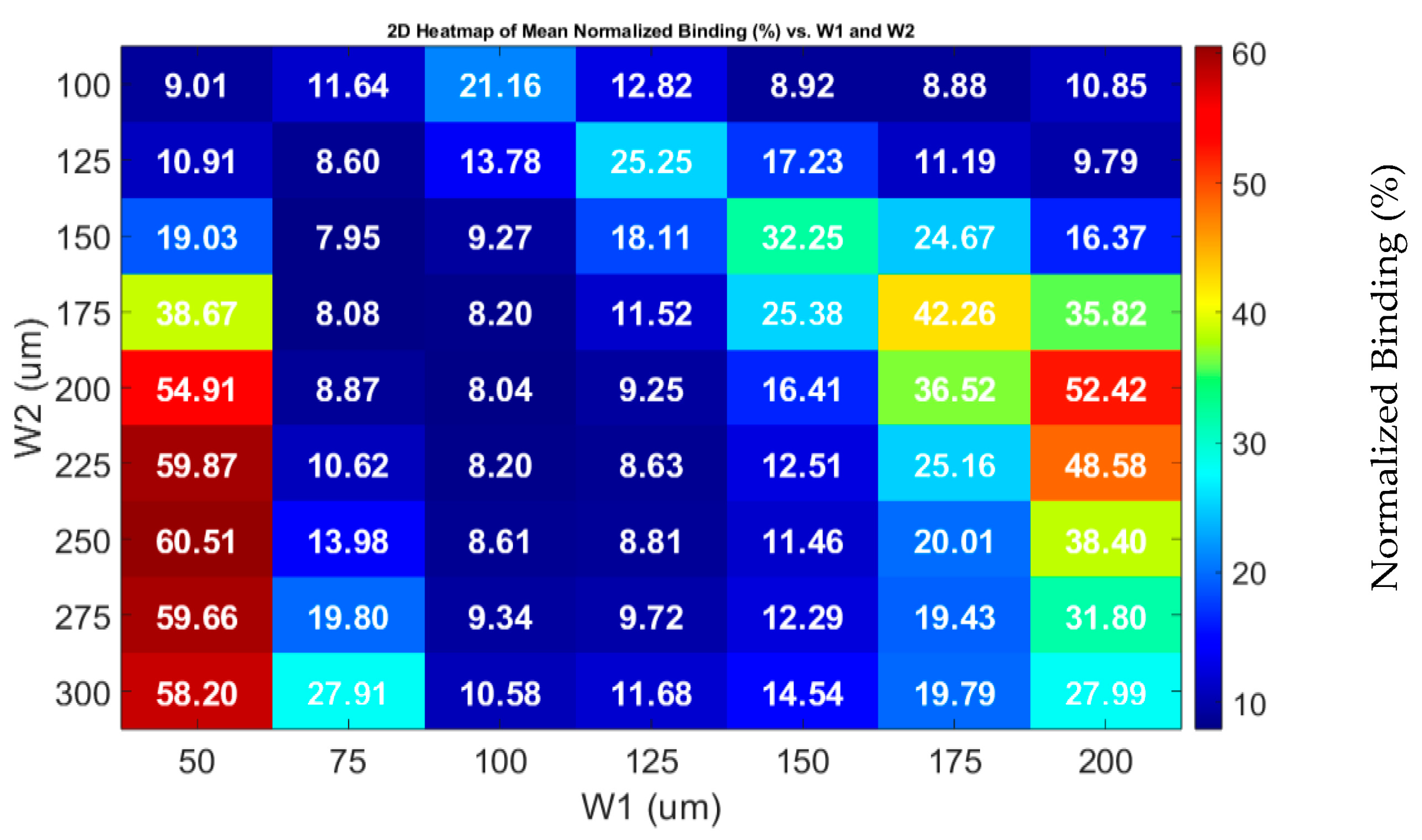
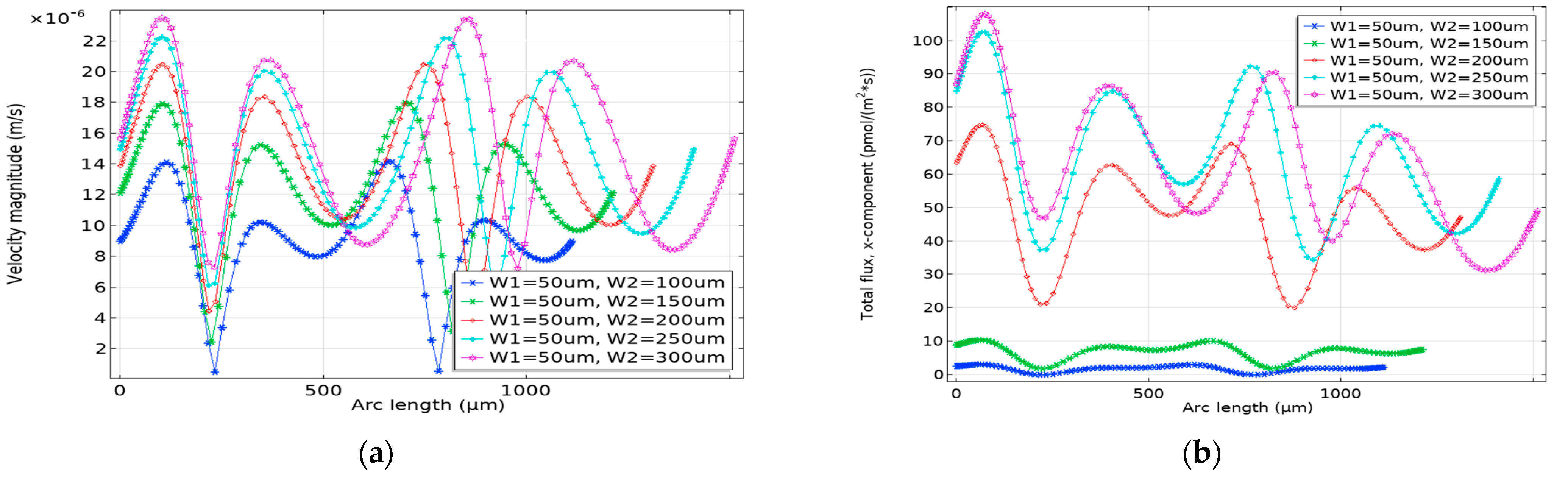
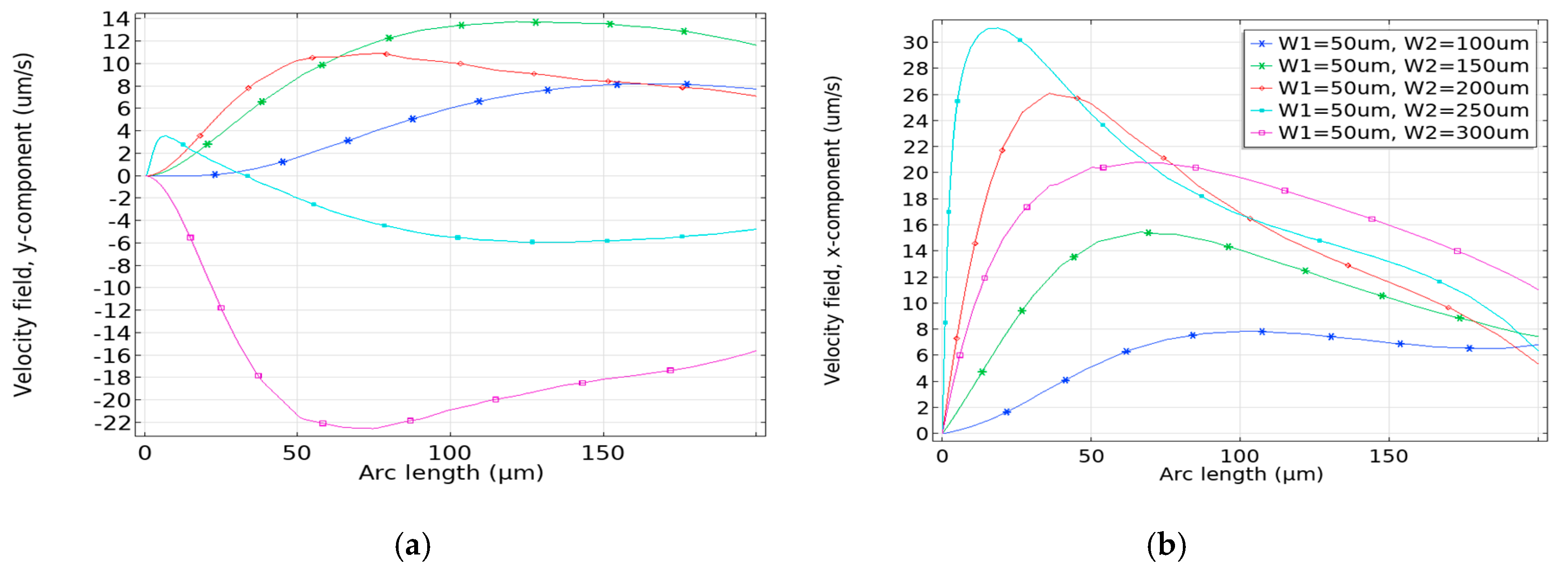
3.1. Impact of Geometrical Optimization
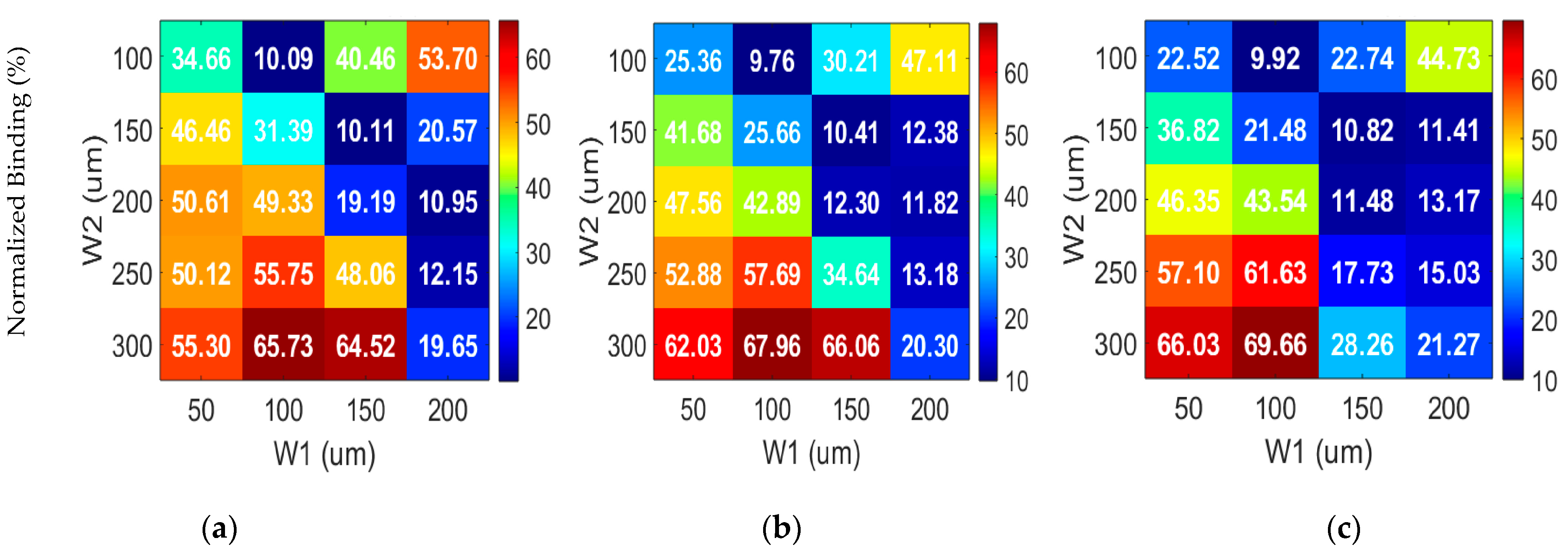
3.2. Optimization Procedure
3.2.1. Effective Width Configuration
3.2.2. Characteristic Length G1 Optimization
3.2.3. Optimization of G2
3.3. Enhancement Factor and Detection Time
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- An, J.; Ding, S.; Hu, X.; Sun, L.; Gu, Y.; Xu, Y.; Hu, Y.; Liu, C.; Zhang, X. Preparation, characterization and application of anti-human OX40 ligand (OX40L) monoclonal antibodies and establishment of a sandwich ELISA for autoimmune diseases detection. Int. Immunopharmacol. 2019, 67, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Alhajj, M.; Zubair, M.; Farhana, A. Enzyme Linked Immunosorbent Assay. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK555922/ (accessed on 10 August 2023).
