An Unusual Cause of Acute Kidney Injury in Pregnancy: Beware of HELLP Look-Alikes
Abstract
1. Background
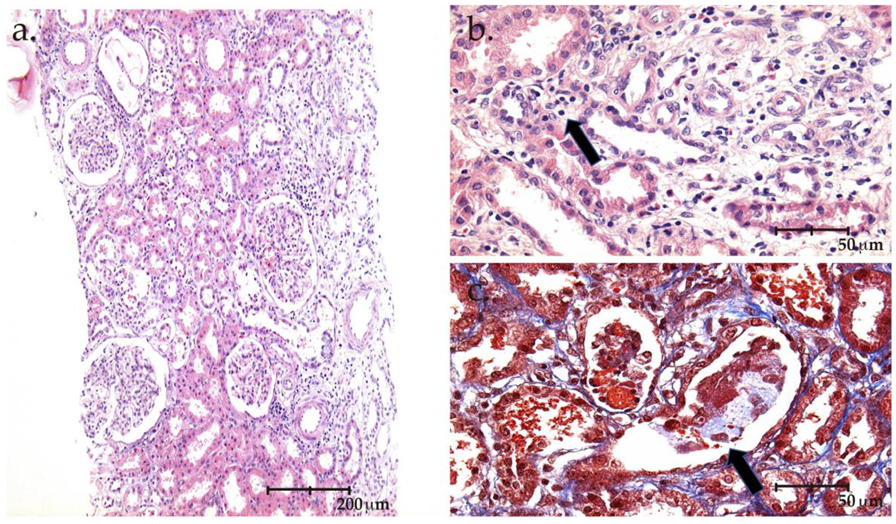
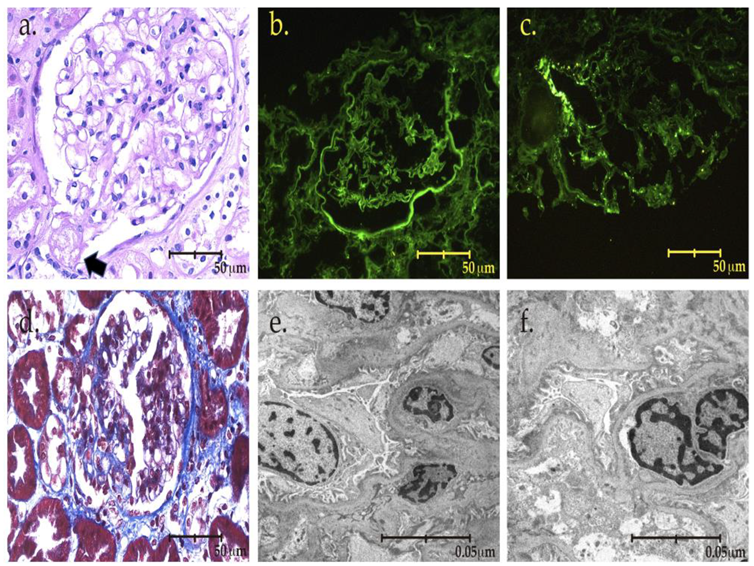
2. The Case
3. Discussion
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Sibai, B.M.; Ramadan, M.K.; Usta, I.; Salama, M.; Mercer, B.M.; Friedman, S.A. Maternal morbidity and mortality in 442 pregnancies with hemolysis, elevated liver enzymes, and low platelets (HELLP syndrome). Am. J. Obs. Gynecol. 1993, 169, 1000–1006. [Google Scholar] [CrossRef]
- Kandukurti, K.; Sun, J.; Venuto, R. Multiple pathologies in the kidney biopsy of a recently pregnant woman. Case Rep. Nephrol. Urol. 2013, 3, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Shu, H.; Wen, Y.; Ye, W.; Li, H.; Qin, Y.; Chen, L.; Li, X. Renal histopathology of prolonged acute kidney injury in HELLP syndrome: A case series and literature review. Int. Urol. Nephrol. 2019, 51, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Ling, G.J.; Zhang, S.Q.; Zhai, W.Q.; Chen, Y.J. Effect of HELLP syndrome on acute kidney injury in pregnancy and pregnancy outcomes: A systematic review and meta-analysis. BMC Pregnancy Childbirth 2020, 20, 657. [Google Scholar] [CrossRef] [PubMed]
- Fakhouri, F.; Scully, M.; Provôt, F.; Blasco, M.; Coppo, P.; Noris, M.; Paizis, K.; Kavanagh, D.; Pène, F.; Quezada, S.; et al. Management of thrombotic microangiopathy in pregnancy and postpartum: Report from an international working group. Blood 2020, 136, 2103–2117. [Google Scholar] [CrossRef]
- Saei Ghare Naz, M.; Rahmati, M.; Azizi, F.; Ramezani Tehrani, F. Risk of chronic kidney disease in women with a history of preterm delivery: Tehran Lipid and Glucose Study. J. Nephrol. 2021, 34, 1621–1629. [Google Scholar] [CrossRef]
- Kountouris, E.; Clark, K.; Kay, P.; Roberts, N.; Bramham, K.; Kametas, N.A. Postnatal assessment for renal dysfunction in women with hypertensive disorders of pregnancy : A prospective observational study. J. Nephrol. 2021, 34, 1641–1649. [Google Scholar] [CrossRef]
- Cabiddu, G.; Mannucci, C.; Fois, A.; Maxia, S.; Chatrenet, A.; Osadolor, S.; Kimani, E.; Torreggiani, M.; Attini, R.; Masturzo, B.; et al. Preeclampsia is a valuable opportunity to diagnose chronic kidney disease: A multicentre study. Nephrol. Dial. Transplant. 2021, 2, gfab225. [Google Scholar]
- Gaber, L.W.; Spargo, B.H. Pregnancy-induced nephropathy: The significance of focal segmental glomerulosclerosis. Am. J. Kidney Dis. 1987, 9, 317–323. [Google Scholar] [CrossRef]
- Molina-Pérez, C.J.; Nolasco-Leaños, A.G.; Carrillo-Juárez, R.I.; Leaños-Miranda, A. Clinical usefulness of angiogenic factors in women with chronic kidney disease and suspected superimposed preeclampsia. J. Nephrol. 2022, 35, 1699–1708. [Google Scholar] [CrossRef]
