GLP-1 Receptor Agonists in the Treatment of Patients with Type 2 Diabetes and Chronic Kidney Disease
Abstract
:1. Introduction
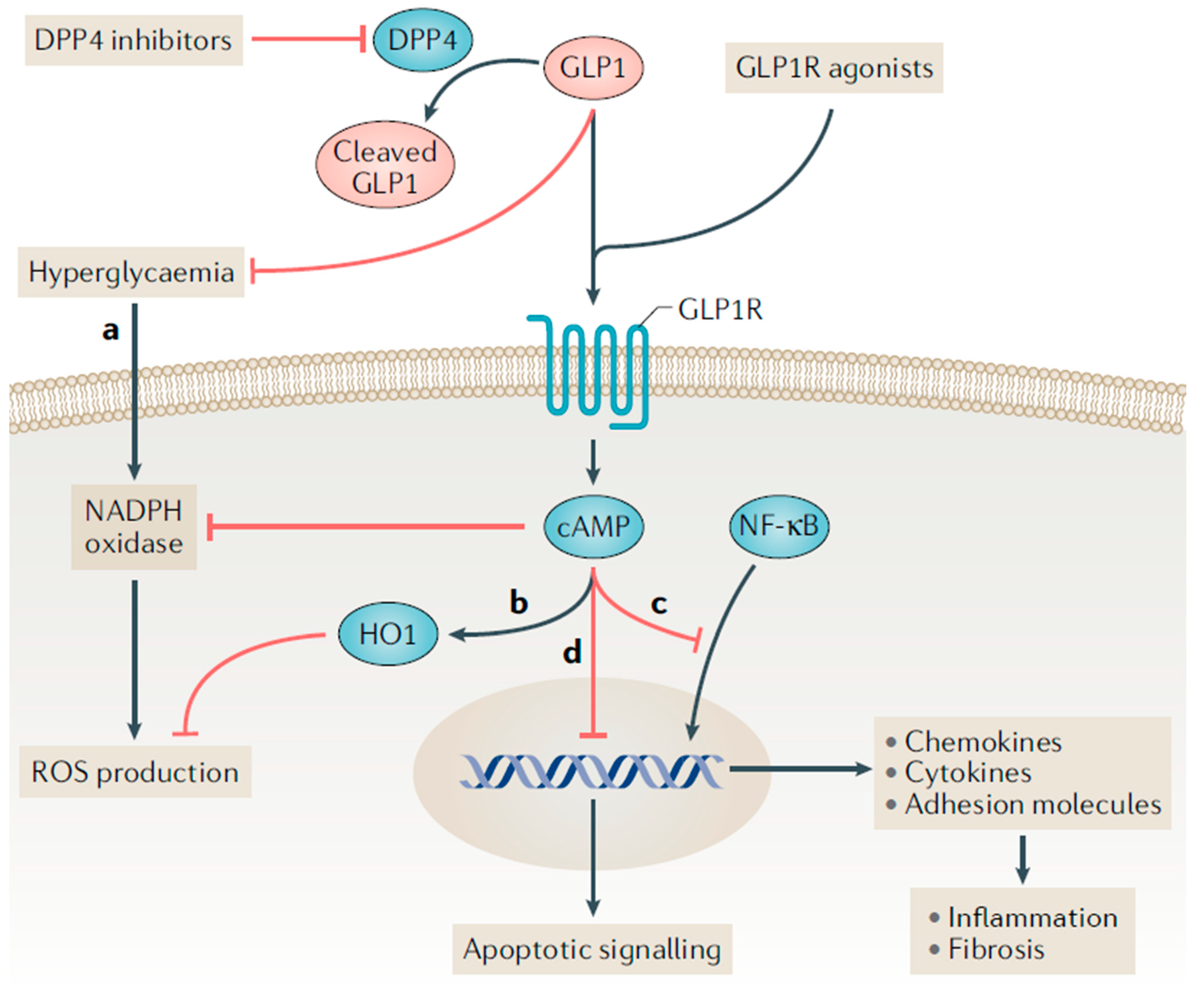
2. Proposed Mechanisms for Kidney Benefit with GLP-1 Receptor Agonists
3. Kidney Disease Outcomes with GLP-1 Receptor Agonists
3.1. Dulaglutide
3.2. Exenatide
3.3. Lixisenatide
3.4. Liraglutide
3.5. Semaglutide
4. Current Recommendations for GLP-1 Receptor Agonist Use in DKD
5. Future Directions and Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Diabetes Foundation. IDF Diabetes Atlas: Tenth Edition 2021, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- American Diabetes Association Professional Practice Committee. 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022, 45 (Suppl. S1), S17–S38. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. 6. Glycemic targets: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022, 45 (Suppl. S1), S83–S96. [Google Scholar]
- Buse, J.B.; Wexler, D.J.; Tsapas, A.; Rossing, P.; Mingrone, G.; Mathieu, C.; D’Alessio, D.A.; Davies, M.J. 2019 Update to: Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2020, 43, 487–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Diabetes Association Professional Practice Committee. 11. Chronic kidney disease and risk management: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022, 45 (Suppl. S1), S175–S184. [Google Scholar] [CrossRef]
- Rao Kondapally Seshasai, S.; Kaptoge, S.; Thompson, A.; Di Angelantonio, E.; Gao, P.; Sarwar, N.; Whincup, P.H.; Mukamal, K.J.; Gillum, R.F.; Holme, I.; et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N. Engl. J. Med. 2011, 364, 829–841. [Google Scholar]
- Afkarian, M.; Sachs, M.C.; Kestenbaum, B.; Hirsch, I.B.; Tuttle, K.R.; Himmelfarb, J.; De Boer, I.H. Kidney disease and increased mortality risk in type 2 diabetes. J. Am. Soc. Nephrol. 2013, 24, 302–308. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 9. Pharmacological approaches to glycemic treatment: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022, 45 (Suppl. S1), S125–S143. [Google Scholar] [CrossRef]
- Trujillo, J.M.; Nuffer, W.; Smith, B.A. GLP-1 receptor agonists: An updated review of head-to-head clinical studies. Ther. Adv. Endocrinol. Metab. 2021, 12, 2042018821997320. [Google Scholar] [CrossRef]
- ADVANCE Collaborative Group; Patel, A.; MacMahon, S.; Chalmers, J.; Neal, B.; Billot, L.; Woodward, M.; Marre, M.; Cooper, M.; Glasziou, P.; et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 2008, 358, 2560–2572. [Google Scholar]
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein, H.C.; Miller, M.E.; Byington, R.P.; Goff, D.C., Jr.; Bigger, J.T.; Buse, J.B.; Cushman, W.C.; Genuth, S.; Ismail-Beigi, F.; et al. Effects of intensive glucose lowering in type 2 diabetes. N. Engl. J. Med. 2008, 358, 2545–2559. [Google Scholar]