- Song, M.; Lin, X.; Peng, Z.; Zhang, M.; Wu, J. Enhancing affinity-based electroanalytical biosensors by integrated AC electrokinetic enrichment—A mini review. Electrophoresis 2022, 43, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Li, D. Electrokinetics in Microfluidics; Interface Science and Technology Series, no. 2. Elsevier-Academic Press: Amsterdam, The Netherlands; Boston, MA, USA; Heidelberg, Germany, 2004. [Google Scholar]
- Sigurdson, M.; Wang, D.; Meinhart, C.D. Electrothermal stirring for heterogeneous immunoassays. Lab Chip 2005, 5, 1366. [Google Scholar] [CrossRef]
- Lian, M.; Islam, N.; Wu, J. AC electrothermal manipulation of conductive fluids and particles for lab-chip applications. IET Nanobiotechnol. 2007, 1, 36. [Google Scholar] [CrossRef]
- Lian, M.; Wu, J. Ultrafast micropumping by biased alternating current electrokinetics. Appl. Phys. Lett. 2009, 94, 064101. [Google Scholar] [CrossRef]
- Ramos, A.; Morgan, H.; Green, N.G.; Castellanos, A. Ac electrokinetics: A review of forces in microelectrode structures. J. Phys. Appl. Phys. 1998, 31, 2338–2353. [Google Scholar] [CrossRef]
- Green, N.G.; Ramos, A.; González, A.; Morgan, H.; Castellanos, A. Fluid flow induced by nonuniform ac electric fields in electrolytes on microelectrodes. I. Experimental measurements. Phys. Rev. E 2000, 61, 4011–4018. [Google Scholar] [CrossRef]
- Wu, J. Biased AC electro-osmosis for on-chip bioparticle processing. IEEE Trans. Nanotechnol. 2006, 5, 84–89. [Google Scholar] [CrossRef]
- Wu, J.J. Ac electro-osmotic micropump by asymmetric electrode polarization. J. Appl. Phys. 2008, 103, 024907. [Google Scholar] [CrossRef]
- Pethig, R. Review Article—Dielectrophoresis: Status of the theory, technology, and applications. Biomicrofluidics 2010, 4, 022811. [Google Scholar] [CrossRef]
- Morgan, H.; Green, N.G. AC Electrokinetics: Colloids and Nanoparticles; Microtechnologies and Microsystems Series, no. 2; Research Studies Press: Baldock, UK, 2003. [Google Scholar]
- Wang, X.-B.; Huang, Y.; Gascoyne, P.; Becker, F. Dielectrophoretic manipulation of particles. IEEE Trans. Ind. Appl. 1997, 33, 660–669. [Google Scholar] [CrossRef]
- Morgan, H.; Hughes, M.P.; Green, N.G. Separation of Submicron Bioparticles by Dielectrophoresis. Biophys. J. 1999, 77, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lillehoj, P.B. Ultrafast Electrothermal Flow-Enhanced Magneto Biosensor for Highly Sensitive Protein Detection in Whole Blood. Angew. Chem. Int. Ed. 2022, 61, e202200206. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Yang, C. Advances in electrokinetics and their applications in micro/nano fluidics. Microfluid. Nanofluidics 2012, 13, 179–203. [Google Scholar] [CrossRef]
- Wu, J.; Lian, M.; Yang, K. Micropumping of biofluids by alternating current electrothermal effects. Appl. Phys. Lett. 2007, 90, 234103. [Google Scholar] [CrossRef]
- AKoklu, A.; El Helou, A.; Raad, P.E.; Beskok, A. Characterization of Temperature Rise in Alternating Current Electrothermal Flow Using Thermoreflectance Method. Anal. Chem. 2019, 91, 12492–12500. [Google Scholar] [CrossRef]
- Lijnse, T.; Cenaiko, S.; Dalton, C. Numerical simulation of a tuneable reversible flow design for practical ACET devices. SN Appl. Sci. 2020, 2, 305. [Google Scholar] [CrossRef]
- Yuan, Q.; Yang, K.; Wu, J. Optimization of planar interdigitated microelectrode array for biofluid transport by AC electrothermal effect. Microfluid. Nanofluidics 2014, 16, 167–178. [Google Scholar] [CrossRef]
- Huang, K.-R.; Chang, J.-S.; Chao, S.D.; Wu, K.-C.; Yang, C.-K.; Lai, C.-Y.; Chen, S.-H. Simulation on binding efficiency of immunoassay for a biosensor with applying electrothermal effect. J. Appl. Phys. 2008, 104, 064702. [Google Scholar] [CrossRef]
- Camillone, N. Diffusion-Limited Thiol Adsorption on the Gold(111) Surface. Langmuir 2004, 20, 1199–1206. [Google Scholar] [CrossRef]
- Kaziz, S.; Saad, Y.; Gazzah, M.H.; Belmabrouk, H. 3D simulation of microfluidic biosensor for SARS-CoV-2 S protein binding kinetics using new reaction surface design. Eur. Phys. J. Plus 2022, 137, 241. [Google Scholar] [CrossRef] [PubMed]
- Sigurdson, M.; Meinhart, C.; Liu, X.; Wang, D. Biosensor Performance Enhancement through Ac Electrokinetics. 2003. Available online: https://www.semanticscholar.org/paper/BIOSENSOR-PERFORMANCE-ENHANCEMENT-THROUGH-AC-Sigurdson-Meinhart/8d12eff79b1f9cc81cecfb3158c4e9dad10e15c5 (accessed on 11 August 2023).
