Fractures in CKD Patients—Risk Analysis in RRT Lombardy Patients
Abstract
1. Introduction
2. Material and Methods
3. Ethical Consideration
4. Statistical Analysis
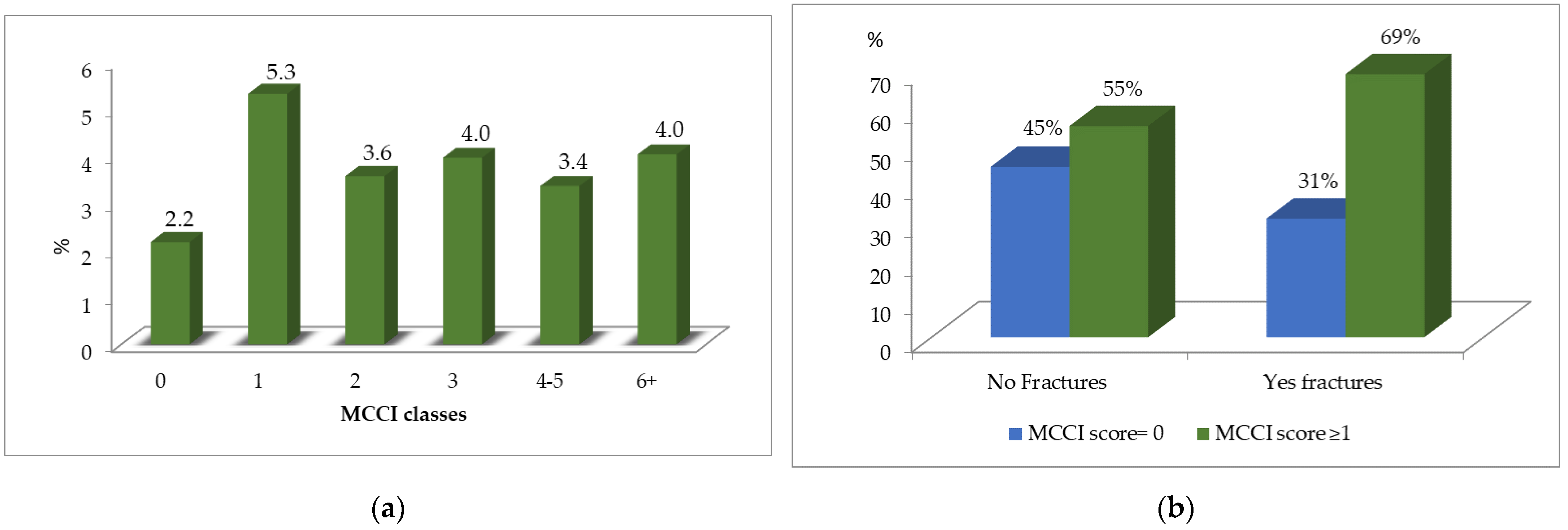
5. Results
6. Discussion
6.1. Epidemiology
6.2. CKD Treatment
6.3. Hospitalization
6.4. Mortality
6.5. Comorbidities
6.6. Drugs Prescription
6.7. Secondary Hyperparathyroidism
6.8. Costs Analysis
6.9. Weakness and Strength
6.10. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johansen, K.L. Life Expectancy Gains for Patients with ESRD. Clin. J. Am. Soc. Nephrol. 2018, 13, 11–12. [Google Scholar] [CrossRef] [PubMed]
- Foster, B.; Mitsnefes, M.; Dahhou, M.; Zhang, X.; Laskin, B.L. Changes in excess mortality from end-stage renal disease in the United States from 1995–2013. Clin. J. Am. Soc. Nephrol. 2018, 13, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Messa, P. Skeletal fractures in patients on renal replacement therapy: How large still is the knowledge gap? Nephrol. Dial. Transpl. 2016, 31, 1554–1556. [Google Scholar] [CrossRef]
- Dhanwal, D.K.; Dennison, E.M.; Harvey, N.C.; Cooper, C. Epidemiology of hip fracture: Worldwide geographic variation. Indian J. Orthop. 2011, 45, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, A.; Urena-Torres, P.; Zillikens, M.C.; Bover, J.; Cohen-Solal, M. Fractures in patients with CKD—Diagnosis, treatment, and prevention: A review by members of the European Calcified Tissue Society and the European Renal Association of Nephrology Dialysis and Transplantation. Kidney Int. 2017, 92, 1343–1355. [Google Scholar] [CrossRef] [PubMed]