- Duckworth, W.; Abraira, C.; Moritz, T.; Reda, D.; Emanuele, N.; Reaven, P.D.; Zieve, F.J.; Marks, J.; Davis, S.N.; Hayward, R.; et al. Glucose control and vascular complications in veterans with type 2 diabetes. N. Engl. J. Med. 2009, 360, 129–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pickup, J.C. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care 2004, 27, 813–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Candales, A.; Hernandez Burgos, P.M.; Hernandez-Suarez, D.F.; Harris, D. Linking Chronic Inflammation with Cardiovascular Disease: From Normal Aging to the Metabolic Syndrome. J. Nat. Sci. 2017, 3, e341. [Google Scholar] [PubMed]
- Tuttle, K.R. Linking metabolism and immunology: Diabetic nephropathy is an inflammatory disease. J. Am. Soc. Nephrol. 2005, 16, 1537–1538. [Google Scholar] [CrossRef] [PubMed]
- Pichler, R.; Afkarian, M.; Dieter, B.P.; Tuttle, K.R. Immunity and inflammation in diabetic kidney disease: Translating mechanisms to biomarkers and treatment targets. Am. J. Physiol. Renal. Physiol. 2017, 312, F716–F731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matoba, K.; Takeda, Y.; Nagai, Y.; Kawanami, D.; Utsunomiya, K.; Nishimura, R. Unraveling the Role of Inflammation in the Pathogenesis of Diabetic Kidney Disease. Int. J. Mol. Sci. 2019, 20, 3393. [Google Scholar] [CrossRef] [Green Version]
- Vilsboll, T.; Christensen, M.; Junker, A.E.; Knop, F.K.; Gluud, L.L. Effects of glucagon-like peptide-1 receptor agonists on weight loss: Systematic review and meta-analyses of randomised controlled trials. BMJ 2012, 344, d7771. [Google Scholar] [CrossRef] [Green Version]
- Davies, M.; Færch, L.; Jeppesen, O.K.; Pakseresht, A.; Pedersen, S.D.; Perreault, L.; Rosenstock, J.; Shimomura, I.; Viljoen, A.; Wadden, T.A.; et al. Semaglutide 2.4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): A randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet 2021, 397, 971–984. [Google Scholar] [CrossRef]
- Wadden, T.A.; Bailey, T.S.; Billings, L.K.; Davies, M.; Frias, J.P.; Koroleva, A.; Lingvay, I.; O’Neil, P.M.; Rubino, D.M.; Skovgaard, D.; et al. Effect of subcutaneous semaglutide vs placebo as an adjunct to intensive behavioral therapy on body weight in adults with overweight or obesity: The STEP 3 randomized clinical trial. JAMA 2021, 325, 1403–1413. [Google Scholar] [CrossRef]
- Rubino, D.; Abrahamsson, N.; Davies, M.; Hesse, D.; Greenway, F.L.; Jensen, C.; Lingvay, I.; Mosenzon, O.; Rosenstock, J.; Rubio, M.A.; et al. Effect of continued weekly subcutaneous semaglutide vs placebo on weight loss maintenance in adults with overweight or obesity: The STEP 4 randomized clinical trial. JAMA 2021, 325, 1414–1425. [Google Scholar] [CrossRef]
- Wegovy (Semaglutide) Injection [Prescribing Information]; Novo Nordisk Inc.: Plansboro, NJ, USA, 2021.
- Saxenda (Liraglutide) Injection [Prescribing Information]; Novo Nordisk Inc.: Plansboro, NJ, USA, 2021.
