Chitin-Derived Silver Nanoparticles for Enhanced Food Preservation: Synthesis, Characterization, and Antimicrobial Potential
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection and Identification of Experimental Specimens
2.2. Extraction
2.2.1. Deproteinization
2.2.2. Decoloration
2.2.3. Demineralization
2.3. Characterization of Chitin
2.4. Characterization of the Chitin Physicochemical Properties
2.5. Synthesis of Silver Nanoparticles using Chitin
2.5.1. Preparation of 1 mm silver nitrate (AgNO3)
2.5.2. Synthesis of Silver Nanoparticles (AgNPs)
2.5.3. Characterization of AgNP-Mediated Chitin Nanoparticles
2.5.4. Preparation of Chitin Composite Film
2.5.5. Antimicrobial Activity of Chitin Film
2.6. Toxicity Assay by Hemolytic Activity
2.6.1. Preparation of RBC Suspension
2.6.2. Hemolysis Assay
2.7. Applications of AgNP-Synthesized Chitin as a Food Preservative
3. Results
3.1. Chitin Yield, Insoluble Content
3.2. UV–vis Spectroscopy Analysis of Chitin
3.3. FTIR Analysis of Chitin
3.4. SEM Analysis of Chitin
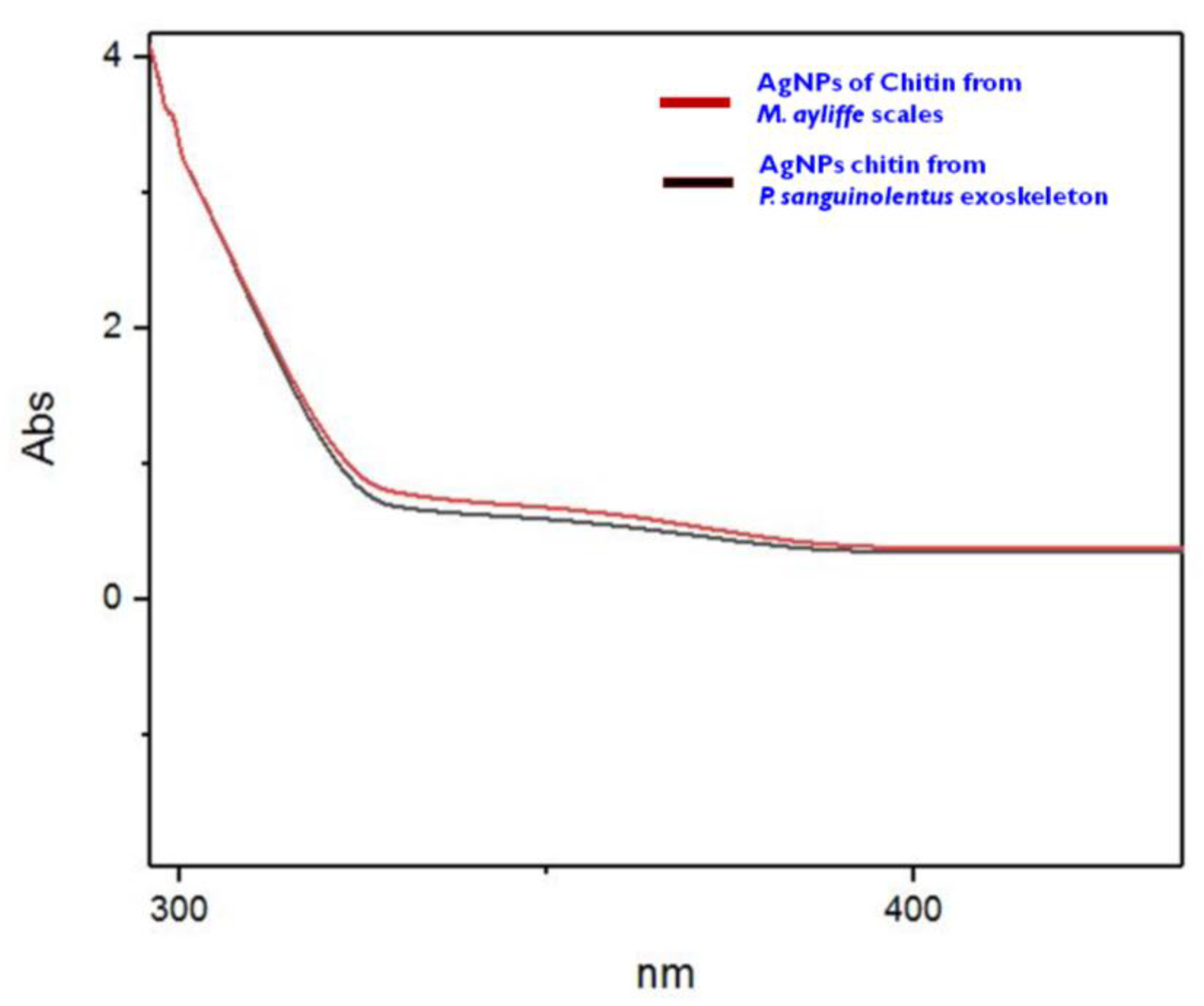
3.5. Synthesis and Characterization of Silver Nanoparticles
3.6. Toxicological Assessment of AgNP-Synthesized Chitin from M. ayliffe Scales
3.7. Antimicrobial Activity of Chitin Film against Food Pathogen Vibrio spp.
3.8. Exploring Chitin AgNPs from M. ayliffe Scales for Food Preservation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Massaglia, S.; Borra, D.; Peano, C.; Sottile, F.; Merlino, V.M. Consumer preference heterogeneity evalua-tion in fruit and vegetable purchasing decisions using the best–worst approach. Foods 2019, 8, 266. [Google Scholar] [CrossRef] [PubMed]
- Berdegué, J.A.; Balsevich, F.; Flores, L.; Reardon, T. Central American supermarkets’ private standards of quality and safety in procurement of fresh fruits and vegetables. Food Policy 2005, 30, 254–269. [Google Scholar] [CrossRef]
- Ziv, C.; Fallik, E. Postharvest Storage Techniques and Quality Evaluation of Fruits and Vegetables for Reducing Food Loss. Agronomy 2021, 11, 1133. [Google Scholar] [CrossRef]
- Duan, C.; Meng, X.; Meng, J.; Khan, M.I.H.; Dai, L.; Khan, A.; Ni, Y. Chitosan as a preservative for fruits and vegetables: A review on chemistry and antimicrobial properties. J. Bioresour. Bioprod. 2019, 4, 11–21. [Google Scholar] [CrossRef]
- Rawat, S. Food Spoilage: Microorganisms and their prevention. Asian J. Plant Sci. Res. 2015, 5, 47–56. [Google Scholar]
- Iversen, L.J.L.; Rovina, K.; Vonnie, J.M.; Matanjun, P.; Erna, K.H.; ‘Aqilah, N.M.N.; Felicia, W.X.L.; Funk, A.A. The Emergence of Edible and Food-Application Coatings for Food Packaging: A Review. Molecules 2022, 27, 5604. [Google Scholar] [CrossRef] [PubMed]
- Baranwal, J.; Barse, B.; Fais, A.; Delogu, G.L.; Kumar, A. Biopolymer: A Sustainable Material for Food and Medical Applications. Polymers 2022, 14, 983. [Google Scholar] [CrossRef]