- Piscitelli, P.; Brandi, M.L.; Chitano, G.; Argentiero, A.; Neglia, C.; Distante, A.; Saturnino, L.; Tarantino, U. Epidemiology of fragility fractures in Italy. Clin. Cases Miner. Bone Metab. 2011, 8, 29–34. [Google Scholar] [PubMed]
- Adami, S.; Giannini, S.; Giorgino, R.; Isaia, G.; Maggi, S.; Sinigaglia, L.; Filipponi, P.; Crepaldi, G.; Di Munno, O. The effect of age, weight, and lifestyle factors on calcaneal quantitative ultrasound: The ESOPO study. Osteoporos. Int. 2003, 14, 198–207. [Google Scholar] [CrossRef]
- ISTAT. Annuario Statistico Italiano; cap.3 Tavola 3.11. 2013. Available online: https://www.istat.it›archivio (accessed on 16 November 2021).
- Tentori, F.; McCullough, K.; Kilpatrick, R.D.; Bradbury, B.D.; Robinson, B.M.; Kerr, P.G.; Pisoni, R.L. High rates of death and hospitalization follow bone fracture among hemodialysis patients. Kidney Int. 2013, 85, 166–173. [Google Scholar] [CrossRef]
- Sidibé, A.; Auguste, D.; Desbiens, L.-C.; Fortier, C.; Wang, Y.P.; Jean, S.; Moore, L.; Mac-Way, F. Fracture Risk in Dialysis and Kidney Transplanted Patients: A Systematic Review. JBMR Plus 2018, 3, 45–55. [Google Scholar] [CrossRef]
- Ball, A.M.; Gillen, D.L.; Sherrard, D.; Weiss, N.S.; Emerson, S.S.; Seliger, S.L.; Kestenbaum, B.R.; Stehman-Breen, C. Risk of hip fracture among dialysis and renal transplant recipients. JAMA 2002, 288, 3014–3018. [Google Scholar] [CrossRef]
- Chen, Y.J.; Kung, P.T.; Wang, Y.H.; Huang, C.C.; Hsu, S.C.; Tsai, W.C.; Hsu, H.C. Greater risk of hip fracture in hemodialysis than in peritoneal dialysis. Osteoporos. Int. 2014, 25, 1513–1518. [Google Scholar] [CrossRef] [PubMed]
- The R Project for Statistical Computing. Available online: https://www.r-project.org (accessed on 30 January 2020).