- Htike, Z.Z.; Zaccardi, F.; Papamargaritis, D.; Webb, D.R.; Khunti, K.; Davies, M.J. Efficacy and safety of glucagon-like peptide-1 receptor agonists in type 2 diabetes: A systematic review and mixed-treatment comparison analysis. Diabetes Obes. Metab. 2017, 19, 524–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alicic, R.Z.; Johnson, E.J.; Tuttle, K.R. Inflammatory Mechanisms as New Biomarkers and Therapeutic Targets for Diabetic Kidney Disease. Adv. Chronic Kidney Dis. 2018, 25, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Alicic, R.Z.; Rooney, M.T.; Tuttle, K.R. Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin. J. Am. Soc. Nephrol. 2017, 12, 2032–2045. [Google Scholar] [CrossRef] [PubMed]
- Yaribeygi, H.; Maleki, M.; Sathyapalan, T.; Jamialahmadi, T.; Sahebkar, A. Anti-inflammatory potentials of incretin-based therapies used in the management of diabetes. Life Sci. 2019, 241, 117152. [Google Scholar] [CrossRef]
- Alicic, R.Z.; Cox, E.J.; Neumiller, J.J.; Tuttle, K.R. Incretin drugs in diabetic kidney disease: Biological mechanisms and clinical evidence. Nat. Rev. Nephrol. 2021, 17, 227–244. [Google Scholar] [CrossRef]
- Cox, E.J.; Alicic, R.; Neumiller, J.J.; Tuttle, K. Clinical evidence and proposed mechanisms for cardiovascular and kidney benefits from glucagon-like peptide-1 receptor agonists. US Endocrinol. 2020, 16, 80–87. [Google Scholar] [CrossRef]
- Turner, J.E.; Becker, M.; Mittrucker, H.W.; Panzer, U. Tissue-Resident Lymphocytes in the Kidney. J. Am. Soc. Nephrol. 2018, 29, 389–399. [Google Scholar] [CrossRef]
- Lee, S.B.; Kalluri, R. Mechanistic connection between inflammation and fibrosis. Kidney Int. 2010, 78 (Suppl. S119), S22–S26. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.M.; Nikolic-Paterson, D.J.; Lan, H.Y. Inflammatory processes in renal fibrosis. Nat. Rev. Nephrol. 2014, 10, 493–503. [Google Scholar] [CrossRef]
- Hogan, A.E.; Gaoatswe, G.; Lynch, L.; Corrigan, M.A.; Woods, C.; O’Connell, J.; O’Shea, D. Glucagon-like peptide 1 analogue therapy directly modulates innate immune-mediated inflammation in individuals with type 2 diabetes mellitus. Diabetologia 2014, 57, 781–784. [Google Scholar] [CrossRef]
- Park, C.W.; Kim, H.W.; Ko, S.H.; Lim, J.H.; Ryu, G.R.; Chung, H.W.; Han, S.W.; Shin, S.J.; Bang, B.K.; Breyer, M.D.; et al. Long-term treatment of glucagon-like peptide-1 analog exendin-4 ameliorates diabetic nephropathy through improving metabolic anomalies in db/db mice. J. Am. Soc. Nephrol. 2007, 18, 1227–1238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kodera, R.; Shikata, K.; Kataoka, H.U.; Takatsuka, T.; Miyamoto, S.; Sasaki, M.; Kajitani, N.; Nishishita, S.; Sarai, K.; Hirota, D.; et al. Glucagon-like peptide-1 receptor agonist ameliorates renal injury through its anti-inflammatory action without lowering blood glucose level in a rat model of type 1 diabetes. Diabetologia 2011, 54, 965–978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pyke, C.; Heller, R.S.; Kirk, R.K.; Ørskov, C.; Reedtz-Runge, S.; Kaastrup, P.; Hvelplund, A.; Bardram, L.; Calatayud, D. GLP-1 receptor localization in monkey and human tissue: Novel distribution revealed with extensively validated monoclonal antibody. Endocrinology 2014, 155, 1280–1290. [Google Scholar] [CrossRef] [PubMed]
