Should We Still Use Therapeutic Plasma Exchange for Rapidly Progressive Glomerulonephritis in ANCA Associated Vasculitis?
Abstract
:1. Introduction
2. Mechanism of Benefit of TPE
3. Are ANCA Pathogenic?
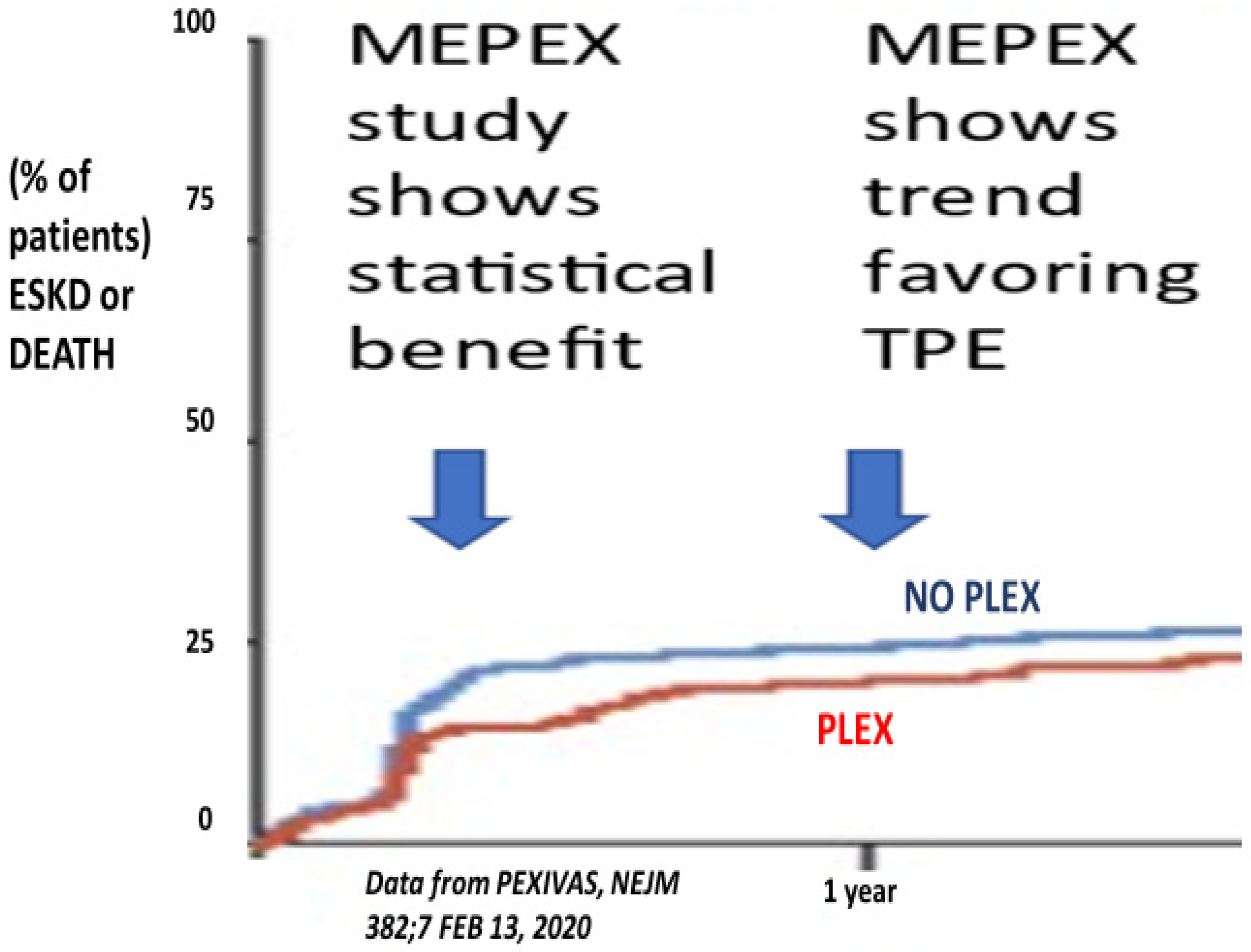
4. Results of Clinical Trials
5. Cost Analysis of Providing TPE
6. Risk of Serious Infection with TPE
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walsh, M.; Merkel, P.A.; Peh, C.A.; Szpirt, W.M.; Puéchal, X.; Fujimoto, S.; Hawley, C.M.; Khalidi, N.; Floßmann, O.; Wald, R.; et al. Plasma exchange and Glucocorticoids in Severe ANCA-Associated Vasculitis (PEXIVAS). N. Engl. J. Med. 2020, 382, 622. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.; Collister, D.; Zeng, L.; Merkel, A.P.; Pusey, C.D.; Guyatt, G.; Peh, C.A.; Szpirt, W.M.; Ito-Hara, T.; Jayne, D.R.W. The effects of plasma exchange in patients with ANCA-associated vasculitis: An updated systematic review and meta-analysis. BMJ 2022, 376, e064604. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Freeman, T. Metabolic heterogenicity of human γ-globulin. Biochem. J. 1960, 76, 475–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaplan, A.A. A Practical Guide to Therapeutic Plasma Exchange; Blackwell Science: Malden, MA, USA, 1999. [Google Scholar]
- Kaplan, A.A. Therapeutic Plasma Exchange: Core Curriculum 2008. Am. J. Kidney Dis. 2008, 52, 1180–1196. [Google Scholar] [CrossRef]
- Lionaki, S.; Falk, R.J. Removing Antibody and Preserving Glomeruli in ANCA Small Vessel Vasculitis. J. Am. Soc. Nephrol. 2007, 18, 987–1989. [Google Scholar] [CrossRef] [Green Version]
- Falk, R.J.; Jennette, J.C. ANCA Are Capable of Activating Leukocytes In Vitro. J. Am. Soc. Nephrol. 2002, 13, 1977–1979. [Google Scholar] [CrossRef]
- Xiao, H.; Heeringa, P.; Hu, P.; Liu, Z.; Zhao, M.; Aratani, Y.; Maeda, N.; Falk, R.J.; Jennette, J.C. Antineutrophil cytoplasmic antibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J. Clin. Investig. 2002, 110, 955–963. [Google Scholar] [CrossRef]
- Schlieben, D.J.; Korbet, S.M.; Kimura, R.E. Pulmonary-renal syndrome in a newborn with placental transmission of ANCAs. Case of transplacental transfer of ANCA resulting in vasculitis in newborn infant. Am. J. Kidney Dis. 2005, 45, 758–761. [Google Scholar] [CrossRef]
- Silva, F.; Specks, U.; Sethi, S.; Irazabal, M.V.; Fervenza, F.C. Successful pregnancy and delivery of a healthy newborn despite transplacental transfer of antimyeloperoxidase antibodies from a mother with microscopic polyangiitis. Am. J. Kidney Dis. 2009, 54, 542–545. [Google Scholar] [CrossRef]
- Silva Xiao, H.; Schreiber, A.; Heeringa, P.; Falk, R.J.; Jennette, J.C. Alternative Complement Pathway in the Pathogenesis of Disease Mediated by Anti-Neutrophil Cytoplasmic Antibodies. Am. J. Pathol. 2007, 170, 52–64. [Google Scholar] [CrossRef] [Green Version]