- Hemmelgarn, B.R.; Manns, B.J.; Quan, H.; Ghali, W.A. Adapting the Charlson Comorbidity Index for Use in Patients with ESRD. Am. J. Kidney Dis. 2003, 42, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Roggeri, D.P.; Roggeri, A.; Zocchetti, C.; Cozzolino, M.; Rossi, C.; Conte, F. Real-world data on healthcare resource consumption and costs before and after kidney transplantation. Clin. Transplant. 2019, 33, e13728. [Google Scholar] [CrossRef]
- Gavrielov-Yusim, N.; Friger, M. Use of administrative medical databases in population-based research. J. Epidemiol. Community Health 2014, 68, 283–287. [Google Scholar] [CrossRef]
- RBrodt, E.D.; Skelly, A.C.; Dettori, J.R.; Hashimoto, R.E. Administrative Database Studies: Goldmine or Goose Chase? Evid. Based Spine Care J. 2014, 5, 74–76. [Google Scholar] [CrossRef]
- Garland, A.; Gershengorn, H.B.; Marrie, R.A.; Reider, N.; Wilcox, M.E. A Practical, Global Perspective on Using Administrative Data to Conduct Intensive Care Unit Research. Ann. Am. Thorac. Soc. 2015, 12, 1373–1386. [Google Scholar] [CrossRef] [PubMed]
- Bello, A.; Hemmelgarn, B.; Manns, B.; Tonelli, M.; for Alberta Kidney Disease Network. Use of administrative databases for health-care planning in CKD. Nephrol. Dial. Transplant. 2012, 27 (Suppl. 3), iii12–iii18. [Google Scholar] [CrossRef]
- Fusaro, M.; D’Arrigo, G.; Pitino, A.; Iervasi, G.; Tentori, F.; Robinson, B.; Aghi, A.; Bieber, B.; Mccullogh, K.; Fabris, F.; et al. Increased Risk of Bone Fractures in Hemodialysis Patients Treated with Proton Pump Inhibitors in Real World: Results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). J. Bone Miner. Res. 2019, 34, 2238–2245. [Google Scholar]
- Chang, N.-T.; Lee, Y.-H.; Hsu, J.-C.; Chan, C.-L.; Huang, G.-S.; Renn, J.-H.; Yang, N.-P. Epidemiological study of orthopedic injuries in hemodialysis patients in Taiwan: A fixed cohort survey,2004–2008. Clin. Interv. Aging 2013, 8, 301–308. [Google Scholar] [CrossRef]
- Wagner, J.; Jhaveri, K.D.; Rosen, L.; Sunday, S.; Mathew, A.T.; Fishbane, S. Increased bone fractures among elderly United States hemodialysis patients. Nephrol. Dial. Transplant. 2014, 29, 146–151. [Google Scholar] [CrossRef][Green Version]
- Bergh, C.; Wennergren, D.; Möller, M.; Brisby, H. Fracture incidence in adults in relation to age and gender: A study of 27,169 fractures in the Swedish Fracture Register in a well-defined catchment area. PLoS ONE 2020, 15, e0244291. [Google Scholar] [CrossRef] [PubMed]
- Dey, V.; Farrah, T.E.; Traynor, J.P.; Spalding, E.M.; Robertson, S.E.; Geddes, C.C. Geddes Symptomatic fracture risk in the renal replacement therapy population. Nephrol. Dial. Transplant. 2017, 32, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Chikritzhs, T. The Effect of Age on Fracture Risk: A Population-Based Cohort Study. J. Aging Res. 2016, 2016, 5071438. [Google Scholar] [CrossRef]
- Wu, S.C.; Rau, C.S.; Kuo, S.C.; Chien, P.C.; Hsieh, C.H. The influence of ageing on the incidence and site of traumatic femoral fractures: A cross-sectional analysis. BMC Musculoskelet. Disord. 2019, 20, 413. [Google Scholar] [CrossRef] [PubMed]
