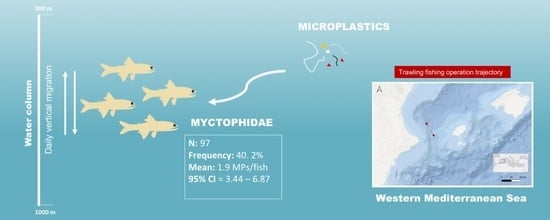
Microplastics in Lampanyctus crocodilus (Risso 1810, Myctophidae), a Common Lanternfish Species from the Ibiza Channel (Western Mediterranean)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling and Study Area
2.2. Laboratory Procedure
2.3. Contamination Control
2.4. Statistical Analyses
3. Results
3.1. Biometric Parameters
3.2. Microplastic Content and Characteristics
3.3. Biometric Parameters and Microplastic Content
4. Discussion
4.1. Microplastics in Lanternfish Species
| Area | Species | Environment | MPs/Individual (Mean ± SD) | % Fish with MPs | Prevalent Shape | Prevalent Colour | Reference |
|---|---|---|---|---|---|---|---|
| W Med. (E Spain) | Mullus barbatus (Linnaeus, 1758) | Demersal | 1.9 ± 1.29 | 10–33% | Fibers (71%) | Black | [28] |
| W Med. (Spain) | Boops boops, Engraulis encrasicolus, Sardina pilchardus and Trachurus mediterraneus | Pelagic | 0 ± 0–1.22 ± 2.08 | 28% | Fibers | Blue | [29] |
| Ligurian Sea | Engraulis encrasicolus | Pelagic | 0.34 ± 0.29 fibres ind−1 and 0.12 ± 0.12 fragments ind−1 | 30–40% | Fibers | Blue and black | [30] |
| E Med. (Turkey) | 28 species | Demersal and pelagic | 2.36 (only fish with MPs, 58%) | 41% | Fibers (70%) | Blue | [31] |
| NE Ionian, N Adriatic | Chelon auratus (Risso, 1810), Mullus barbatus, Mullus surmuletus, Pagellus erythrinus, Sparus aurata, Sardina pilchardus, Solea solea (Linnaeus, 1758). | Demersal and pelagic | 6.7 ± 3.5; 2.5 ± 0.2; 1.7 ± 0.2 | 40–87% | – | – | [32] |
| N Ionian | Sardina pilchardus, Pagellus erythrinus and Mullus barbatus | Pelagic | 0.8 ± 0.2, 0.8 ± 0.2, 0.5 ± 0.2, respectively. | 47.2%, 42.1%, 32%, respectively. | Fragments (80%) | Blue | [33] |
| Central Med. | Electrona risso (Cocco 1829), Hygophum benoiti (Cocco, 1838), Myctophum punctatum (Rafinesque, 1810), Diaphus metopoclampus (Cocco, 1829) | Pelagic | 1.09 ± 0.30, 4.10 ± 3.08, 1.91 ± 0.55, respectively. | 2.7% | Small microplastics | Hyaline | [36] |
| E Med. (Lebanon) | Engraulis encrasicolus | Pelagic | 2.9 ± 1.9 | 83.4% | Fragments | Blue | [48] |
| Spanish Med. | Sardina pilchardus and Engraulis encrasicolus | Pelagic | 0.18 ± 0.20 | 14.8% | Fibers (83%) | Blue | [49] |
| Thyrrenian Sea | Pagellus spp. | Demersal | MPs in the stomach of 4 specimens. Amount not specified. | 10.25% | Fibers (100%, Nylon 66) | Black | [50] |
| Balearic Sea | Mullus surmuletus (Linnaeus, 1758) | Demersal | 0.42 ± 0.04 | 27.3% | Filament (97%) | Blue | [51] |
| Balearic Sea | Galeus melastomus (Rafinesque, 1810) | Demersal | 0.34 ± 0.07 | 16.8% | Filament (86.36%) | Transparent | [52] |
| W Med. (Spain) | Boops boops (Linnaeus, 1758). | Pelagic | 1.68 ± 0.31 0.50 ± 0.14 0.53 ± 0.14 | 46% | Fragments | Blue | [53] |
| Adriatic, Thyrrenian and Ionian Seas | Mullus barbatus and Merluccius merluccius (Linnaeus, 1758). | Demersal | 0–1.75 | 23.3% | Fibers | Blue | [54] |
| SE Med. (Egypt) | Caranx crysos (Mitchill, 1815), Liza aurata (Risso, 1810), Signus rivulatus (Forsskål & Niebuhr, 1775) and Epinephelus caninus (Valenciennes, 1843) | Demersal | 8.6 ± 1.52–2 ± 2.64 | – | Fibers (70%) | Blue | [55] * |