- Jayne, D.R.W.; Marshall, P.D.; Jones, S.J.; Lockwood, C.M. Autoantibodies to GBM and neutrophil cytoplasm in rapidly progressive glomerulonephritis. Kidney Int. 1990, 37, 965–970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lockwood, C.M.; Rees, A.J.; Pinching, A.J.; Pussell, B.; Sweny, P.; Uff, J.; Peters, D.K. Plasma exchange and immunosuppression in the treatment of fulminating immune-complex crescentic nephritis. Lancet 1977, 309, 63–67. [Google Scholar] [CrossRef]
- Kincaid-Smith, P.; D’Apice, A.J.F. Plasmapheresis in rapidly progressive glomerulonephritis. Am. J. Med. 1978, 65, 564–566. [Google Scholar] [CrossRef]
- Hind, C.R.K.; Paraskevakou, H.; Lockwood, C.M.; Evans, D.J.; Peters, D.K.; Rees, A.J. Prognosis after immunosuppression of patients with crescentic nephritis requiring dialysis. Lancet 1983, 321, 263–265. [Google Scholar] [CrossRef]
- Mauri, J.M.; Gonzales, M.T.; Poveda, R.; Seron, D.; Torras, J.; Andujar, J.; Andres, E.; Alsina, J. Therapeutic plasma exchange in the treatment of rapidly progressive glomerulonephritis. Plasma Transfus. Technol. 1985, 6, 587–591. [Google Scholar]
- Glockner, W.M.; Sieberth, H.G.; Wichmann, H.E.; Backes, E.; Bambauer, R.; Boesken, W.H.; Bohle, A.; Daul, A.; Graben, N.; Keller, F.; et al. Plasma exchange and immunosuppression in rapidly progressive glomerulonephritis: A controlled multi-center study. Clin. Nephrol. 1988, 29, 1–8. [Google Scholar] [PubMed]
- Pusey, C.D.; Rees, A.J.; Evans, D.J.; Peters, D.K.; Lockwood, C.M. Plasma exchange in focal necrotizing glomerulonephritis without anti-GBM antibodies. Kidney Int. 1991, 40, 757–763. [Google Scholar] [CrossRef] [Green Version]
- Cole, E.; Cattran, D.; Magil, A.; Greenwood, C.; Churchill, D.; Sutton, D.; Clark, W.; Morrin, P.; Posen, G.; Bernstein, K.; et al. A prospective randomized trial of plasma exchange as additive therapy in idiopathic crescentic glomerulonephritis. Am. J. Kidney Dis. 1992, 20, 261–269. [Google Scholar] [CrossRef]
- Kaplan, A.A. Therapeutic plasma exchange for the treatment of rapidly progressive glomerulonephritis (RPGN). Ther. Apher. 1997, 1, 255–259. [Google Scholar] [CrossRef]
- Jayne, D.R.; Gaskin, G.; Rasmussen, N.; Abramowicz, D.; Ferrario, F.; Guillevin, L.; Mirapeix, E.; Savage, C.O.; Sinico, R.A.; Stegeman, C.A.; et al. Randomized trial of plasma exchange or high-dosage methylprednisolone as adjunctive therapy for severe renal vasculitis MEPEX. J. Am. Soc. Nephrol. 2007, 18, 2180–2188. [Google Scholar] [CrossRef]
- Walsh, M.; Catapano, F.; Szpirt, W.; Thorlund, K.; Bruchfeld, A.; Guillevin, L.; Haubitz, M.; Merkel, P.A.; Peh, C.A.; Pusey, C.; et al. Plasma exchange for renal vasculitis and idiopathic rapidly progressive glomerulonephritis: A meta-analysis. Am. J. Kidney Dis. 2011, 57, 566–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szpirt, W.; Heaf, J.; Pedersen, J. Plasma exchange for induction and cyclosporine A for maintenance of remission in Wegener’s granulomatosis—A clinical randomized controlled trial. Nephrol. Dial. Transpl. 2011, 26, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Derebail, V.K.; Falk, R.J. Editorial ANCA-Associated Vasculitis—Refining Therapy with Plasma Exchange and Glucocorticoids. Ther. Apher. 2020, 382, 255–259. [Google Scholar]