- Bullock, B.P.; Heller, R.S.; Habener, J.F. Tissue distribution of messenger ribonucleic acid encoding the rat glucagon-like peptide-1 receptor. Endocrinology 1996, 137, 2968–2978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erdogdu, Ö.; Nathanson, D.; Sjöholm, Å.; Nyström, T.; Zhang, Q. Exendin-4 stimulates proliferation of human coronary artery endothelial cells through eNOS-, PKA- and PI3K/Akt-dependent pathways and requires GLP-1 receptor. Mol. Cell. Endocrinol. 2010, 325, 26–35. [Google Scholar] [CrossRef] [Green Version]
- Schlatter, P.; Beglinger, C.; Drewe, J.; Gutmann, H. Glucagon-like peptide 1 receptor expression in primary porcine proximal tubular cells. Regul. Pept. 2017, 141, 120–128. [Google Scholar] [CrossRef]
- Crajoinas, R.O.; Oricchio, F.T.; Pessoa, T.D.; Pacheco, B.P.; Lessa, L.M.; Malnic, G.; Girardi, A.C. Mechanisms mediating the diuretic and natriuretic actions of the incretin hormone glucagon-like peptide-1. Am. J. Physiol.-Ren. Physiol. 2011, 301, F355–F363. [Google Scholar] [CrossRef] [Green Version]
- Moreno, C.; Mistry, M.; Roman, R.J. Renal effects of glucagon-like peptide in rats. Eur. J. Pharmacol. 2002, 434, 163–167. [Google Scholar] [CrossRef]
- Jensen, E.P.; Poulsen, S.S.; Kissow, H.; Holstein-Rathlou, N.H.; Deacon, C.F.; Jensen, B.L.; Holst, J.J.; Sorensen, C.M. Activation of GLP-1 receptors on vascular smooth muscle cells reduces the autoregulatory response in afferent arterioles and increases renal blood flow. Am. J. Physiol.-Ren. Physiol. 2015, 308, F867–F877. [Google Scholar] [CrossRef] [Green Version]
- Muskiet, M.H.; Tonneijck, L.; Smits, M.M.; Kramer, M.H.H.; Diamant, M.; Joles, J.A.; van Raalte, D.H. Acute renal haemodynamic effects of glucagon-like peptide-1 receptor agonist exenatide in healthy overweight men. Diabetes Obes. Metab. 2016, 18, 178–185. [Google Scholar] [CrossRef]
- Gutzwiller, J.P.; Tschopp, S.; Bock, A.; Zehnder, C.E.; Huber, A.R.; Kreyenbuehl, M.; Gutmann, H.; Drewe, J.; Henzen, C.; Goeke, B.; et al. Glucagon-like peptide 1 induces natriuresis in healthy subjects and in insulin-resistant obese men. J. Clin. Endocrinol. Metab. 2004, 89, 3055–3061. [Google Scholar] [CrossRef] [PubMed]