- Moshood, T.D.; Nawanir, G.; Mahmud, F.; Mohamad, F.; Ahmad, M.H.; AbdulGhani, A. Sustainability of biodegradable plastics: New problem or solution to solve the global plastic pollution? Curr. Res. Green Sustain. Chem. 2022, 5, 100273. [Google Scholar] [CrossRef]
- Jianglian, D.; Shaoying, Z. Application of chitosan based coating in fruit and vegetable preservation: A review. J. Food Process. Technol 2013, 4, 227. [Google Scholar] [CrossRef]
- Zambrano-Zaragoza, M.L.; González-Reza, R.; Mendoza-Muñoz, N.; Miranda-Linares, V.; Bernal-Couoh, T.F.; Mendoza-Elvira, S.; Quintanar-Guerrero, D. Nanosystems in edible coatings: A novel strategy for food preservation. Int. J. Mol. Sci. 2018, 19, 705. [Google Scholar] [CrossRef]
- Seidi Damyeh, M.; Mereddy, R.; Netzel, M.E.; Sultanbawa, Y. An insight into curcumin-based photo-sensitization as a promising and green food preservation technology. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1727–1759. [Google Scholar] [CrossRef] [PubMed]
- Shiekh, R.A.; Malik, M.A.; Al-Thabaiti, S.A.; Shiekh, M.A. Chitosan as a novel edible coating for fresh fruits. Food Sci. Technol. Res. 2013, 19, 139–155. [Google Scholar] [CrossRef]
- Dhall, R.K. Advances in edible coatings for fresh fruits and vegetables: A review. Crit. Rev. Food Sci. Nutr. 2013, 53, 435–450. [Google Scholar] [CrossRef] [PubMed]
- Muhamad, I.I.; Zulkifli, N.; Lazim, N.A.M. Bioactive algal-derived polysaccharides: Multi-functionalization, therapeutic potential and biomedical applications. Curr. Pharm. Des. 2019, 25, 1147–1162. [Google Scholar] [CrossRef] [PubMed]
- Bealer, E.J.; Kavetsky, K.; Dutko, S.; Lofland, S.; Hu, X. Protein and polysaccharide-based magnetic composite materials for medical applications. Int. J. Mol. Sci. 2020, 21, 186. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, C.; González Auza, L.; Koberidze, D.; Rasche, S.; Fischer, R.; Bortesi, L. Conversion of chitin to defined chitosan oligomers: Current status and future prospects. Mar. Drugs 2019, 17, 452. [Google Scholar] [CrossRef] [PubMed]
- Balassa, L.L.; Prudden, J.F. Applications of chitin and chitosan in wound-healing acceleration. In Proceedings of the First International Conference on Chitin/Chitosan, Boston, MA, USA, 11 April 1978; National Technical Information: Springfield, VA, USA, 1978; pp. 296–305. [Google Scholar]
- Huang, X.; Xie, W.J.; Gong, Z.Z. Characteristics and antifungal activity of a chitin binding protein from Ginkgo biloba. FEBS Lett. 2000, 478, 123–126. [Google Scholar] [CrossRef]
- Benhabiles, M.S.; Salah, R.; Lounici, H.; Drouiche, N.; Goosen, M.F.A.; Mameri, N. Antibacterial activity of chitin, chitosan and its oligomers prepared from shrimp shell waste. Food Hydrocoll. 2012, 29, 48–56. [Google Scholar] [CrossRef]
- Carpenter, K.E. The Living Marine Resources of the Western Central Atlantic; Volume 1: Introduction, molluscs, crustaceans, hagfishes, sharks, batoid fishes, and chimaeras; Food and Agricultural Organization of the United Nations: Rome, Italy, 2002. [Google Scholar]
