Abstract
Protein-energy wasting (PEW) increases the morbidity and mortality in maintenance hemodialysis (MHD) patients. The existing screening tools (e.g., Malnutrition-Inflammation Score (MIS)) are time-consuming and require expertise. Consequently, assessing a more practical and reliable tool such as handgrip strength (HGS) is important, as it strongly correlates with the PEW status in patients undergoing MHD, in whom increased protein and fat breakdown leads to muscle strength and function loss. A systematic search of five databases identified studies assessing HGS’s predictive value for PEW in patients undergoing MHD, using MIS as the reference. The quality of the studies was evaluated with the updated QUADAS tool. A diagnostic meta-analysis was carried out to estimate the pooled sensitivity, specificity, sROC, and sAUC using a two-level mixed-effects model. From 350 records, five studies were obtained which were included for analysis. The pooled sensitivity and specificity of the HGS among male patients were 68% (95%CI: 63–73%) and 66% (95%CI: 53–77%), respectively. Among female patients, the pooled sensitivity and specificity were 73% (95%CI: 62–82%) and 65% (95%CI: 48–79%), respectively. The positive likelihood ratio and negative likelihood ratio for male patients were 2.0 (95%CI: 1.4 to 2.9) and 0.48 (95%CI: 0.38 to 0.60), respectively. Meanwhile, among female patients, the positive likelihood ratio and negative likelihood ratio were 2.1 (95%CI: 1.4–3.1) and 0.41 (95%CI: 0.29–0.59), respectively. The sAUC for males and females was estimated to be 0.69 (95%CI: 0.65 to 0.73) and 0.75 (95%CI: 0.71–0.79). In summary, the sensitivity and specificity of the HGS were modest for all sexes, with females being more sensitive. HGS may be useful for triaging during daily screening and guiding further examination, but it requires supporting measurements to be employed as a diagnostic tool. PROSPERO: CRD42024595677 as of 1 October 2024.
1. Introduction
Protein-energy waste (PEW) has emerged as a critical issue among patients with chronic kidney disease (CKD), particularly those undergoing hemodialysis. It is associated with a diminished quality of life and poor prognosis [1]. A meta-analysis estimated that the prevalence of PEW among patients who are on maintenance dialysis is 42% [2]. The International Society of Renal Nutrition and Metabolism (ISRNM) defines PEW as a condition marked by nutritional and metabolic deficiencies resulting in the depletion of systemic body protein and energy reserves, which ultimately leads to a reduction in muscle and fat mass [3]. As CKD progresses, patients experience nutritional deficiencies and muscle breakdown, resulting in PEW [4,5,6]. PEW is becoming a growing concern, as the number of individuals undergoing dialysis is projected to exceed 5 million by 2030 [7]. Research indicates that only 57% of patients who undergo maintenance hemodialysis (MHD) survive three years post-initial treatment, which is influenced by several factors, including malnutrition conditions such as PEW [4,8]. In such cases, nutritional support is essential and screening for malnutrition during hemodialysis is crucial.
Several established screening tools are currently used in clinical settings, including the Malnutrition Inflammation Score (MIS), which assesses the nutritional status of patients with CKD based on hospitalization rates and mortality, as well as indicators of anemia, inflammation, and nutritional status in patients who are undergoing dialysis [9]. The MIS serves as a standard tool for detecting PEW; however, its practicality for routine screening is limited due to the extensive assessment and expert review that it necessitates [10]. Researchers and clinicians have investigated alternative methods to create a more effective screening tool for PEW, including the application of handgrip strength (HGS) [11,12]. HGS is believed to have a strong correlation with the PEW status of patients, as increased protein and fat breakdown in this condition leads to the loss of muscle strength and function. The latest Kidney Disease Outcomes Quality Initiative (KDOQI) recommendations incorporate HGS as a screening method for PEW [13]. Previous research has consistently demonstrated a significant correlation between HGS and the MIS [14,15,16]. However, the application, accuracy, and effectiveness of using HGS remain unclear. To demonstrate the benefits of HGS in screening for PEW and its potential to replace the MIS in clinical settings, a meta-analysis is required to evaluate its potential across diverse patient populations and settings. This systematic review and meta-analysis aimed to estimate the predictive value of HGS in identifying PEW in patients undergoing MHD.
2. Materials and Methods
2.1. Study Design and Search Strategy
The research question of this study was “What are the sensitivity, specificity, and accuracy of HGS in predicting PEW among individuals undergoing MHD?” This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [17]. The PRISMA item and abstract checklists are provided in Tables S1 and S2. The review protocol was registered with PROSPERO (CRD42024595677) on 1 October 2024. The search was conducted across multiple databases, including Scopus, Web of Science, Europe PMC, Google Scholar, and Scilit, with the aim of identifying articles published and indexed on or before 6 October 2024. The combination of keywords included Boolean operators and MeSH terms when appropriate. The primary terms “hand grip” and “protein-energy wasting” were subsequently expanded into a comprehensive search strategy, as detailed in Table 1.

Table 1.
Combinations of keywords used across different databases to identify records related to HGS and malnutrition.
2.2. Eligibility Criteria
The eligibility criteria were primarily based on the population, index test, reference test, and target condition used in the analyzed studies. The study population consisted of patients undergoing MHD who were diagnosed with PEW, as determined by an MIS of 6 or higher by a nutritionist. The index test was HGS measurement using a digital dynamometer, an analog dynamometer, or a Jamar dynamometer. For the reference test, it was checked if the study used a standard assessment tool for PEW. The target condition was PEW, and a the reported findings were required to encompass the predictive value of HGS, as recommended by the Standards for Reporting of Diagnostic Accuracy Studies (STARD). The included studies were diagnostic, observational, and randomized controlled trials (RCTs); conference abstracts, review articles, case reports, and case series were excluded. Non-peer-reviewed articles (e.g., preprints and theses) were excluded from analysis. The screening process imposed no restrictions on the publication year or language. Articles reported in languages other than English were translated using multiple translators such as Google Translate and DeepL.
2.3. Screening and Selection
After retrieval from each database, the identified literature was uploaded to Rayyan.ai (https://www.rayyan.ai accessed on 1 October 2024) for preliminary screening. Duplicates were identified automatically with a minimum similarity threshold of 90% and their removal was performed manually. Subsequently, two reviewers (M. H. G. and M. I.) independently screened the titles and abstracts of the identified studies and evaluated the correspondence between their research topic and the existing literature. The eligibility criteria were applied to the full texts of the retrieved studies. Disputes arising from the final findings were resolved through consensus or, when necessary, consultation with a third reviewer (S. T. A. G. and M.H.).
2.4. Quality Appraisal
This study employed the Quality Assessment of Diagnostic Accuracy Study (QUADAS)-2 to evaluate the quality of observational diagnostic studies [18,19]. This assessment tool consists of four domains: “patient selection”, “index test”, “reference standard”, and “flow and timing”. The results of the QUADAS-2 were represented in a “traffic light” diagram, categorizing the quality level of each item as “low risk”, “some concerns”, or “high risk”.