| Central Med. (Italy) | Trachurus trachurus | Pelagic | 112.86 ± 38.93 | 90.6% | Filament (84.9%) | Blue | [56] |
| SW Med. (Mar Menor) | Sparus aurata (Linnaeus, 1758) | Demersal | 20.11 ± 2.94 MP/kg | 100% | Fibers (71.68%) | White | [57] |
| W Med. (E Spain) | Lampanyctus crocodilus (Risso, 1810) | Pelagic | 1.907 ± 4.023 | 40.21% | Fibers (97.75%) | Blue | This study |
4.2. Microplastics and Fish Fitness
4.3. Influence of the Environment in Microplastic Concentration in Lanternfishes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pham, C.K.; Ramirez-Llodra, E.; Alt, C.H.S.; Amaro, T.; Bergmann, M.; Canals, M.; Company, J.B.; Davies, J.; Duineveld, G.; Galgani, F.; et al. Marine Litter Distribution and Density in European Seas, from the Shelves to Deep Basins. PLoS ONE 2014, 9, e95839. [Google Scholar] [CrossRef] [Green Version]
- Jamieson, A.J.; Brooks, L.S.R.; Reid, W.D.K.; Piertney, S.B.; Narayanaswamy, B.E.; Linley, T.D. Microplastics and synthetic particles ingested by Deep-sea amphipods in six of the deepest marine ecosystems on Earth. R. Soc. Open Sci. 2019, 6, 180667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, J.; Park, J.; Eo, S.; Choi, J.; Song, Y.K.; Cho, Y.; Hong, S.H.; Shim, W.J. Ecological risk assessment of microplastics in coastal, shelf, and deep sea waters with a consideration of environmentally relevant size and shape. Environ. Pollut. 2021, 270, 116217. [Google Scholar] [CrossRef] [PubMed]
- Kaandorp, M.L.A.; Dijkstra, H.A.; van Sebille, E. Closing the Mediterranean marine floatinc plastic mass budget: Inverse modeling of sources and sinks. Environ. Sci. Technol. 2020, 54, 11980–11989. [Google Scholar] [CrossRef]
- de la Fuente, R.; Drótos, G.; Hernández-García, E.; Jópez, C.; van Sebille, E. Sinking microplastics in the water column: Simulations in the Mediterranean Sea. Ocean Sci. 2021, 17, 431–453. [Google Scholar] [CrossRef]
- Boucher, J.; Bilard, G. The Mediterranean: Mare Plasticum; IUCN: Gland, Switzerland, 2020. [Google Scholar]
- van Sebille, E.; Wilcox, C.; Lebreton, L.; Maximenko, N.; Hardesty, B.D.; van Franeker, J.A.; Eriksen, M.; Siegel, D.; Galgani, F.; Law, K.L. A global inventory of small floating plastic debris. Environ. Res. Lett. 2015, 10, 124006. [Google Scholar] [CrossRef]
- Kaiser, D.; Kowalski, N.; Waniek, J.J. Effects of biofouling on the sinking behavior of microplastics. Environ. Res. Lett. 2017, 12, 124003. [Google Scholar] [CrossRef] [Green Version]
- Farrell, P.; Nelson, K. Trophic level transfer of microplastic: Mytilus edulis (L.) to Carcinus maenas (L.). Environ. Pollut. 2013, 177, 1–3. [Google Scholar] [CrossRef]
- Setälä, O.; Fleming-Lehtinen, V.; Lehtiniemi, M. Ingestion and transfer of microplastics in the planktonic food web. Environ. Pollut. 2014, 185, 77–83. [Google Scholar] [CrossRef]
- Nelms, S.E.; Galloway, T.S.; Godley, B.J.; Jarvis, D.S.; Lindeque, P.K. Investigating microplastic trophic transfer in marine top predators. Environ. Pollut. 2018, 238, 999–1007. [Google Scholar] [CrossRef]