- Tonneijck, L.; Smits, M.M.; Muskiet, M.H.; Hoekstra, T.; Kramer, M.H.; Danser, A.H.; Diamant, M.; Joles, J.A.; van Raalte, D.H. Acute renal effects of the GLP-1 receptor agonist exenatide in overweight type 2 diabetes patients: A randomised, double-blind, placebo-controlled trial. Diabetologia 2016, 59, 1412–1421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonneijck, L.; Smits, M.M.; Muskiet, M.H.; Hoekstra, T.; Kramer, M.H.; Danser, A.J.; Ter Wee, P.M.; Diamant, M.; Joles, J.A.; Van Raalte, D.H. Renal effects of DPP-4 inhibitor sitagliptin or GLP-1 receptor agonist liraglutide in overweight patients with type 2 diabetes: A 12-week, randomized, double-blind, placebo-controlled trial. Diabetes Care 2016, 39, 2042–2050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonneijck, L.; Muskiet, M.H.; Blijdorp, C.J.; Smits, M.M.; Twisk, J.W.; Kramer, M.H.; Danser, A.J.; Diamant, M.; Joles, J.A.; Hoorn, E.J.; et al. Renal tubular effects of prolonged therapy with the GLP-1 receptor agonist lixisenatide in patients with type 2 diabetes mellitus. Am. J. Physiol.-Ren. Physiol. 2019, 316, F231–F240. [Google Scholar] [CrossRef]
- FDA. Guidance for Industry: Diabetes Mellitus—Evaluating Cardiovascular Risk in New Antidiabetic Therapies to Treat Type 2 Diabetes; Food and Drug Administration: Silver Spring, MD, USA, 2008.
- Gerstein, H.C.; Colhoun, H.M.; Dagenais, G.R.; Diaz, R.; Lakshmanan, M.; Pais, P.; Probstfield, J.; Riesmeyer, J.S.; Riddle, M.C.; Rydén, L.; et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): A double-blind, randomized placebo-controlled trial. Lancet 2019, 394, 121–130. [Google Scholar] [CrossRef]
- Holman, R.R.; Bethel, M.A.; Mentz, R.J.; Thompson, V.P.; Lokhnygina, Y.; Buse, J.B.; Chan, J.C.; Choi, J.; Gustavson, S.M.; Iqbal, N.; et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2017, 377, 1228–1239. [Google Scholar] [CrossRef]
- Pfeffer, M.A.; Claggett, B.; Diaz, R.; Dickstein, K.; Gerstein, H.C.; Køber, L.V.; Lawson, F.C.; Ping, L.; Wei, X.; Lewis, E.F.; et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N. Engl. J. Med. 2015, 373, 2247–2257. [Google Scholar] [CrossRef]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef] [Green Version]
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschewitz, F.G.; Jódar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; Warren, M.L.; et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 2016, 375, 1834–1844. [Google Scholar] [CrossRef] [Green Version]
- Husain, M.; Birkenfeld, A.L.; Donsmark, M.; Dungan, K.; Eliaschewitz, F.G.; Franco, D.R.; Jeppesen, O.K.; Lingvay, I.; Mosenzon, O.; Pedersen, S.D.; et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 2019, 381, 841–851. [Google Scholar] [CrossRef] [Green Version]
- Tuttle, K.R.; Lakshmanan, M.C.; Rayner, B.; Busch, R.S.; Zimmermann, A.G.; Woodward, D.B.; Botros, F.T. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): A multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol. 2018, 6, 605–617. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine. A Research Study to See How Semaglutide Works Compared to Placebo in People with Type 2 Diabetes and Chronic Kidney Disease (FLOW). Available online: https://clinicaltrials.gov/ct2/show/NCT03819153 (accessed on 25 April 2022).