2.5. Data Extraction
Data extraction was conducted on a pre-designed standard table by two independent reviewers (S. T. A. G. and M. I.). Disagreements between the two reviewers were addressed by re-examining the individual study, with consultation from a third reviewer (M. H. G. and M.H.) occurring when consensus was unattainable. The study characteristics included the first author’s name, publication year, research location, and study design. The characteristics of the research subjects included the sample size (both male and female), age, and BMI of patients, as well as the dynamometer type, timing of HGS measurements, and established cut-off values. The cut-off values of each study were determined based on maximizing sensitivity and specificity and closest-to-(0,1) criterion. The extracted outcomes were false positive (FP), false negative (FN), true positive (TP) and true negative (TN). In the absence of the provided information, data on sensitivity, specificity, and PEW prevalence were obtained.
2.6. Quantitative Synthesis
A diagnostic meta-analysis was performed using the ‘midas’ package in STATA version 17. The pooled analysis assessed the specificity and sensitivity of the chosen studies using a logistic regression model employing two-level mixed effects. A summarized receiver operating characteristic (sROC) curve was constructed to ascertain the summarized area under the curve (sAUC). The analysis was conducted independently for male and female patients. Publication bias was analyzed using a Deek’s funnel plot that was contingent upon the inclusion of more than 10 studies from the systematic search.
3. Results
3.1. Searching Results
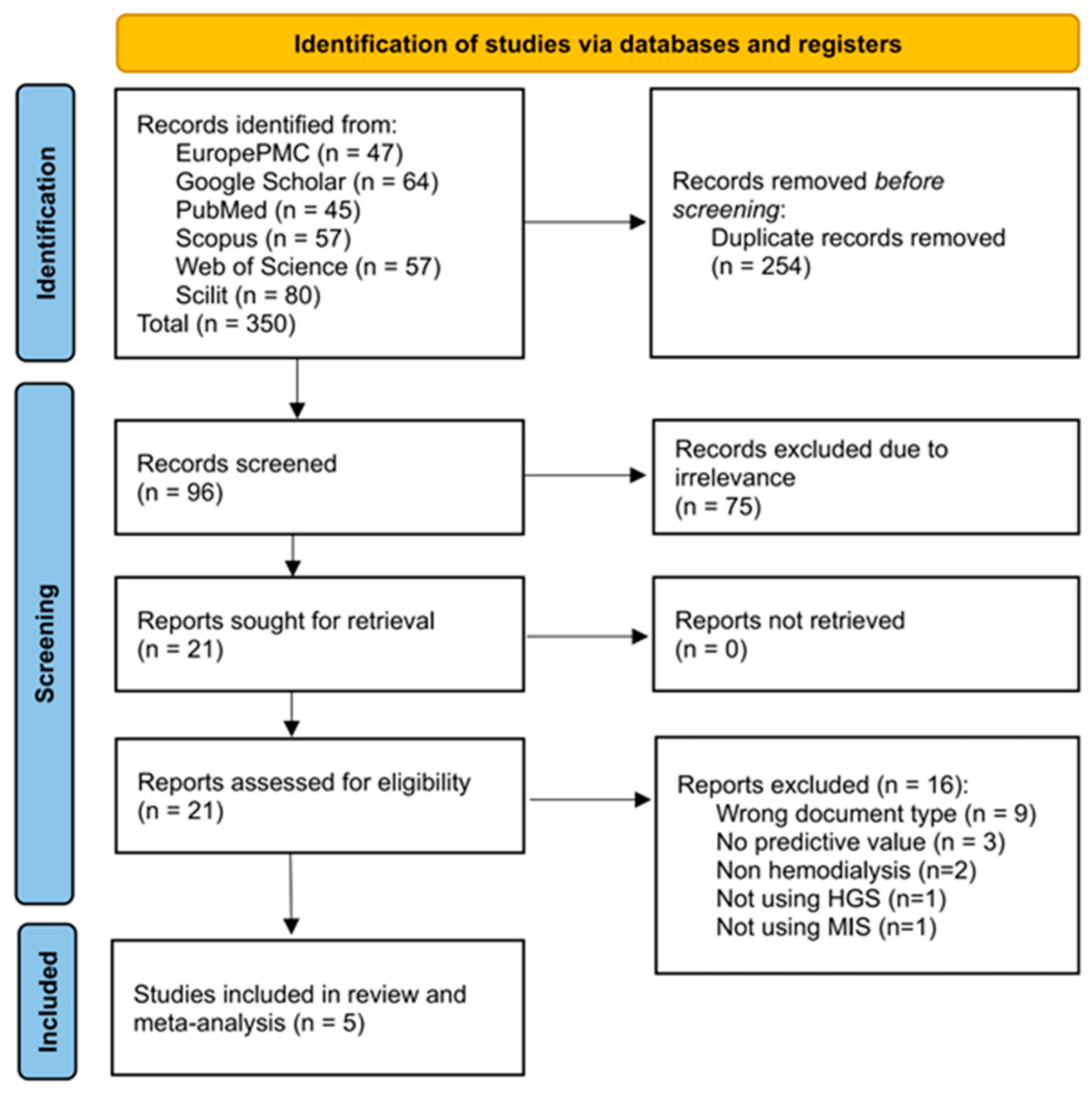
The initial identification of studies across the six databases resulted in 350 records, of which 254 were identified as duplicates using the screening tool. A total of 96 records were screened based on their title and abstract for relevance to the study, resulting in the exclusion of 75 records at this stage. Subsequently, full access was requested for 21 records, and all records were made available. The eligibility criteria were implemented, resulting in the exclusion of studies based on their document type (n = 9) and the availability of their predictive value data (n = 3). Two studies involved patients with CKD who were not undergoing MHD [14,20]. One study developed a predictive model using the combination of patients’ HGS and their hemoglobin level; thus, it could not be applied in the current review [21]. One study was excluded because it focused on the predictive value of mortality despite utilizing HGS as a predictor [22]. A total of five studies were included in the systematic review and meta-analysis [12,23,24,25,26]. On the other hand, all papers included in the review were in English. The screening and selection processes for this systematic review are presented in Figure 1.
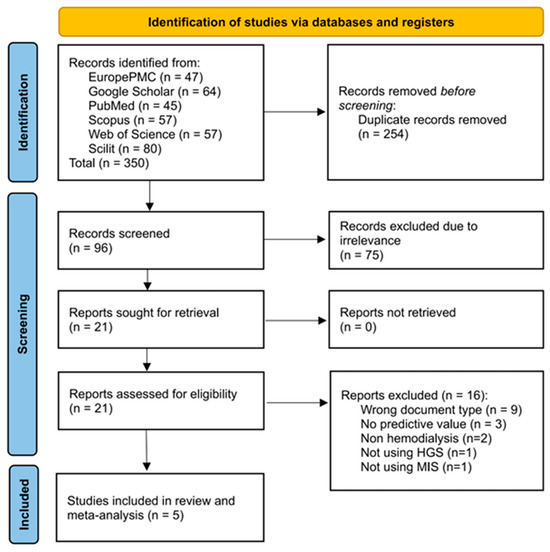
Figure 1.