- Vijayaraj, R.; Altaff, K.; Lakshmanan, G.; Jayaprakashvel, M.; Mickymaray, S.; Gunapriya, R.; Alothaim, A.S. Inhibitory Activity of Chitin,(2-Acetamido-2-Deoxy-Hexopyranose) against Penicillin-Binding Proteins of Staphylococcus aureus. Coatings 2022, 12, 1854. [Google Scholar] [CrossRef]
- Kumaran, N.; Vijayaraj, R.; Swarnakal. BIosynthesis of silver nano particles from Leucas aspera (willd.) link and its anti-inflammatory potential against carrageen induced paw edema in rats. Int. J. Pharm. Sci. Res. 2017, 8, 2588–2593. [Google Scholar]
- Nadarajah, S.K.; Vijayaraj, R.; Mani, J. Therapeutic significance of Loligo vulgaris (Lamarck, 1798) ink extract: A biomedical approach. Pharmacogn. Res. 2017, 9 (Suppl. S1), S105. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraj, R.; Sri Kumaran, N.; Altaff, K. Toxicity evaluation of novel antidiabetic compound (11-methoxy-2-methyltridecane-4-ol) from marine macro alga, Gracilaria edulis. J. Biol. Act. Prod. Nat. 2022, 12, 223–231. [Google Scholar] [CrossRef]
- Valdes, A.; Ramos, M.; Beltrán, A.; Jiménez, A.; Garrigós, M.C. State of the art of antimicrobial edible coatings for food packaging applications. Coatings 2017, 7, 56. [Google Scholar] [CrossRef]
- Kurita, K. Controlled functionalization of the polysaccharide chitin. Prog. Polym. Sci. 2001, 26, 1921–1971. [Google Scholar] [CrossRef]
- Sharp, R.G. A review of the applications of chitin and its derivatives in agriculture to modify plant-microbial interactions and improve crop yields. Agronomy 2013, 3, 757–793. [Google Scholar] [CrossRef]
- Zhang, M.; Haga, A.; Sekiguchi, H.; Hirano, S. Structure of insect chitin isolated from beetle larva cuticle and silkworm (Bombyx mori) pupa exuvia. Int. J. Biol. Macromol. 2000, 27, 99–105. [Google Scholar] [CrossRef]
- Sajomsang, W.; Gonil, P. Preparation and characterization of α-chitin from cicada sloughs. Mater. Sci. Eng. C 2010, 30, 357–363. [Google Scholar] [CrossRef]
- Dahmane, E.M.; Taourirte, M.; Eladlani, N.; Rhazi, M. Extraction and characterization of chitin and chitosan from Parapenaeus longirostris from Moroccan local sources. Int. J. Polym. Anal. Charact. 2014, 19, 342–351. [Google Scholar] [CrossRef]
- Povea, M.B.; Monal, W.A.; Cauich-Rodríguez, J.V.; Pat, A.M.; Rivero, N.B.; Covas, C.P. Interpenetrated chitosan-poly (acrylic acid-co-acrylamide) hydrogels. Synthesis, characterization and sustained protein release studies. Mater. Sci. Appl. 2011, 2, 509. [Google Scholar] [CrossRef]
- Qu, X.; Wirsen, A.; Albertsson, A.C. Effect of lactic/glycolic acid side chains on the thermal degradation kinetics of chitosan derivatives. Polymer 2000, 41, 4841–4847. [Google Scholar] [CrossRef]
- Brugnerotto, J.; Lizardi, J.; Goycoolea, F.M.; Argüelles-Monal, W.; Desbrieres, J.; Rinaudo, M. An infrared investigation in relation with chitin and chitosan characterization. Polymer 2001, 42, 3569–3580. [Google Scholar] [CrossRef]