- Orozco Guillén, O.A.; Velazquez Silva, R.I.; Gonzalez, B.M.; Becerra Gamba, T.; Gutiérrez Marín, A.; Paredes, N.R.; Cardona Pérez, J.A.; Soto Abraham, V.; Piccoli, G.B.; Madero, M. Collapsing Lesions and Focal Segmental Glomerulosclerosis in Pregnancy: A Report of 3 Cases. Am. J. Kidney Dis. 2019, 74, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Smyth, A.; Wall, C.A. Nephrotic syndrome due to focal segmental glomerulosclerosis occurring in early pregnancy. Obs. Med. 2011, 4, 80–82. [Google Scholar] [CrossRef] [PubMed]
- Kopp, J.B.; Rosenberg, A.Z. One Actor, Many Roles: Histopathologies Associated With APOL1 Genetic Variants. Adv. Anat. Pathol. 2019, 26, 215–219. [Google Scholar] [CrossRef] [PubMed]
- De Castro, I.; Easterling, T.R.; Bansal, N.; Jefferson, J.A. Nephrotic syndrome in pregnancy poses risks with both maternal and fetal complications. Kidney Int. 2017, 91, 1464–1472. [Google Scholar] [CrossRef]
- Cabiddu, G.; Longhitano, E.; Cataldo, E.; Lepori, N.; Chatrenet, A.; Torreggiani, M.; Attini, R.; Masturzo, B.; Rossini, M.; Versino, E.; et al. History of Preeclampsia in Patients Undergoing a Kidney Biopsy: A Biphasic, Multiple-Hit Pathogenic Hypothesis. Kidney Int. Rep. 2021, 7, 547–557. [Google Scholar] [CrossRef]
- World Health Organization. The World Health Report 2005: Make Every Mother and Child Count; World Health Organization: Geneva, Switzerland, 2005. [Google Scholar]
- Weinstein, L. Syndrome of hemolysis, elevated liver enzymes, and low platelet count: A severe consequence of hypertension in pregnancy. Am. J. Obstet. Gynecol. 1982, 142, 159–167. [Google Scholar] [CrossRef]
- Jamme, M.; Rafat, C.; Gaugain, S.; Chousterman, B.G. Postpartum renal cortical necrosis. Kidney Int. 2018, 94, 1240. [Google Scholar] [CrossRef]
- Dai, D.F.; Sasaki, K.; Lin, M.Y.; Smith, K.D.; Nicosia, R.F.; Alpers, C.E.; Najafian, B. Interstitial eosinophilic aggregates in diabetic nephropathy: Allergy or not? Nephrol Dial. Transplant. 2015, 30, 1370–1376. [Google Scholar] [CrossRef]
- Rolfo, A.; Attini, R.; Tavassoli, E.; Neve, F.V.; Nigra, M.; Cicilano, M.; Nuzzo, A.M.; Giuffrida, D.; Biolcati, M.; Nichelatti, M.; et al. Is It Possible to Differentiate Chronic Kidney Disease and Preeclampsia by means of New and Old Biomarkers? A Prospective Study. Dis. Markers 2015, 2015, 127083. [Google Scholar] [CrossRef]
- Wiles, K.; Bramham, K.; Seed, P.T.; Kurlak, L.O.; Mistry, H.D.; Nelson-Piercy, C.; Lightstone, L.; Chappell, L.C. Diagnostic Indicators of Superimposed Preeclampsia in Women With CKD. Kidney Int. Rep. 2019, 4, 842–853. [Google Scholar] [CrossRef]