PRISMA flow diagram of selection process of eligible studies for the qualitative and quantitative review of HGS performance in predicting PEW among patients undergoing hemodialysis.
3.2. Study Characteristics
The characteristics of the included studies are summarized in Table 2. All studies used a cross-sectional design. The selected reports were published from 2011 to 2020, originating in Turkey (n = 1), Brazil (n = 3), and the Philippines (n = 1). The mean age and BMI ranges of the recruited patients were generally consistent across studies (24.9), with the exception of one study, which reported the lowest mean age and BMI (22.76 kg/m2) [25]. Most studies reported that the cut-off determination was based on maximal sensitivity and specificity without further explanation of how this was performed [23,24,25,26]. Only one study explicitly reported the use of a closest-to-(0, 1) criterion [12]. The sensitivity of HGS in predicting PEW among male patients was found to be ≥70% in four of the studies [23,24,25], whereas one study reported a specificity higher than 80% [12]. The sensitivity and specificity among female patients ranged from 63.5% to 87% and 43% to 88.2%, respectively [12,23,24,25,26].

Table 2.
Characteristics and HGS predictive performance for PEW among patients undergoing maintenance from individual studies.
3.3. Quality of the Included Studies
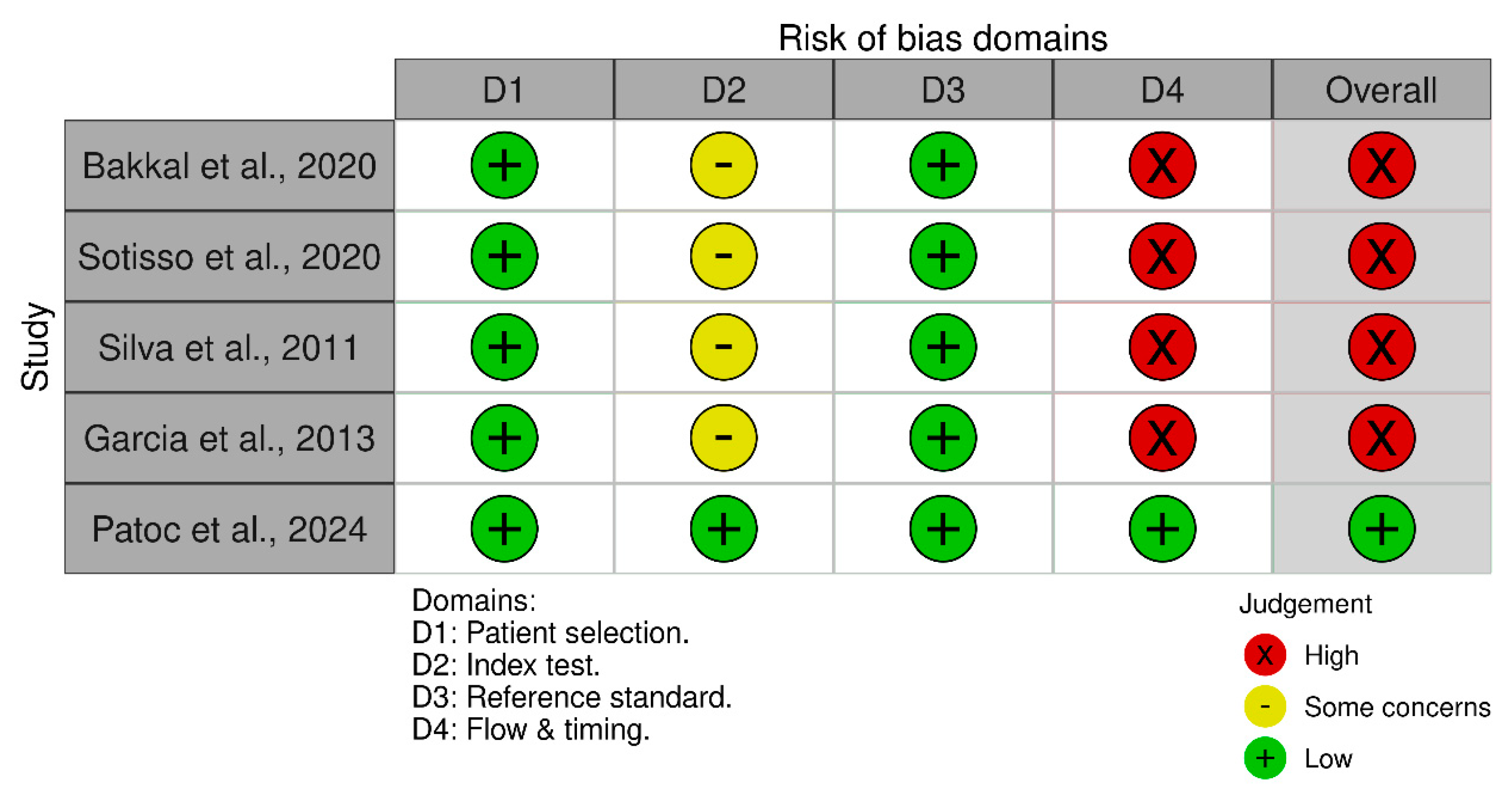
All of the studies demonstrated high quality in their patient selection, as they provided comprehensive definitions of the clinical condition of their research subjects, along with explicit criteria for their inclusion or exclusion [12,23,24,25,26]. All included studies were cross-sectional, making them vulnerable to biases related to baseline characteristics, including age [12,23,24,25,26]. The influence of sex bias was not relevant as all studies conducted a stratified analysis according to this covariate. Statistical tests conducted in the individual studies indicated that the bias associated with BMI was negligible [12,23,24,25,26]. Regarding the comparator for HGS, all reviewed studies used the MIS as the standard tool to identify PEW among patients who were undergoing hemodialysis [12,23,24,25,26]. The timing effect in measuring HGS can impact its predictive performance. Studies that measured HGS at only one time point (either pre- or post-hemodialysis) were considered to have a higher risk of bias, as this approach may not fully capture the variability in muscle strength across different stages of dialysis [23,24,25,26]. Only one study gathered HGS data before and after hemodialysis [12]. A summary of the QUADAS-2 assessment results is presented in Figure 2.
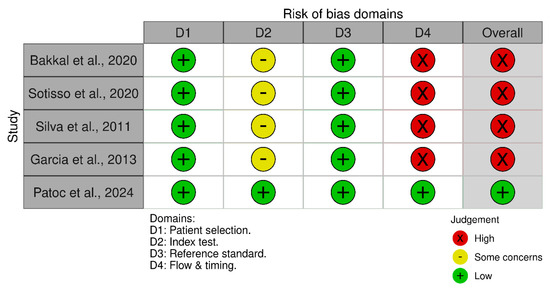
Figure 2.
Traffic-light plot visualizing the results from the QUADAS-2 assessment on the included studies [12,23,24,25,26].