- Roggeri, A.; Conte, F.; Rossi, C.; Cozzolino, M.; Zocchetti, C.; Roggeri, D.P. Cinacalcet adherence in dialysis patients with secondary hyperparathyroidism in Lombardy Region: Clinical implications and costs. Drugs Context 2020, 9, 2020-1-1. [Google Scholar] [CrossRef] [PubMed]
- Boonpheng, B.; Thongprayoon, C.; A Mao, M.; Wijarnpreecha, K.; Bathini, T.; Kaewput, W.; Ungprasert, P.; Cheungpasitporn, W. Risk of hip fracture in patients on hemodialysis versus peritoneal dialysis: A meta-analysis of observational studies. J. Evid. Based Med. 2019, 12, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Signorelli, S.S.; Scuto, S.; Marino, E.; Giusti, M.; Xourafa, A.; Gaudio, A. Anticoagulants and Osteoporosis. Int. J. Mol. Sci. 2019, 20, 5275. [Google Scholar] [CrossRef]
- Nickolas, T.L.; Stein, E.M.; Dworakowski, E.; Nishiyama, K.K.; Komandah-Kosseh, M.; A Zhang, C.; McMahon, D.J.; Liu, X.S.; Boutroy, S.; Cremers, S.; et al. Rapid cortical bone loss in patients with chronic kidney disease. J. Bone Miner. Res. 2013, 28, 18–20. [Google Scholar] [CrossRef]
- Wei, M.; Esbaei, K.; Bargman, J.M.; Oreopoulos, D.G. Inverse correlation between serum magnesium and parathyroid hormone in peritoneal dialysis patients: A contributing factor to adynamic bone disease? Int. Urol. Nephrol. 2006, 38, 317–322. [Google Scholar] [CrossRef]
- Tan, J.; Li, Y.; Wu, Z.; Zhao, J. Risk of hip fracture in patients on dialysis or kidney transplant: A meta-analysis of 14 cohort studies. Ther. Clin. Risk Manag. 2018, 14, 1747–1755. [Google Scholar] [CrossRef]
- Iyer, S.P.; Nikkel, L.E.; Nishiyama, K.K.; Dworakowski, E.; Cremers, S.; Zhang, C.; McMahon, D.J.; Boutroy, S.; Liu, X.S.; Ratner, L.E.; et al. Kidney transplantation with early corticosteroid withdrawal: Paradoxical effects at the central and peripheral skeleton. J. Am. Soc. Nephrol. 2014, 25, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Nikkel, L.E.; Mohan, S.; Zhang, A.; McMahon, D.J.; Boutroy, S.; Dube, G.; Tanriover, B.; Cohen, D.; Ratner, L.; Hollenbeak, C.S.; et al. Reduced fracture risk with early corticosteroid withdrawal after kidney transplant. Am. J. Transplant. 2012, 12, 649–659. [Google Scholar] [CrossRef] [PubMed]
- RaWestenfeld, R.; Schlieper, G.; Wöltje, M.; Gawlik, A.; Brandenburg, V.; Rutkowski, P.; Floege, J.; Jahnen-Dechent, W.; Ketteler, M. Impact of sirolimus, tacrolimus and mycophenolate mofetil on osteoclastogenesis—Implications for post-transplantation bone disease. Nephrol. Dial. Transplant. 2011, 26, 4115–4123. [Google Scholar] [CrossRef] [PubMed]
- Hickson, L.J.; Farah, W.H.; Johnson, R.L.; Thorsteinsdottir, B.; Ubl, D.S.; Yuan, B.J.; Albright, R.; Rule, A.D.; Habermann, E. Death and Postoperative Complications After Hip Fracture Repair: Dialysis Effect. Kidney Int. Rep. 2018, 3, 1294–1303. [Google Scholar] [CrossRef] [PubMed]
- ANNUARIO-STATISTICO-ITALIANO-2012:ISBN978-88-458-1731-1. Available online: https//ebiblio.istat.it/digibib/Annuario%20Statistico%20Italiano/RAV0040597ASI2012.pd (accessed on 25 March 2021).