- Nezam, D.; Porcher, R.; Grolleau, F.; Morel, P.; Titeca-Beauport, D.; Faguer, S.; Karras, A.; Solignac, J.; Jourde-Chiche, N.; Maurier, F.; et al. Kidney histopathology can predict kidney in ANCA-associated vasculitides with acute kidney injury treated with plasma exchanges. J. Am. Soc. Nephrol. 2022, 33, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Moura, M.C.; Soler, M.J.; Sethi, S.; Fervenza, F.C.; Specks, U. Predicting kidney response to plasma exchange in ANCA-associated vasculitis: Need for plausible models. J. Am. Soc. Nephrol. 2022, 33, 1223–1224. [Google Scholar] [CrossRef]
- Esposito, P.; Cipriani, L.; Viazzi, F. Correspondance. N. Engl. J. Med. 2020, 382, 2168–2169. [Google Scholar]
- Zeng, L.; Walsh, M.; Guyatt, G.H.; Siemieniuk, R.A.C.; Collister, D.; Booth, M.; Brown, P.; Farrar, L.; Farrar, M.; Firth, T.; et al. Plasma exchange and glucocorticoid dosing for patients with ANCA-associated vasculitis: A clinical practice guideline. BMJ 2022, 376, e064597. [Google Scholar] [CrossRef]
- USRDS-2018 (United States Renal Data System Annual Data Report, Executive Summary) Chapter 9: Healthcare Expenditures for Persons with ESRD. Available online: https://www.usrds.org (accessed on 20 April 2022).
- Mokrzycki, M.H.; Kaplan, A.A. Therapeutic plasma exchange: Complications and management. Am. J. Kidney Dis. 1994, 23, 817–827. [Google Scholar] [CrossRef]
- Pohl, M.A.; Lan, S.P.; Berl, T.; The Lupus Nephritis Collaborative Study Group. Plasmapheresis does not increase the risk of infection in immunocompromised patients with severe lupus nephritis. Ann. Intern. Med. 1991, 114, 924–929. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaplan, A.A.; Szpirt, W.M. Should We Still Use Therapeutic Plasma Exchange for Rapidly Progressive Glomerulonephritis in ANCA Associated Vasculitis? Kidney Dial. 2022, 2, 399-406. https://doi.org/10.3390/kidneydial2030035
Kaplan AA, Szpirt WM. Should We Still Use Therapeutic Plasma Exchange for Rapidly Progressive Glomerulonephritis in ANCA Associated Vasculitis? Kidney and Dialysis. 2022; 2(3):399-406. https://doi.org/10.3390/kidneydial2030035
Chicago/Turabian StyleKaplan, Andre A., and Wladimir M. Szpirt. 2022. "Should We Still Use Therapeutic Plasma Exchange for Rapidly Progressive Glomerulonephritis in ANCA Associated Vasculitis?" Kidney and Dialysis 2, no. 3: 399-406. https://doi.org/10.3390/kidneydial2030035
APA StyleKaplan, A. A., & Szpirt, W. M. (2022). Should We Still Use Therapeutic Plasma Exchange for Rapidly Progressive Glomerulonephritis in ANCA Associated Vasculitis? Kidney and Dialysis, 2(3), 399-406. https://doi.org/10.3390/kidneydial2030035