- Kumari, S.; Rath, P.; Kumar, A.S.H.; Tiwari, T.N. Extraction and characterization of chitin and chitosan from fishery waste by chemical method. Environ. Technol. Innov. 2015, 3, 77–85. [Google Scholar] [CrossRef]
- Gbenebor, O.P.; Adeosun, S.O.; Lawal, G.I.; Jun, S.; Olaleye, S.A. Acetylation, crystalline and morpho-logical properties of structural polysaccharide from shrimp exoskeleton. Eng. Sci. Technol. Int. J. 2017, 20, 1155–1165. [Google Scholar]
- Amjed, N.; Bhatti, I.A.; Zia, K.M.; Iqbal, J.; Jamil, Y. Synthesis and characterization of stable and biological active chitin-based polyurethane elastomers. Int. J. Biol. Macromol. 2020, 154, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Jan, H.; Zaman, G.; Usman, H.; Ansir, R.; Drouet, S.; Gigliolo-Guivarc’h, N.; Abbasi, B.H. Biogenically proficient synthesis and characterization of silver nanoparticles (Ag-NPs) employing aqueous extract of Aquilegia pubiflora along with their in vitro antimicrobial, anti-cancer and other biological applications. J. Mater. Res. Technol. 2021, 15, 950–968. [Google Scholar] [CrossRef]
- Ashraf, H.; Anjum, T.; Riaz, S.; Naseem, S. Microwave-assisted green synthesis and characterization of silver nanoparticles using Melia azedarach for the management of Fusarium wilt in tomato. Front. Microbiol. 2020, 11, 238. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Li, Y.; Liu, Y.; Li, N.; Zhang, X.; Yan, C. Antimicrobial Properties of Chitosan and Chitosan Derivatives in the Treatment of Enteric Infections. Molecules 2021, 26, 7136. [Google Scholar] [CrossRef]
- Ashbolt, N.J. Microbial contamination of drinking water and disease outcomes in developing regions. Toxicology 2004, 198, 229–238. [Google Scholar] [CrossRef]
- Azwai, S.M.; Alfallani, E.A.; Abolghait, S.K.; Garbaj, A.M.; Naas, H.T.; Moawad, A.A.; Eldaghayes, I.M. Isolation and molecular identification of Vibrio spp. by sequencing of 16S rDNA from seafood, meat and meat products in Libya. Open Vet. J. 2016, 6, 36–43. [Google Scholar] [CrossRef]
- Gxalo, O.; Digban, T.O.; Igere, B.E.; Olapade, O.A.; Okoh, A.I.; Nwodo, U.U. Virulence and antibiotic re-sistance characteristics of Vibrio isolates from rustic environmental freshwaters. Front. Cell. Infect. Microbiol. 2021, 11, 732001. [Google Scholar] [CrossRef]
- Mondal, A.; Dhar, A.K.; Banerjee, S.; Hasnain, M.S.; Nayak, A.K. Antimicrobial uses of chitosan. In Chitosan in Biomedical Applications; Academic Press: Cambridge, MA, USA, 2022; Volume 1, pp. 13–36. [Google Scholar]
- Almasi, H.; Jafarzadeh, P.; Mehryar, L. Fabrication of novel nanohybrids by impregnation of CuO nanoparticles into bacterial cellulose and chitosan nanofibers: Characterization, antimicrobial and release properties. Carbohydr. Polym. 2018, 186, 273–281. [Google Scholar] [CrossRef]
- Galus, S.; Kadzińska, J. Food applications of emulsion-based edible films and coatings. Trends Food Sci. Technol. 2015, 45, 273–283. [Google Scholar] [CrossRef]