- Trulicity (Dulaglutide) Injection. [Prescribing Information]; Eli Lilly and Company: Indianapolis, IN, USA, 2021.
- Tuttle, K.R.; Rayner, B.; Lakshmanan, M.C.; Kwan, A.Y.; Konig, M.; Shurzinske, L.; Botros, F.T. Clinical outcomes by albuminuria status with dulaglutide versus insulin glargine in participants with diabetes and CKD: AWARD-7 exploratory analysis. Kidney360 2021, 2, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Bethel, M.A.; Mentz, R.J.; Merrill, P.; Buse, J.B.; Chan, J.C.; Goodman, S.G.; Iqbal, N.; Jakuboniene, N.; Katona, B.; Lokhnygina, Y. Microvascular and Cardiovascular Outcomes According to Renal Function in Patients Treated with Once-Weekly Exenatide: Insights from the EXSCEL Trial. Diabetes Care 2019, 43, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Muskiet, M.H.; Tonneijck, L.; Huang, Y.; Liu, M.; Saremi, A.; Heerspink, H.J.; van Raalte, D.H. Lixisenatide and renal outcomes in patients with type 2 diabetes and acute coronary syndrome: An exploratory analysis of the ELIXA randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2018, 6, 859–869. [Google Scholar] [CrossRef]
- Mann, J.F.; Ørsted, D.D.; Brown-Frandsen, K.; Marso, S.P.; Poulter, N.R.; Rasmussen, S.; Tornøe, K.; Zinman, B. Liraglutide and Renal Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 839–848. [Google Scholar] [CrossRef] [Green Version]
- Muskiet, M.H.A.; Bunck, M.C.; Heine, R.J.; Corner, A.; Yki-Järvinen, H.; Eliasson, B.; Joles, J.A.; Diamant, M.; Tonneijck, L. Exenatide twice-daily does not affect renal function or albuminuria compared to titrated insulin glargine in patients with type 2 diabetes mellitus: A post-hoc analysis of a 52-week randomised trial. Diabetes Res. Clin. Pract. 2019, 153, 14–22. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhang, H.; Zhang, Q.; Guan, M.; Sheng, S.; Mo, W.; Zou, M.; Li, J.; Bi, J.; Tang, X.; et al. Exenatide and renal outcomes in patients with type 2 diabetes and diabetic kidney disease. Am. J. Nephrol. 2020, 51, 806–814. [Google Scholar] [CrossRef]
- Davies, M.J.; Bain, S.C.; Atkin, S.L.; Rossing, P.; Scott, D.; Shamkhalova, M.S.; Bosch-Traberg, H.; Syrén, A.; Umpierrez, G.E. Efficacy and Safety of Liraglutide Versus Placebo as Add-on to Glucose-Lowering Therapy in Patients with Type 2 Diabetes and Moderate Renal Impairment (LIRA-RENAL): A Randomized Clinical Trial. Diabetes Care 2016, 39, 222–230. [Google Scholar] [CrossRef] [Green Version]
- U.S. National Library of Medicine. A Heart Disease Study of Semaglutide in Patients with Type 2 Diabetes (SOUL). Available online: https://clinicaltrials.gov/ct2/show/NCT03914326 (accessed on 24 April 2022).