- Ahn, E.-J.; Bang, S.R. Effect of renal dialysis on mortality and complications following hip fracture surgery in elderly patients: A population based retrospective cohort study. Medicine 2020, 99, e21676. [Google Scholar] [CrossRef]
- Lin, J.C.-F.; Liang, W.-M. Mortality and complications after hip fracture among elderly patients undergoing hemodialysis. BMC Nephrol. 2015, 16, 100. [Google Scholar] [CrossRef]
- Xue, Q.-L. The frailty syndrome: Definition and natural history. Clin. Geriatr. Med. 2011, 27, 1–5. [Google Scholar] [CrossRef]
- Hung, L.W.; Hwang, Y.T.; Huang, G.S.; Liang, C.C.; Lin, J. The influence of renal dialysis and hip fracture sites on the 10-year mortality of elderly hip fracture patients A nationwide population-based observational study. Medicine 2017, 96, e7618. [Google Scholar] [CrossRef]
- Maravic, M.; Ostertag, A.; Torres, P.U.; Cohen-Solal, M. Incidence and risk factors for hip fractures in dialysis patients. Osteoporos. Int. 2014, 25, 159–165. [Google Scholar] [CrossRef]
- Lunde, A.; Tell, G.S.; Pedersen, A.B.; Scheike, T.H.; Apalset, E.M.; Ehrenstein, V.; Sørensen, H.T. The Role of Comorbidity in Mortality After Hip Fracture: A Nationwide Norwegian Study of 38,126 Women with Hip Fracture Matched to a General-Population Comparison Cohort. Am. J. Epidemiol. 2019, 188, 398–407. [Google Scholar] [CrossRef]
- Iseri, K.; Carrero, J.J.; Evans, M.; Felländer-Tsai, L.; E Berg, H.; Runesson, B.; Stenvinkel, P.; Lindholm, B.; Qureshi, A.R. Incidence of Fractures Before and After Dialysis Initiation. J. Bone Miner. Res. 2020, 35, 2372–2380. [Google Scholar] [CrossRef] [PubMed]
- MaggiMa, M.K.; Yap, D.Y.; Yip, T.P.; Lui, S.L.; Lo, W.K. Charlson co-morbidity index and albumin significantly associated with fracture risk in peritoneal dialysis patients. Nephrology 2013, 18, 365–368. [Google Scholar] [CrossRef]
- Cardone, K.E.; Parker, W.M. Medication management in dialysis: Barriers and strategies. Semin. Dial. 2020, 33, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Vangala, C.; Niu, J.; Lenihan, C.R.; Mitch, W.E.; Navaneethan, S.D.; Winkelmayer, W.C. Proton Pump Inhibitors, Histamine-2 Receptor Antagonists, and Hip Fracture Risk among Patients on Hemodialysis. Clin. J. Am. Soc. Nephrol. 2018, 13, 1534–1541. [Google Scholar] [CrossRef]
- Thong, B.K.S.; Ima-Nirwana, S.; Chin, K.-Y. Proton Pump Inhibitors and Fracture Risk: A Review of Current Evidence and Mechanisms Involved. Int. J. Environ. Res. Public Health 2019, 16, 1571. [Google Scholar] [CrossRef]
- January, C.T.; Wann, L.S.; Alpert, J.S.; Calkins, H.; Cigarroa, J.E.; Cleveland, J.C.; Conti, J.; Ellinor, P.; Ezekowitz, M.; Field, M.; et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2014, 64, e1–e76. [Google Scholar] [CrossRef]
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castella, M.; Diener, H.-C.; Heidbuchel, H.; Hendriks, J.; et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS: The Task Force for the management of atrial fibrillation of the European Society of Cardiology (ESC). Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2016, 37, 2893–2962. [Google Scholar]