3.4. Predictive Values of HGS for Males
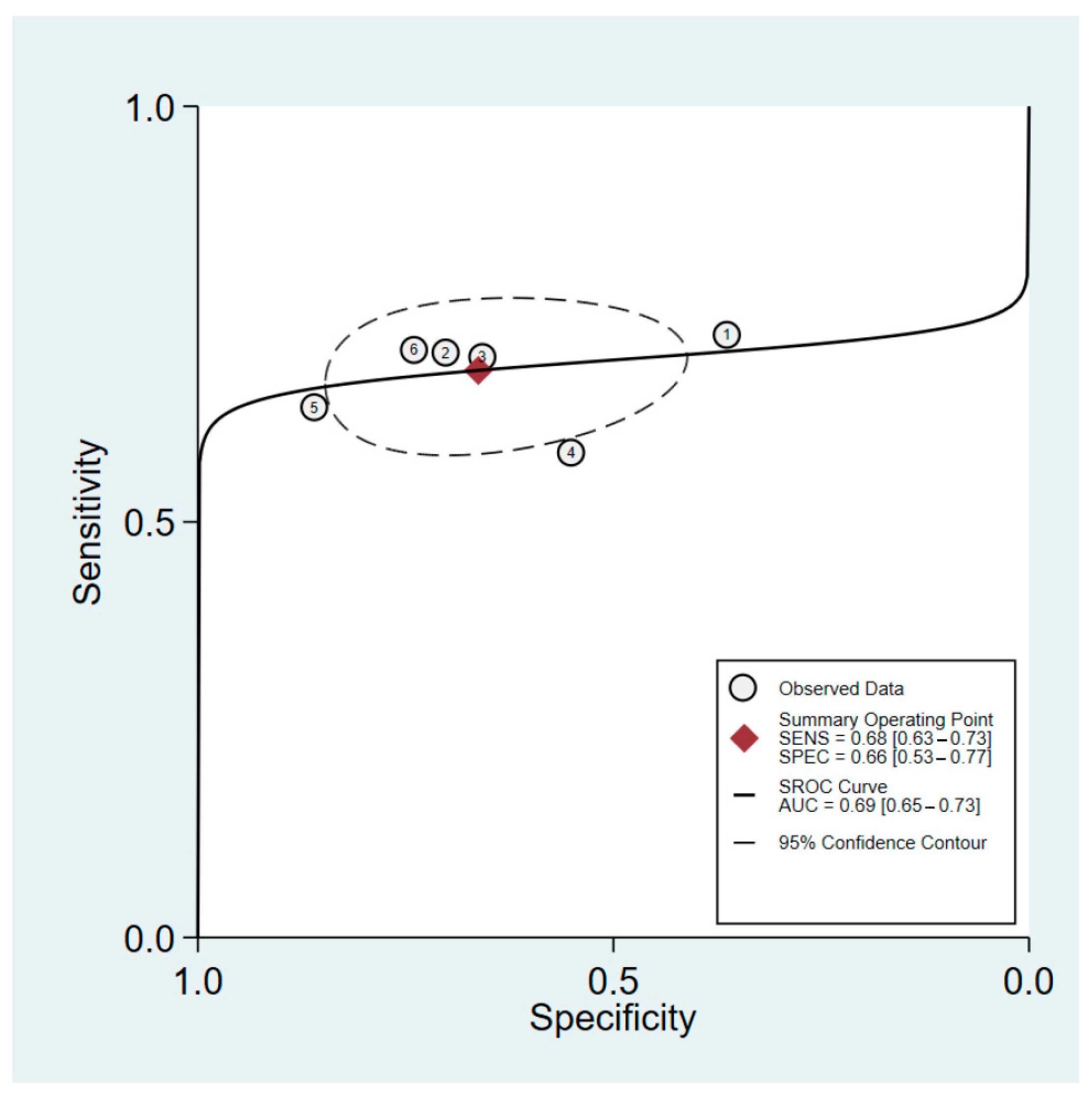
The sensitivity and specificity of HGS in predicting PEW among male patients were 68% (95%CI: 63–73%) and 66% (95%CI: 53–77%), respectively. The heterogeneity in sensitivity was negligible (I2 = 0%), whereas the specificity was significantly higher (I2 = 81.3%). The positive likelihood ratio for male patients was 2.0 (95%CI: 1.4 to 2.9), while the negative likelihood ratio was 0.48 (95%CI: 0.38 to 0.60). The pooled estimate for this group yielded a diagnostic odds ratio of 4 (95%CI: 2–7). An sROC curve for the performance of HGS in predicting PEW in male patients is presented in Figure 3. The AUC was found to be 0.69 (95%CI: 0.65 to 0.73).
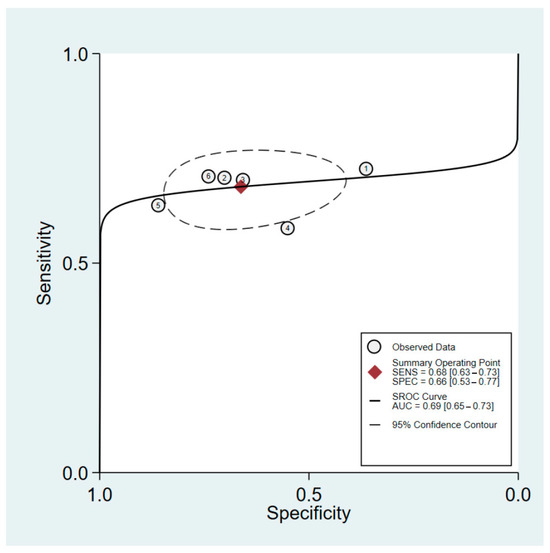
Figure 3.
sROC of HGS predictive performance for PEW among male patients with maintenance hemodialysis. The six data points represent the five studies included in the analysis, with one study contributing data for both pre- and post-hemodialysis measurements. This accounts for the additional data point.