- Bramham, K.; Seed, P.T.; Lightstone, L.; Nelson-Piercy, C.; Gill, C.; Webster, P.; Poston, L.; Chappell, L.C. Diagnostic and predictive biomarkers for pre-eclampsia in patients with established hypertension and chronic kidney disease. Kidney Int. 2016, 89, 874–885. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, S.; Kwiatkowska, E.; Rzepka, R.; Kurkiewicz, V.; Mikołajek-Bedner, W.; Torbè, A. Development of a focal segmental glomerulosclerosis after pregnancy complicated by preeclampsia: Case report and review of literature. J. Matern. Fetal Neonatal Med. 2016, 29, 1566–1569. [Google Scholar] [CrossRef] [PubMed]
| At Referral | Puerperium | 3 Weeks after Delivery, Kidney Biopsy | 8 Weeks after Delivery | |
|---|---|---|---|---|
| S Cr (mg/dL) | 3.3 | 6.3 | 3.3 | 0.9 |
| BUN (mg/dL) | 30.8 | 54.7 | 67.8 | 12.1 |
| e-GFR (mL/min) | - | - | 19 | 89 |
| Albumin (g/dL) | 4.55 | 4.1 | 3.7 | 4 |
| Total proteins | 7.5 | 7.2 | 7 | 7.3 |
| Proteinuria (g/24 h) | - | - | 11.39 | 0.486 |
| Haemoglobin (g/dL) | 14.6 | 11.2 | 11.4 | 12.9 |
| Platelets (mm3) | 48,000 | 142 | 397 | 396 |
| LDH (UI/L) | 978 | 432 | 242 | 260 |
| AST (UI/L) | 750 | 363 | 46 | 25 |
| ALT (UI/L) | 1265 | 273 | 51 | 43 |
| Total bilirubin(mg/dL) | 3.2 | 0.6 | 0.3 | 0.4 |
| Potassium (mEq/L) | 4.7 | 5.7 | 4.2 | 4 |
| HCO3 (mEq/L) | 14 | 10.4 | 19.2 | 25 |
| Immunologic markers: C3 96.5 mg/dL (90–180 mg/dL), C4 12.20 mg/dL (10–40 mg/dL), ANA negative B2 glycoprotein (IgG) 3.9 CU, b2 Glycoprotein (IgM) 1.4 CU, anticardiolipin (IgM) 1.4 CU, anti-SM antibodies >3.3 CU, DNA double-strain antibodies >9.8 UI/ml | ||||
| Thrombotic microangiopathic screening: IgG antibodies ADAMS-13(J) 1.83 U/mL negative (negative <12); positive >15 Adams-13 activity (j) 0.75 (0.4–1.3 U/mL) Adams-13 activity 75% (40–130%) Haptoglobin 27.1 (30–200 mg/dL) | ||||
| Viral markers: Hepatitis B, C, HIV negative | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orozco-Guillen, O.A.; Soto-Abram, V.; Moguel-Gonzalez, B.; Madero, M.; Piccoli, G.B. An Unusual Cause of Acute Kidney Injury in Pregnancy: Beware of HELLP Look-Alikes. Kidney Dial. 2022, 2, 588-594. https://doi.org/10.3390/kidneydial2040053
Orozco-Guillen OA, Soto-Abram V, Moguel-Gonzalez B, Madero M, Piccoli GB. An Unusual Cause of Acute Kidney Injury in Pregnancy: Beware of HELLP Look-Alikes. Kidney and Dialysis. 2022; 2(4):588-594. https://doi.org/10.3390/kidneydial2040053
Chicago/Turabian StyleOrozco-Guillen, Oralia Alejandra, Virgilia Soto-Abram, Bernardo Moguel-Gonzalez, Magdalena Madero, and Giorgina Barbara Piccoli. 2022. "An Unusual Cause of Acute Kidney Injury in Pregnancy: Beware of HELLP Look-Alikes" Kidney and Dialysis 2, no. 4: 588-594. https://doi.org/10.3390/kidneydial2040053
APA StyleOrozco-Guillen, O. A., Soto-Abram, V., Moguel-Gonzalez, B., Madero, M., & Piccoli, G. B. (2022). An Unusual Cause of Acute Kidney Injury in Pregnancy: Beware of HELLP Look-Alikes. Kidney and Dialysis, 2(4), 588-594. https://doi.org/10.3390/kidneydial2040053