- Van Der Meersch, H.; De Bacquer, D.; De Vriese, A.S. Vitamin K antagonists for stroke prevention in hemodialysis patients with atrial fibrillation: A systematic review and meta-analysis. Am. Heart J. 2017, 184, 37–46. [Google Scholar] [CrossRef]
- Voskamp, P.W.M.; Rookmaaker, M.B.; Verhaar, M.C.; Dekker, F.; Ocak, G. Vitamin K antagonist use and mortality in dialysis patients. Nephrol. Dial. Transplant. 2018, 33, 170–176. [Google Scholar] [CrossRef]
- De Vriese, A.S.; Caluwé, R.; Pyfferoen, L.; De Bacquer, D.; De Boeck, K.; Delanote, J.; De Surgeloose, D.; Van Hoenacker, P.; Van Vlem, B.; Verbeke, F. Multicenter Randomized Controlled Trial of Vitamin K Antagonist Replacement by Rivaroxaban with or without Vitamin K2 in Hemodialysis Patients with Atrial Fibrillation: The Valkyrie Study. J. Am. Soc. Nephrol. 2020, 31, 186–196. [Google Scholar] [CrossRef]
- Kohlmeier, M.; Saupe, J.; Shearer, M.J.; Schaefer, K.; Asmus, G. Bone health of adult hemodialysis patients is related to vitamin K status. Kidney Int. 1997, 51, 1218–1221. [Google Scholar] [CrossRef] [PubMed]
- Fusaro, M.; Noale, M.; Viola, V.; Galli, F.; Tripepi, G.; Vajente, N.; Plebani, M.; Zaninotto, M.; Guglielmi, G.; Miotto, D.; et al. Vitamin K, vertebral fractures, vascular calcifications, and mortality: Vitamin K Italian (VIKI) dialysis study. J. Bone Miner. Res. 2012, 27, 2271–2278. [Google Scholar] [CrossRef]
- Fusaro, M.; Tripepi, G.; Noale, M.; Plebani, M.; Zaninotto, M.; Piccoli, A.; Naso, A.; Miozzo, D.; Giannini, S.; Avolio, M.; et al. Prevalence of vertebral fractures, vascular calcifications, and mortality in warfarin treated hemodialysis patients. Curr. Vasc. Pharmacol. 2013, 13, 248–258. [Google Scholar] [CrossRef]
- Miller, P.D.; A Jamal, S.; Evenepoel, P.; Eastell, R.; Boonen, S. Renal Safety in Patients Treated with Bisphosphonates for Osteoporosis: A Review. J. Bone Miner. Res. J. Bone Miner. Res. 2013, 28, 2049–2059. [Google Scholar] [CrossRef] [PubMed]
- Evenepoel, P.; Cunningham, J.; Ferrari, S.; Haarhaus, M.; Javaid, M.K.; Lafage-Proust, M.-H.; Prieto-Alhambra, D.; Torres, P.U.; Cannata-Andia, J.; Vervloet, M.; et al. European Consensus Statement on the diagnosis and management of osteoporosis in chronic kidney disease stages G4–G5D. Nephrol. Dial. Transplant. 2021, 36, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Drueke, T.B. Cell biology of parathyroid gland hyperplasia in chronic renal failure. J. Am. Soc. Nephrol. 2000, 11, 1141–1152. [Google Scholar] [CrossRef] [PubMed]
- Danese, M.D.; Kim, J.; Doan, Q.V.; Dylan, M.; Griffiths, R.; Chertow, G.M. PTH and the risks for hip, vertebral, and pelvic fractures among patients on dialysis. Am. J. Kidney Dis. 2006, 47, 149–156. [Google Scholar] [CrossRef]
- Cunningham, J.; Danese, M.; Olson, K.; Klassen, P.; Chertow, G.M. Effects of the calcimimetic cinacalcet HCl on cardiovascular disease, fracture, and health-related quality of life in secondary hyperparathyroidism. Kidney Int. 2005, 68, 1793–1800. [Google Scholar] [CrossRef]