3.5. Predictive Values of HGS for Females
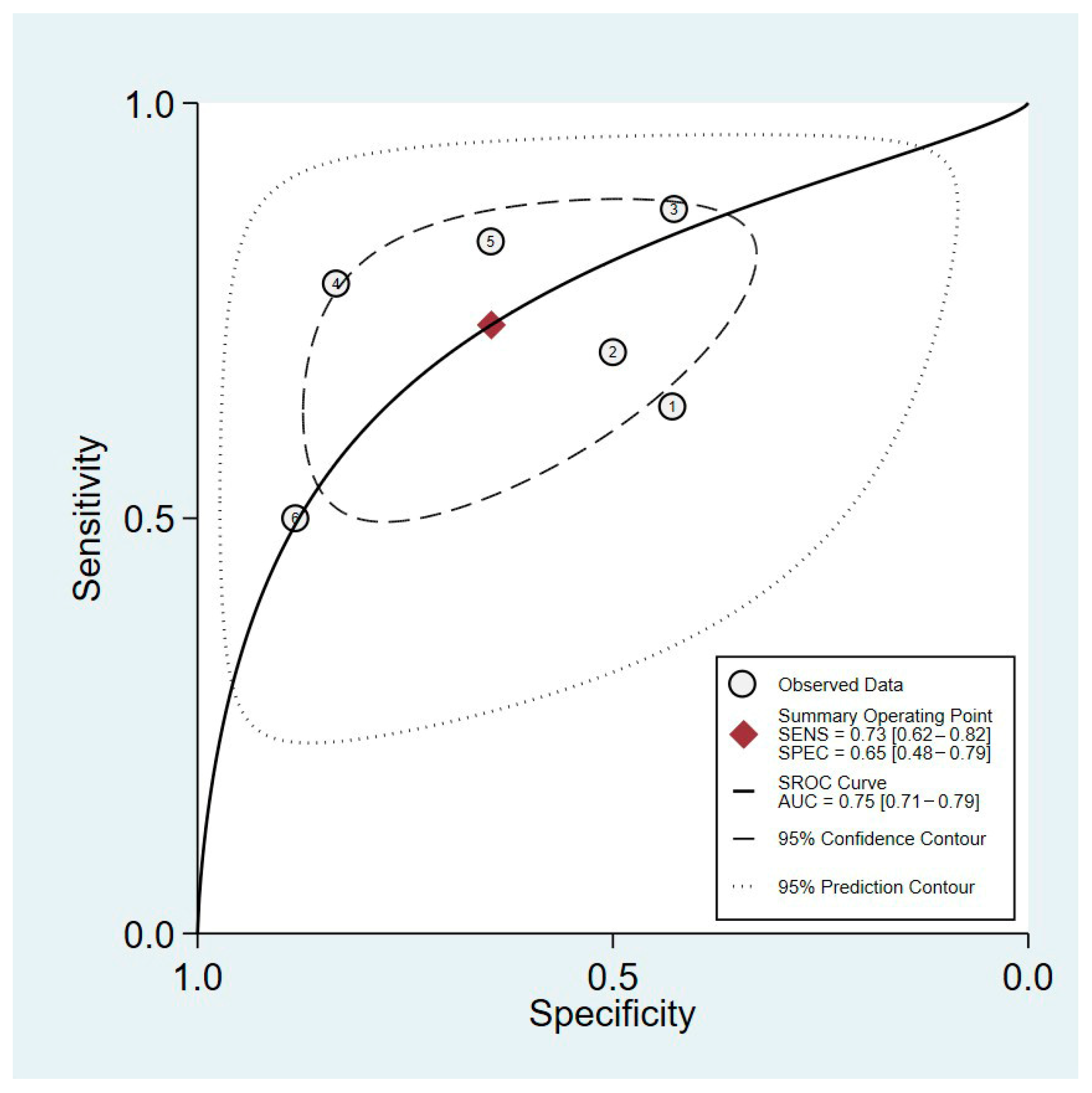
In the female group, the sensitivity and specificity of HGS as a predictive measure for PEW were 73% (95%CI, 62–82%) and 65% (95%CI, 48–0.79%), respectively (Figure 4). Heterogeneity was evident in both the observed sensitivity and specificity, which had I2 values of 74.87% and 78.67%, respectively. The positive likelihood ratio was determined to be 2.1 (95%CI: 1.4 to 3.1), while the negative likelihood ratio was found to be 0.41 (95%CI: 0.29–0.59). The diagnostic odds ratio was 5 (95%CI, 3–10). The pooled estimate indicated that the sAUC of this predictor was 0.75 (95%CI: 0.71 to 0.79) (Figure 4).
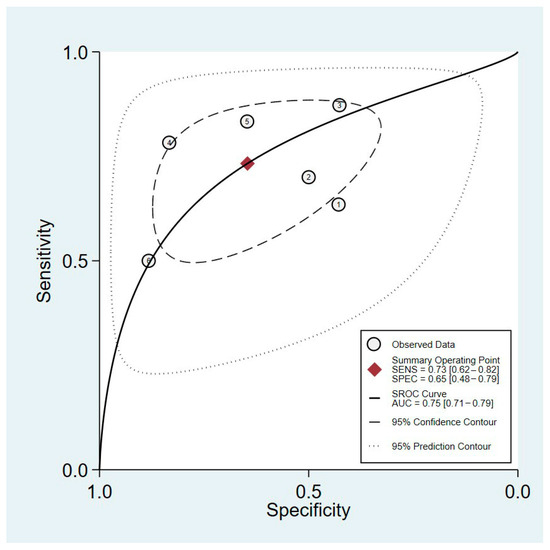
Figure 4.
sROC of HGS predictive performance for PEW among female patients undergoing maintenance hemodialysis. The six data points represent the five studies included in the analysis, with one study contributing data for both pre- and post-hemodialysis measurements. This accounts for the additional data point.
3.6. Publication Bias
In terms of publication bias, a Deek’s funnel plot analysis was not conducted because the minimum number of studies required was not met. Performing a funnel plot analysis on pooled estimates with fewer than 10 studies may yield misleading results. This aligns with the recommendations from prior studies [27,28]. A sensitivity analysis via stratification was not conducted because of the comparable values of covariates such as age and BMI across individual studies.
4. Discussion
The present meta-analysis indicates that HGS demonstrates moderate accuracy in distinguishing between patients with and without PEW among those undergoing MHD. The sensitivity and specificity in males were 68% and 66%, respectively. This means that the HGS accurately identified approximately two-thirds of the patients with PEW and demonstrated a comparable rate of correctly identifying those without the condition. On the other hand, in female patients, the HGS exhibited a slightly higher sensitivity of 73% but a similar specificity of 65% compared to the male patients. The sAUCs for males and females were 0.69 (95%CI: 0.65 to 0.73) and 0.75 (95%CI: 0.71 to 0.79), respectively.
Despite its potential, the heterogeneity in the specificity of this method indicates that the results may differ markedly across various studies. A previous study compared the ability of the Global Leadership Initiative on Malnutrition (GLIM) criteria with that of other screening tools, including the MIS, objective score of nutrition on dialysis, and Geriatric Nutritional Risk Index (GNRI), to assess the malnutrition status among hemodialysis patients [9]. The findings indicated that the highest AUC was 0.70 (95%CI: 0.65 to 0.75), derived from GNRI, while the MIS exhibited an AUC of 0.65 (95%CI: 0.59 to 0.70) [9]. Another recent study also showed that the bioelectrical impedance analysis (BIA)-derived phase angle (PhA) has shown potential for detecting PEW in patients undergoing hemodialysis. A multi-center study in Malaysia found the PhA to be an independent predictor of PEW (adjusted OR = 0.308, p = 0.001) with excellent discriminative performance (adjusted AUC for males = 0.809, females = 0.719). Gender-specific cut-offs were also identified, with males having a higher PhA threshold (4.26°) compared to females (3.30°). However, BIA’s practicality may be limited by factors such as hydration status, which can affect its accuracy in patients with HD [29]. Given these limitations, HGS is a more practical and reliable alternative for assessing PEW, due to its simplicity, low cost, and ease of use in clinical settings. Overall, although the diagnostic accuracy of HGS is moderate, it is deemed acceptable for PEW screening among patients undergoing MHD, especially in resource-limited settings. The HGS of a patient can be measured quickly and easily using a handheld dynamometer, making it accessible for the daily monitoring of nutritional status in patients with chronic HD.