- Ng, W.Y.; Goh, S.; Lam, Y.C.; Yang, C.; Rodríguez, I. DC-biased AC-electroosmotic and AC-electrothermal flow mixing in microchannels. Lab Chip 2009, 9, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Wilber, H.W. Alternating Current Electrolysis with Zinc Electrodes in Sodium Thiosulphate Solution; Rice University: Houston, TX, USA, 1917. [Google Scholar]
- Tibbe, M.; Loessberg-Zahl, J.; Do Carmo, M.P.; van der Helm, M.; Bomer, J.; Van Den Berg, A.; Le-The, H.; Segerink, L.; Eijkel, J. Large-scale fabrication of free-standing and sub-μm PDMS through-hole membranes. Nanoscale 2018, 10, 7711–7718. [Google Scholar] [CrossRef]
- How to Choose the Most Appropriate Technology: FDM, SLA and SLS. Available online: https://filament2print.com/gb/blog/92_3d-printing-fdm-sla-sls-technology.html (accessed on 11 August 2023).

| Parameter Name (Unit) | SARS-CoV-2 | C-Reactive Protein | Immunoglobulin |
|---|---|---|---|
| Analyte diffusivity, D (m2/s) | 1 × 10−11 | 2.175 × 10−11 | 5 × 10−11 |
| Active Site concentration, γs (mol/m2) | 3.3 × 10−8 | 1.4 × 10−8 | 1.4 × 10−8 |
| Association Constant, Kas (m3/(mol.s)) | 1 × 103 | 1 × 104 | 250 |
| Dissociation Constant, Kdes (1/s) | 1 × 10−3 | 2.6 × 10−2 | 3 × 10−4 |
| G1(um) | Applied Voltage (V) | Optimized Ratio | Normalized Binding (%) | Average Bulk Velocity (um/s) |
|---|---|---|---|---|
| 100 | 5 | 1:2:5:4.8 | 61.73 | 14.45 |
| 80 | 4 | 1:2:5:3.6 | 54.01 | 6.18 |
| 60 | 3 | 1:2:5:2.8 | 35.04 | 2.44 |
| G1(um) | Applied Voltage (V) | Optimized Ratio | Normalized Binding (%) | Velocity (um/s) |
|---|---|---|---|---|
| 50 | 5 | 1:1:5:2.2 | 71.22 | 21.25 |
| 60 | 5 | 1:1.2:5:2.6 | 70.41 | 18.83 |
| 70 | 5 | 1:1.4:5:3.2 | 68.92 | 16.79 |
| 80 | 5 | 1:1.6:5:3.6 | 66.84 | 15.11 |
| 90 | 5 | 1:1.8:5:4.2 | 64.35 | 13.67 |
| 100 | 5 | 1:2:5:4.8 | 61.73 | 12.44 |
| Binding Efficiency Parameters | CRP | IgG | SARS-COV-2 |
|---|---|---|---|
| Enhancement Factor Slope | 3.21 | 2.15 | 3.09 |
| Detection Time (s) | 2144 | 2580 | 2820 |
| Normalized Binding (%) | 71.22 | 75.80 | 57.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, S.; Wu, J. Optimization of Planar Interdigitated Microelectrode Array for Enhanced Sensor Responses. Micro 2023, 3, 763-774. https://doi.org/10.3390/micro3040054
Islam S, Wu J. Optimization of Planar Interdigitated Microelectrode Array for Enhanced Sensor Responses. Micro. 2023; 3(4):763-774. https://doi.org/10.3390/micro3040054
Chicago/Turabian StyleIslam, Sakib, and Jie Wu. 2023. "Optimization of Planar Interdigitated Microelectrode Array for Enhanced Sensor Responses" Micro 3, no. 4: 763-774. https://doi.org/10.3390/micro3040054
APA StyleIslam, S., & Wu, J. (2023). Optimization of Planar Interdigitated Microelectrode Array for Enhanced Sensor Responses. Micro, 3(4), 763-774. https://doi.org/10.3390/micro3040054