- Moe, S.M.; Abdalla, S.; Chertow, G.M.; Parfrey, P.S.; Block, G.A.; Correa-Rotter, R.; Floege, J.; Herzog, C.A.; London, G.M.; Mahaffey, K.W.; et al. Effects of Cinacalcet on Fracture Events in Patients Receiving Hemodialysis: The EVOLVE Trial. J. Am. Soc. Nephrol. 2015, 26, 1466–1475. [Google Scholar] [CrossRef]
- Block, G.A.; Martin, K.J.; de Francisco, A.L.; Turner, S.A.; Avram, M.M.; Suranyi, M.G.; Hercz, G.; Cunningham, J.; Abu-Alfa, A.K.; Messa, P.; et al. Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N. Engl. J. Med. 2004, 350, 1516–1525. [Google Scholar] [CrossRef]
- Alfieri, C.; Regalia, A.; Zanoni, F.; Vettoretti, S.; Cozzolino, M.; Messa, P. The importance of adherence in the treatment of secondary hyperparathyroidism. Blood Purif. 2019, 47, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Roggeri, A.; Roggeri, D.P.; Zocchetti, C.; Bersani, M.; Conte, F.; ReNe (Renal Lombardy Network); Additional contributors from ReNe Network. Healthcare costs of the progression of chronic kidney disease and different dialysis techniques estimated through administrative database analysis. J. Nephrol. 2017, 30, 263–269. [Google Scholar] [CrossRef] [PubMed]

| ICD-9-CM Diagnosis | ICD-9-CM Procedures | ||
|---|---|---|---|
| Codes | Description | Codes | Description |
| 820 | Fracture of neck of femur | 78.55 | Internal fixation of bone without fracture reduction femur |
| 821 | Fracture of other parts of femur | 79.25 | Open reduction of fracture without internal fixation femur |
| 808 | Fracture of pelvis | 79.45 | Closed reduction of separated epiphysis femur |
| 805 | Fracture of vertebral column | 79.55 | Open reduction of separated epiphysis femur |
| 733.14 | Pathological fracture of neck of femur | 81.51 | Total hip replacement |
| 733.15 | Pathological fracture of other parts of femur | 81.52 | Partial hip replacement |
| 733.13 | Pathological fracture of vertebra | 81.53/81.40 | Revision of hip replacement/Repair of hip |
| Drugs Groups (ATC Classification Codes) | n (%) Patients Using Drugs | % Fractures in Users | % Fractures in Non-Users | p Value (CMH) |
|---|---|---|---|---|
| Active on bone structure and mineralization (M05B) | 317 (3.9) | 7.9 | 2.9 | <0.001 |
| Aspirin | 4793 (59.1) | 3.3 | 2.9 | 0.3517 |
| Cardiac glycosides (C01AA05) | 305 (3.8) | 3.0 | 3.1 | 1.0000 |
| Lipid-lowering drugs (C10A) | 4447 (54.8) | 2.7 | 3.5 | 0.0375 |
| Steroid drugs (H02A, H02B) | 2229 (27.5) | 3.4 | 3.0 | 0.3502 |
| Renin-angiotensin inhibitors (C09A, C09B, C09CA, C09D, C09X) | 5479 (67.6) | 2.7 | 3.9 | 0.0039 |
| Vit D drugs (A11CB, A11CC) | 6305 (77.8) | 3.1 | 2.9 | 0.7183 |
| Cinacalcet (H05BX01) | 1728 (21.3) | 2.5 | 3.2 | 0.1594 |
| Proton pump inhibitors (A02BC) | 7168 (88.4) | 3.3 | 1.8 | 0.0199 |
| Vit K antagonists (B01AA) | 1681 (20.7) | 3.8 | 2.9 | 0.0697 |
| Comorbidities | n (%) Patients Affected | % Fractures in Affected | % Fractures in Non-Affected | p Value (CMH) |
|---|---|---|---|---|
| Secondary hyperparathyroidism | 2497 (30.8) | 2.8 | 3.2 | 0.4213 |