One of the primary mechanisms linking PEW to muscle atrophy in uremia is increased protein breakdown mediated by the ubiquitin-proteasome system (UPS) and activation of the musculoskeletal myostatin. The UPS degrades muscle proteins, whereas myostatin, a member of the transforming growth factor-β family, inhibits cellular proliferation and muscle synthesis [30]. In a previous study, HGS measurements were found to be reliable indicators of muscle mass in older Asian adults [31].
The higher sensitivity of HGS compared to its specificity estimated in this study indicates its appropriateness for rule-out triage rather than rule-in application. This high sensitivity indicates that HGS is effective in accurately detecting the true PEW, allowing for confident exclusion of the condition when the HGS produces a negative PEW result. The high sensitivity of HGS is attributable to the established correlation between muscle weakness and adequate nutritional intake. A prior study reported that 86.7% of patients undergoing hemodialysis demonstrated muscle weakness, particularly diminished quadricep strength [32]. This condition may result from insufficient nutritional intake, heightened catabolism, and metabolic disturbances, which are frequently observed in patients with CKD [33,34]. HGS is well-recognized as a screening tool for assessing the nutritional status of patients, particularly among patients experiencing rapid fluctuations in nutritional deprivation and recovery [35]. In the general elderly population, HGS showed a significant correlation with nutritional status, as estimated by the GLIM [34]. In one RCT, the provision of nutritional support to patients with low handgrip strength was effective in decreasing mortality (adjusted odds ratio: 0.29 [95%CI: 0.10 to 0.82]) [36].
The moderate accuracy of HGS identified in the present study represents a significant limitation for its application. The objective of daily screening is to exclude patients from the need for nutritional support, thereby preventing unnecessary testing with the MIS or other standard tools. Therefore, to improve the accuracy of HGS, clinicians should consider several approaches, such as performing patient stratification by their age, sex, frailty, and underlying comorbidities, which could contribute to PEW beyond CKD. This strategy aims to prevent false negatives and ensure that patients who truly need assistance are not neglected. Additionally, the present study indicates that the HGS is a more accurate marker of PEW in women, suggesting that its reliability is limited to a specific sex. These results are consistent with those of previous studies, indicating that the predictive value of HGS is significantly greater in females compared to males [36,37]. However, in predicting malnutrition among patients with decompensated heart failure, the accuracy was higher in male patients [38].
Due to the varying characteristics of patients undergoing MHD, future studies should consider stratifying patients based on clinical characteristics when conducting HGS research. Several parameters can influence muscle strength and should be taken into account in future studies. Factors such as age, sex, nutritional status, inflammation, comorbidities (e.g., diabetes, cardiovascular disease), physical activity levels, dialysis adequacy, and biochemical markers (e.g., albumin, C-reactive protein, and creatinine) are important to consider [39,40]. Moreover, the dialysis regimen of a patient, including its frequency and duration, may play a significant role in influencing improvements in nutritional status. The dialysis process plays a key role in chronic nutrient loss, especially that of protein and amino acids.
The dialysis regimen, particularly its frequency and duration, may influence the nutritional status of patients undergoing MHD and potentially affect the predictive performance of their HGS. All studies included in this meta-analysis reported a thrice-weekly dialysis frequency, except for Patoc et al. [12], which included 19% of patients receiving dialysis twice weekly. The observed median dialysis durations ranged from 22 to 51.4 months (Table 2). However, across studies, the differences in HGS sensitivity and specificity were generally within a 10% range, indicating no clinically significant variation in predictive performance that could be attributed solely to the frequency or duration of dialysis. For instance, the observed male sensitivity values ranged from 59.6% to 72.5%, and the specificity from 55.3% to 70%, regardless of dialysis exposure. Although these findings suggest relatively uniform performance, it remains important to consider dialysis-related nutrient loss (estimated at 6–12 g of amino acids and 7–8 g of protein per session), which contributes to hypoalbuminemia and PEW risk [41]. Thus, future models aiming to improve the predictive accuracy of HGS should still account for potential nutritional impacts related to dialysis intensity.
It is worth noting that there is currently no single PEW marker, and multiple markers are typically used. Another strategy to improve the accuracy of HGS is to combine it with other simple markers. One study found that the combination of HGS with other markers, such as hemoglobin, achieved a sensitivity and specificity of 72% and 76%, respectively (an improvement of 4% for sensitivity and 10% for specificity compared with our results) [21]. Other studies integrated HGS with the phase angle, yielding an AUC of 0.67 (95%CI: 0.56–0.78) for predicting outcomes of cardiac surgery [42].
There is a need for a multi-marker approach or a single marker with high predictive value, and the findings from analyzing the HGS of patients could complement existing markers. We recommend future investigation into the integration of HGS with other parameters (such as laboratory values or the clinical characteristics of patients) that may enhance the predictive accuracy of a scoring model for predicting PEW among patients undergoing MHD.
Strength and Limitations
This systematic review and meta-analysis is the first to present the pooled predictive value of HGS in distinguishing between PEW and non-PEW status in patients undergoing MHD. These findings indicate that the accuracy of screening tools may differ across populations and settings. Several limitations of this study must be acknowledged when interpreting its findings regarding the analyzed studies. The limited number of available studies, and particularly the restricted range of countries that they are reported from, constitutes an inherent limitation to the generalizability of their findings. We exclusively included peer-reviewed and published journal articles, thereby excluding findings from unpublished investigations or non-formal publications such as institutional reports. It is also essential to consider the significant heterogeneity in the pooled estimates, particularly in the specificity analysis, when interpreting our findings.
5. Conclusions
HGS exhibited moderate sensitivity and specificity in predicting the PEW status in patients undergoing MHD. This assessment demonstrated strong concordant validity, characterized by high sensitivity and adequate specificity, making it suitable for rule-in-triage. To fulfill its role as a daily screening tool for PEW, critical consideration must be given to the use of HGS as a diagnostic tool. This systematic review and meta-analysis highlights the need for additional research on related topics. In addition, future studies should consider integrating HGS with other laboratory and clinical measurements.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/kidneydial5020016/s1, Table S1: PRISMA item checklist; Table S2: PRISMA 2020 for abstracts checklist. Reference [43] is cited in the supplementary materials.