- Garber, A.J.; Handelsman, Y.; Grunberger, G.; Einhorn, D.; Abrahamson, M.J.; Barzilay, J.I.; Blonde, L.; Bush, M.A.; DeFronzo, R.A.; Garber, J.R.; et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm—2020 Executive Summary. Endocr. Pract. 2020, 26, 107–139. [Google Scholar] [CrossRef]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2020, 41, 255–323. [Google Scholar] [CrossRef] [Green Version]
- De Boer, I.H.; Caramori, M.L.; Chan, J.C.; Heerspink, H.J.; Hurst, C.; Khunti, K.; Liew, A.; Michos, E.D.; Navaneethan, S.D.; Olowu, W.A.; et al. Kidney Disease: Improving Global Outcomes. KDIGO 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2020, 98, S1–S115. [Google Scholar] [CrossRef] [PubMed]

| REWIND (N = 9901) | EXSCEL (N = 14,752) | ELIXA (N = 6068) | LEADER (N = 9340) | SUSTAIN-6 (N = 3297) | PIONEER-6 (N = 3183) | |
|---|---|---|---|---|---|---|
| Agent | Dulaglutide | Exenatide XR | Lixisenatide | Liraglutide | Semaglutide | Oral semaglutide |
| Median follow-up (years) | 5.4 | 3.2 | 2.1 | 3.8 | 2.1 | 1.3 |
| Metformin use (%) | 81 | 77 | 66 | 76 | 73 | 77 |
| Prior CVD (%) | 32 | 73 | 100 | 81 | 60 | 85 |
| Mean baseline A1C (%) | 7.4 | 8.0 | 7.7 | 8.7 | 8.7 | 8.2 |
| Primary CV Outcome * | 3-point MACE 0.88 (0.79–0.99) | 3-point MACE 0.91 (0.83–1.00) | 4-point MACE 1.02 (0.89–1.17) | 3-point MACE 0.87 (0.78–0.97) | 3-point MACE 0.74 (0.58–0.95) | 3-point MACE 0.79 (0.57–1.11) |
| Key Secondary Outcomes * | ||||||
| CV death | 0.91 (0.78–1.06) | 0.88 (0.76–1.02) | 0.98 (0.78–1.22) | 0.78 (0.66–0.93) | 0.98 (0.65–1.48) | 0.49 (0.27–0.92) |
| MI | 0.96 (0.79–1.15) | 0.97 (0.85–1.10) | 1.03 (0.87–1.22) | 0.86 (0.73–1.00) | 0.74 (0.51–1.08) | 1.18 (0.73–1.90) |
| Stroke | 0.76 (0.61–0.95) | 0.85 (0.70–1.03) | 1.12 (0.79–1.58) | 0.86 (0.71–1.06) | 0.61 (0.38–0.99) | 0.74 (0.35–1.57) |
| HF hospitalization | 0.93 (0.77–1.12) | 0.94 (0.78–1.13) | 0.96 (0.75–1.23) | 0.87 (0.73–1.05) | 1.11 (0.77–1.61) | 0.86 (0.48–1.55) |
| All-cause mortality | 0.90 (0.80–1.01) | 0.86 (0.77–0.97) | 0.94 (0.78–1.13) | 0.85 (0.74–0.97) | 1.05 (0.74–1.50) | 0.51 (0.31–0.84) |
| Worsening nephropathy | 0.85 (0.77–0.93) | - | - | 0.78 (0.67–0.92) | 0.64 (0.46–0.88) | - |
| Agent | Secondary Kidney Outcomes |
|---|---|
| Dulaglutide | REWIND
|
AWARD-7
| |
| Exenatide | EXSCEL
|
| Lixisenatide | ELIXA
|
| Liraglutide | LEADER
|
| Semaglutide | SUSTAIN-6
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neumiller, J.J.; Alicic, R.Z.; Tuttle, K.R. GLP-1 Receptor Agonists in the Treatment of Patients with Type 2 Diabetes and Chronic Kidney Disease. Kidney Dial. 2022, 2, 386-398. https://doi.org/10.3390/kidneydial2030034
Neumiller JJ, Alicic RZ, Tuttle KR. GLP-1 Receptor Agonists in the Treatment of Patients with Type 2 Diabetes and Chronic Kidney Disease. Kidney and Dialysis. 2022; 2(3):386-398. https://doi.org/10.3390/kidneydial2030034
Chicago/Turabian StyleNeumiller, Joshua J., Radica Z. Alicic, and Katherine R. Tuttle. 2022. "GLP-1 Receptor Agonists in the Treatment of Patients with Type 2 Diabetes and Chronic Kidney Disease" Kidney and Dialysis 2, no. 3: 386-398. https://doi.org/10.3390/kidneydial2030034
APA StyleNeumiller, J. J., Alicic, R. Z., & Tuttle, K. R. (2022). GLP-1 Receptor Agonists in the Treatment of Patients with Type 2 Diabetes and Chronic Kidney Disease. Kidney and Dialysis, 2(3), 386-398. https://doi.org/10.3390/kidneydial2030034