| Name of the Sample | Yield (g) | Insolubility Content (%) |
|---|---|---|
| M. ayliffe | 7.72 ± 0.04 | 20.66 ± 0.94 |
| P. sanguinolentus | 2.93 ± 0.04 | 90.33 ± 0.47 |
| Frequency, cm−1 | Bond | Functional Group |
|---|---|---|
| Chitin from M. ayliffe scales | ||
| 3490 | O–H stretch | Alcohols |
| 1700 | C=O stretch | Carbonyls |
| 1400 | N–O asymmetric stretch | Nitro compounds |
| 1100 | C–N stretch | Aliphatic amines |
| Chitin from P. sanguinolentus exoskeleton | ||
| 2900 | H–C=O: C–H stretch | Aldehydes |
| 1700 | C=O stretch | Carbonyls |
| 1400 | N–O asymmetric stretch | Nitro compounds |
| 1100 | C–N stretch | Aliphatic amines |
| Frequency, cm−1 | Bond | Functional Group |
|---|---|---|
| Chitin from M. ayliffe scales | ||
| 3200 | H–bonded | Alcohols |
| 2700 | H–C=O: C–H stretch | Aldehydes |
| 1500 | N–O asymmetric stretch | Nitro compounds |
| 1000 | =C–H bend | Aalkenes |
| 900 | C–H | Aromatics |
| Chitin from P. sanguinolentus exoskeleton | ||
| 3400 | H–bonded | Alcohol |
| 1700 | C=O stretch | Aldehyde and Ketone |
| 1600 | N–O asymmetric stretch | Nitro compounds |
| 1000 | =C–H bend | Aalkenes |
| Zone of Inhibition (mm) | ||||
|---|---|---|---|---|
| Standard (Tetracycline) | Chitin from M. ayliffe Scales | Chitin from P. sanguinolentus Exoskeleton | AgNPS from M. ayliffe Scales Chitin | AgNPs from P. sanguinolentus Exoskeleton Chitin |
| 22 ± 0.74 | 15 ± 0.48 | 15 ± 0.92 | 20 ± 1.67 | 10 ± 0.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vijayaraj, R.; Altaff, K.; Jayaprakashvel, M.; Muthezhilan, R.; Saran, B.; Kurinjinathan, P.; Jeyaperumal, S.; Perumal, V.; Kumar, R.M.S.; Govindan, L. Chitin-Derived Silver Nanoparticles for Enhanced Food Preservation: Synthesis, Characterization, and Antimicrobial Potential. Micro 2023, 3, 912-929. https://doi.org/10.3390/micro3040062
Vijayaraj R, Altaff K, Jayaprakashvel M, Muthezhilan R, Saran B, Kurinjinathan P, Jeyaperumal S, Perumal V, Kumar RMS, Govindan L. Chitin-Derived Silver Nanoparticles for Enhanced Food Preservation: Synthesis, Characterization, and Antimicrobial Potential. Micro. 2023; 3(4):912-929. https://doi.org/10.3390/micro3040062
Chicago/Turabian StyleVijayaraj, R., K. Altaff, M. Jayaprakashvel, R. Muthezhilan, B. Saran, P. Kurinjinathan, Selvakumari Jeyaperumal, Venkatesan Perumal, R. M. Saravana Kumar, and Lakshmanan Govindan. 2023. "Chitin-Derived Silver Nanoparticles for Enhanced Food Preservation: Synthesis, Characterization, and Antimicrobial Potential" Micro 3, no. 4: 912-929. https://doi.org/10.3390/micro3040062
APA StyleVijayaraj, R., Altaff, K., Jayaprakashvel, M., Muthezhilan, R., Saran, B., Kurinjinathan, P., Jeyaperumal, S., Perumal, V., Kumar, R. M. S., & Govindan, L. (2023). Chitin-Derived Silver Nanoparticles for Enhanced Food Preservation: Synthesis, Characterization, and Antimicrobial Potential. Micro, 3(4), 912-929. https://doi.org/10.3390/micro3040062