Author Contributions
Conceptualization, M.H.G. and M.I.; methodology, M.H.G., S.T.A.-G., D.D.C.H.R. and J.F.W.; software, M.I.; validation, M.S., A.Y., M.H. and M.I.; formal analysis, M.S., A.Y., M.H. and M.I.; investigation, M.H.G., S.T.A.-G., D.D.C.H.R. and J.F.W.; resources, M.I.; data curation, M.H.G.; writing—original draft preparation, M.H.G., S.T.A.-G. and M.I.; writing—review and editing, M.S., A.Y. and M.H.; visualization, M.I.; supervision, M.S., A.Y., M.H. and M.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
We would appreciate the support from the Universitas Syiah Kuala during the research and preparation of this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CKD | Chronic kidney disease |
| ISRNM | International Society of Renal Nutrition and Metabolism |
| MHD | Maintenance hemodialysis |
| MIS | Malnutrition Inflammation Score |
| HGS | Handgrip strength |
| KDOQI | Kidney Disease Outcomes Quality Initiative |
| PEW | Protein-energy wasting |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| RCT | Randomized controlled trial |
| STARD | Standards for Reporting of Diagnostic Accuracy Studies |
| QUADAS-2 | Quality Assessment of Diagnostic Accuracy Study-2 |
| FP | False positive |
| FN | False negative |
| TP | True positive |
| TN | True negative |
| sROC | Summarized receiver operating characteristic |
| sAUC | Summarized area under the curve |
| GNRI | Geriatric Nutritional Risk Index |
| GLIM | Global Leadership Initiative on Malnutrition |
| UPS | Ubiquitin-proteasome system |
References
- Chen, S.; Ma, X.; Zhou, X.; Wang, Y.; Liang, W.; Zheng, L.; Zang, X.; Mei, X.; Qi, Y.; Jiang, Y. An updated clinical prediction model of protein-energy wasting for hemodialysis patients. Front. Nutr. 2022, 9, 933745. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.J.; Thomas, F.; Nagy, K.; Arogundade, F.; Avesani, C.M.; Chan, M.; Chmielewski, M.; Cordeiro, A.C.; Espinosa-Cuevas, A.; Fiaccadori, E. Global prevalence of protein-energy wasting in kidney disease: A meta-analysis of contemporary observational studies from the international society of renal nutrition and metabolism. J. Ren. Nutr. 2018, 28, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Gracia-Iguacel, C.; González-Parra, E.; Mahillo, I.; Ortiz, A. Criteria for classification of protein–energy wasting in dialysis patients: Impact on prevalence. Br. J. Nutr. 2019, 121, 1271–1278. [Google Scholar] [CrossRef]
- Turkmen, K.; Ozer, H.; Kusztal, M. The relationship of epicardial adipose tissue and cardiovascular disease in chronic kidney disease and hemodialysis patients. J. Clin. Med. 2022, 11, 1308. [Google Scholar] [CrossRef] [PubMed]
- MacLaughlin, H.L.; Friedman, A.N.; Ikizler, T.A. Nutrition in kidney disease: Core curriculum 2022. Am. J. Kidney Dis. 2022, 79, 437–449. [Google Scholar] [CrossRef]
- Iseki, K. Nutrition and quality of life in chronic kidney disease patients: A practical approach for salt restriction. Kidney Res. Clin. Pract. 2022, 41, 657. [Google Scholar] [CrossRef]
- Filipska, A.; Bohdan, B.; Wieczorek, P.P.; Hudz, N. Chronic kidney disease and dialysis therapy: Incidence and prevalence in the world. Pharmacia 2021, 68, 463–470. [Google Scholar] [CrossRef]
- Bailey, A.; Brody, R.; Sackey, J.; Parrott, J.S.; Peters, E.; Byham-Gray, L. Current methods for developing predictive energy equations in maintenance dialysis are imprecise. Ann. Med. 2022, 54, 909–920. [Google Scholar] [CrossRef]
- Cohen-Cesla, T.; Azar, A.; Hamad, R.A.; Shapiro, G.; Stav, K.; Efrati, S.; Beberashvili, I. Usual nutritional scores have acceptable sensitivity and specificity for diagnosing malnutrition compared to GLIM criteria in hemodialysis patients. Nutr. Res. 2021, 92, 129–138. [Google Scholar] [CrossRef] [PubMed]
- García-Almeida, J.M.; García-García, C.; Ballesteros-Pomar, M.D.; Olveira, G.; Lopez-Gomez, J.J.; Bellido, V.; Bretón Lesmes, I.; Burgos, R.; Sanz-Paris, A.; Matia-Martin, P. Expert consensus on morphofunctional assessment in disease-related malnutrition. Grade review and Delphi study. Nutrients 2023, 15, 612. [Google Scholar] [CrossRef]
- Chrástecká, M.; Blanař, V.; Pospíchal, J. Risk of malnutrition assessment in hospitalised adults: A scoping review of existing instruments. J. Clin. Nurs. 2023, 32, 3397–3411. [Google Scholar] [CrossRef] [PubMed]
- Patoc, G.R., Jr.; Fajutag, J.D.; Blanco, J.L.J.; Villanueva, A.R.T.; Briones, M.V.A. Diagnostic value of the handgrip strength in detecting protein-energy wasting among patients on maintenance hemodialysis at National Kidney and Transplant Institute, Philippines. Clin. Nutr. Open Sci. 2024, 55, 48–56. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Cuppari, L. The 2020 updated KDOQI clinical practice guidelines for nutrition in chronic kidney disease. Blood Purif. 2021, 50, 667–671. [Google Scholar] [CrossRef]
- Amparo, F.C.; Cordeiro, A.C.; Carrero, J.J.; Cuppari, L.; Lindholm, B.; Amodeo, C.; Kamimura, M.A. Malnutrition-inflammation score is associated with handgrip strength in nondialysis-dependent chronic kidney disease patients. J. Ren. Nutr. 2013, 23, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Hasheminejad, N.; Namdari, M.; Mahmoodi, M.R.; Bahrampour, A.; Azmandian, J. Association of handgrip strength with malnutrition-inflammation score as an assessment of nutritional status in hemodialysis patients. Iran. J. Kidney Dis. 2016, 10, 30. [Google Scholar]
- Marini, A.C.; Pimentel, G.D. Is body weight or muscle strength correlated with the Malnutrition Inflammation Score (MIS)? A cross-sectional study in hemodialysis patients. Clin. Nutr. ESPEN 2019, 33, 276–278. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, 160. [Google Scholar] [CrossRef]
- Huang, Q.-X.; Huang, X.-W. QUADAS-2 tool for quality assessment in diagnostic meta-analysis. Ann. Palliat. Med. 2022, 11, 1844845. [Google Scholar] [CrossRef]
- Iqhrammullah, M.; Yusnaini, R.; Amirah, S.; Mulya, I.C.; Tsurayya, G.; Naufal, M.A.; Santosa, S.F.; Harapan, H.; Zulkifli, B. Effect of tuberculosis-specific antigen stimulation on the diagnostic accuracy of interferon-γ inducible protein-10 in distinguishing active and latent tuberculosis infection: A meta-analysis. Microbes Infect. 2024, 26, 105396. [Google Scholar] [CrossRef]