| Diabetes | 2219 (27.4) | 3.3 | 3.0 | 0.5833 |
| Peripheral vascular disease | 893 (11.0) | 3.6 | 3.0 | 0.4293 |
| Myocardial infarction | 2024 (25.0) | 2.9 | 3.2 | 0.5387 |
| Congestive heart failure | 1724 (21.3) | 3.5 | 3.0 | 0.3362 |
| Cerebral vascular disease | 1195 (14.7) | 5.4 | 2.7 | <0.001 |
| Dementia | 100 (1.2) | 7.0 | 3.0 | 0.03508 |
| MCC*I (cat ≥1 vs. 0) | 4521 (55.8) | 3.8 | 2.2 | <0.001 |
| SHPT n(%) | NO_SHPT n(%) | p Value | |
|---|---|---|---|
| Patients | 2497 (30.8) | 5612 (69.2) | --------- |
| Gender (females) | 1028 (41.2) | 1952 (34.8) | <0.001 |
| Age (mean ± SD) | 62.4 ± 15.0 | 69.3 ± 13.8 | <0.001 |
| Renal replacement therapy HD | 2291 (91.8) | 5173 (92.2) | 0.5404 |
| Kidney transplantation | 228 (9.1) | 310 (5.5) | <0.001 |
| Fractures | 71 (2.8) | 180 (3.2) | 0.4213 |
| Fractures | Number of Patients (n) | Total Costs for All Patients (€) | Mean Per-Patient Costs (€) | |
|---|---|---|---|---|
| All drugs | No | 7858 | 42,966,512.4 | 5467.9 |
| Yes | 251 | 1,039,559.5 | 4141.7 | |
| Territorial Drugs | No | 7858 | 32,261,086.1 | 4105.5 |
| Yes | 251 | 773,330.6 | 3081.0 | |
| Drugs distributed directly by RHS | No | 7858 | 10,705,426.3 | 1362.4 |
| Yes | 251 | 266,228.9 | 1060.7 | |
| Diagnostic and therapeutic procedures | No | 7858 | 400,579,033.0 | 50,977.2 |
| Yes | 251 | 12,961,263.5 | 51,638.5 | |
| Hospitalizations (all) | No | 7858 | 98,239,011.5 | 12,501.8 |
| Yes | 251 | 5,283,639.7 | 21,050.4 | |
| Total costs | No | 7858 | 541,784,556.9 | 68,946.9 |
| Yes | 251 | 19,284,462.6 | 76,830.5 |
| Variable | OR | 95% Wald CI | p Value |
|---|---|---|---|
| Age > 65 | 2.608 | 1.846–3.685 | p < 0.01 |
| Gender (F, M) | 1.403 | 1.086–1.811 | p < 0.01 |
| Kidney Transplant (Yes, No) | 0.287 | 0.090–0.916 | p < 0.05 |
| PPI (Yes, No) | 1.904 | 1.154–3.140 | p < 0.05 |
| CVD (/Yes, No) | 1.766 | 1.316–2.370 | p < 0.05 |
| Myocardial Infarction (Yes, No) | 0.771 | 0.568–1.045 | p < 0.1 |
| Observations: 8109; log–likelihood: −1074.519; Akaike inf. crit.: 2163.037 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conte, F.; Roggeri, D.P.; Cozzolino, M.G.; Rossi, C.; Zocchetti, C.; Roggeri, A. Fractures in CKD Patients—Risk Analysis in RRT Lombardy Patients. Kidney Dial. 2023, 3, 95-110. https://doi.org/10.3390/kidneydial3010009
Conte F, Roggeri DP, Cozzolino MG, Rossi C, Zocchetti C, Roggeri A. Fractures in CKD Patients—Risk Analysis in RRT Lombardy Patients. Kidney and Dialysis. 2023; 3(1):95-110. https://doi.org/10.3390/kidneydial3010009
Chicago/Turabian StyleConte, Ferruccio, Daniela Paola Roggeri, Mario Gennaro Cozzolino, Carlotta Rossi, Carlo Zocchetti, and Alessandro Roggeri. 2023. "Fractures in CKD Patients—Risk Analysis in RRT Lombardy Patients" Kidney and Dialysis 3, no. 1: 95-110. https://doi.org/10.3390/kidneydial3010009
APA StyleConte, F., Roggeri, D. P., Cozzolino, M. G., Rossi, C., Zocchetti, C., & Roggeri, A. (2023). Fractures in CKD Patients—Risk Analysis in RRT Lombardy Patients. Kidney and Dialysis, 3(1), 95-110. https://doi.org/10.3390/kidneydial3010009