- Abdulan, I.M.; Ștefăniu, R.; Maștaleru, A.; Lefter, N.; Alexa, I.D.; Mocanu, V. Cut-off values of anthropometric measurements, handgrip strength, physical activity and geriatric scores for the malnutrition risk among older patients with chronic kidney disease. Med. Surg. J. 2020, 124, 19–26. [Google Scholar]
- Lonardo, M.S.; Cacciapuoti, N.; Chiurazzi, M.; Di Lauro, M.; Guida, B.; Damiano, S.; Cataldi, M. Combined use of handgrip strength and hemoglobin as markers of undernutrition in patients with stage 3–5 chronic kidney disease. Nutr. Metab. Cardvasc. Dis. 2023, 33, 2169–2178. [Google Scholar] [CrossRef] [PubMed]
- Vogt, B.P.; Borges, M.C.C.; de Goés, C.R.; Caramori, J.C.T. Handgrip strength is an independent predictor of all-cause mortality in maintenance dialysis patients. Clin. Nutr. 2016, 35, 1429–1433. [Google Scholar] [CrossRef]
- Bakkal, H.; Dizdar, O.S.; Erdem, S.; Kulakoğlu, S.; Akcakaya, B.; Katırcılar, Y.; Uludag, K. The relationship between hand grip strength and nutritional status determined by malnutrition inflammation score and biochemical parameters in hemodialysis patients. J. Ren. Nutr. 2020, 30, 548–555. [Google Scholar] [CrossRef]
- Sostisso, C.F.; Olikszechen, M.; Sato, M.N.; Oliveira, M.d.A.S.C.; Karam, S. Handgrip strength as an instrument for assessing the risk of malnutrition and inflammation in hemodialysis patients. Braz. J. Nephrol. 2020, 42, 429–436. [Google Scholar] [CrossRef]
- Silva, L.F.; Matos, C.M.; Lopes, G.B.; Martins, M.T.S.; Martins, M.S.; Arias, L.U.; Pisoni, R.L.; Lopes, A.A. Handgrip strength as a simple indicator of possible malnutrition and inflammation in men and women on maintenance hemodialysis. J. Ren. Nutr. 2011, 21, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.F.; Wazlawik, E.; Moreno, Y.M.F.; Führ, L.M.; González-Chica, D.A. Diagnostic accuracy of handgrip strength in the assessment of malnutrition in hemodialyzed patients. e-SPEN J. 2013, 8, e181–e186. [Google Scholar] [CrossRef]
- Duta, T.F.; Zulfa, P.O.; Alina, M.; Henira, N.; Tsurayya, G.; Fakri, F.; Acharya, Y. Efficacy of acetazolamide and loop diuretics combinatorial therapy in congestive heart failure: A meta-analysis. Narra X 2024, 2, e124. [Google Scholar] [CrossRef]
- Song, F.; Khan, K.S.; Dinnes, J.; Sutton, A.J. Asymmetric funnel plots and publication bias in meta-analyses of diagnostic accuracy. Int. J. Epidemiol. 2002, 31, 88–95. [Google Scholar] [CrossRef]
- Lim, C.-K.-M.; Lim, J.-H.; Ibrahim, I.; Chan, Y.-M.; Zakaria, N.F.; Yahya, R.; Daud, Z.A.M. Bioelectrical impedance analysis derived-phase angle as a pragmatic tool to detect protein energy wasting among multi-ethnic hemodialysis patients. Diagnostics 2021, 11, 1745. [Google Scholar] [CrossRef]
- Mitch, W.E.; Goldberg, A.L. Mechanisms of muscle wasting—The role of the ubiquitin–proteasome pathway. N. Engl. J. Med. 1996, 335, 1897–1905. [Google Scholar] [CrossRef]
- Chan, J.; Lu, Y.-C.; Yao, M.M.-S.; Kosik, R.O. Correlation between hand grip strength and regional muscle mass in older Asian adults: An observational study. BMC Geriatr. 2022, 22, 206. [Google Scholar] [CrossRef] [PubMed]
- Vanden Wyngaert, K.; Celie, B.; Calders, P.; Eloot, S.; Holvoet, E.; Van Biesen, W.; Van Craenenbroeck, A.H. Markers of protein-energy wasting and physical performance in haemodialysis patients: A cross-sectional study. PLoS ONE 2020, 15, e0236816. [Google Scholar] [CrossRef] [PubMed]
- Chan, W. Chronic kidney disease and nutrition support. Nutr. Clin. Pract. 2021, 36, 312–330. [Google Scholar] [CrossRef]
- Ko, G.-J.; Kalantar-Zadeh, K. How important is dietary management in chronic kidney disease progression? A role for low protein diets. Korean J. Intern. Med. 2021, 36, 795. [Google Scholar] [CrossRef]
- Norman, K.; Stobäus, N.; Gonzalez, M.C.; Schulzke, J.-D.; Pirlich, M. Hand grip strength: Outcome predictor and marker of nutritional status. Clin. Nutr. 2011, 30, 135–142. [Google Scholar] [CrossRef]
- Kaegi-Braun, N.; Tribolet, P.; Baumgartner, A.; Fehr, R.; Baechli, V.; Geiser, M.; Deiss, M.; Gomes, F.; Kutz, A.; Hoess, C.; et al. Value of handgrip strength to predict clinical outcomes and therapeutic response in malnourished medical inpatients: Secondary analysis of a randomized controlled trial. Am. J. Clin. Nutr. 2021, 114, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Fountotos, R.; Munir, H.; Goldfarb, M.; Lauck, S.; Kim, D.; Perrault, L.; Arora, R.; Moss, E.; Rudski, L.G.; Bendayan, M. Prognostic value of handgrip strength in older adults undergoing cardiac surgery. Can. J. Cardiol. 2021, 37, 1760–1766. [Google Scholar] [CrossRef]
- Parahiba, S.M.; Spillere, S.R.; Zuchinali, P.; Padilha, G.d.R.; Duarte, M.B.; da Silveira, I.V.; Dias, L.H.; Knobloch, I.d.S.; Perry, I.S.; Souza, G.C. Handgrip strength in patients with acute decompensated heart failure: Accuracy as a predictor of malnutrition and prognostic value. Nutrition 2021, 91–92, 111352. [Google Scholar] [CrossRef]
- Nogueira, Á.; Álvarez, G.; Barril, G. Impact of the Nutrition–Inflammation Status on the Functionality of Patients with Chronic Kidney Disease. Nutrients 2022, 14, 4745. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.-J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J. KDOQI clinical practice guideline for nutrition in CKD: 2020 update. Am. J. Kidney Dis. 2020, 76, S1–S107. [Google Scholar] [CrossRef]
- Sahathevan, S.; Khor, B.-H.; Ng, H.-M.; Abdul Gafor, A.H.; Mat Daud, Z.A.; Mafra, D.; Karupaiah, T. Understanding development of malnutrition in hemodialysis patients: A narrative review. Nutrients 2020, 12, 3147. [Google Scholar] [CrossRef] [PubMed]
- Panagidi, M.; Papazoglou, A.S.; Moysidis, D.V.; Vlachopoulou, E.; Papadakis, M.; Kouidi, E.; Galanos, A.; Tagarakis, G.; Anastasiadis, K. Prognostic value of combined preoperative phase angle and handgrip strength in cardiac surgery. J. Cardiothorac. Surg. 2022, 17, 227. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).