- Wang, W.H.; Gao, H.; Jin, S.; Li, R.; Na, G. The ecotoxicological effects of microplastics on aquatic food web, from primary producer to human: A review. Ecotoxicol. Environ. Saf. 2019, 173, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Novillo-Sanjuan, O.; Raga, J.A.; Tomás, J. Microdebris in three Spanish Mediterranean beaches located at a sporadic loggerhead turtles’ (Caretta caretta) nesting area. Reg. Stud. Mar. Sci. 2022, 49, 102116. [Google Scholar] [CrossRef]
- Roch, S.; Friedrich, C.; Brinker, A. Uptake routes of microplastics in fishes: Practical and theoretical approaches to test existing theories. Sci. Rep. 2020, 10, 3896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, J.P.; Duarte, A.C.; Santos-Echeandía, J.; Rocha-Santos, T. Significance of interactions between microplastics and POPs in the marine environment: A critical overview. TrAC 2019, 111, 252–260. [Google Scholar] [CrossRef]
- Hossain, M.A.; Olden, J.D. Global meta-analysis reveals diverse effect of microplastics on freshwater and marine fishes. Fish Fish. 2022, 23, 1439–1454. [Google Scholar] [CrossRef]
- Fanelli, E.; Papiol, V.; Cartes, J.E.; Rodriguez-Romeu, O. Trophic ecology of Lampanyctus crocodilus on north-west Mediterranean Sea slopes in relation to reproductive cycle and environmental variables. J. Fish Biol. 2014, 84, 1654–1688. [Google Scholar] [CrossRef]
- Gómez de Segura, A.; Crespo, E.A.; Pedraza, S.N.; Hammond, P.S.; Raga, J.A. Abundance of small cetaceans in waters of the central Spanish Mediterranean. Mar. Biol. 2006, 150, 149–160. [Google Scholar] [CrossRef]
- Aznar, F.J.; Míguez-Lozano, R.; Ruiz, B.; de Castro, A.; Raga, J.A.; Blanco, C. Long-term changes (1990–2012) in the diet of striped dolphins Stenella coeruleoalba from the western Mediterranean. Mar. Ecol. Progr. Ser. 2017, 568, 231–247. [Google Scholar] [CrossRef]
- Lusher, A.L.; O’Donnell, C.; Officer, R.; O’Connor, I. Microplastic interactions with North Atlantic mesopelagic fish. ICES Mar. Sci. Symp. 2016, 73, 1214–1225. [Google Scholar] [CrossRef] [Green Version]
- Foekema, E.M.; De Gruijter, C.; Mergia, M.T.; van Franeker, J.A.; Murk, A.J.; Koelmans, A.A. Plastic in North Sea fish. Environ. Sci. Technol. 2013, 47, 8818–8824. [Google Scholar] [CrossRef]
- Wang, Z.M.; Wagner, J.; Ghosal, S.; Bedi, G.; Wall, S. SEM/EDS and optical microscopy analyses of microplastics in ocean trawl and fish guts. Sci. Total Environ. 2017, 15, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.B.; Bastos, A.S.; Justino, C.I.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T.A. Microplastics in the environment: Challenges in analytical chemistry—A review. Anal. Chim. Acta. 2018, 1017, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Reiczigel, J.; Marozzi, M.; Fabian, I.; Rozsa, L. Biostatistics for parasitologists—A primer to Quantitative Parasitology. Trends Parasitol. 2019, 35, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Froese, R. Cube law, condition factor and weight–length relationships: History, meta-analysis and recommendations. J. Appl. Ichthyol. 2006, 22, 241–253. [Google Scholar] [CrossRef]
- Stefanescu, C.; Cartes, J.E. Benthopelagic habits of adult specimens of Lampanyctus crocodilus (Risso, 1810) (Osteichthyes, Myctophidae) in the western Mediterranean deep slope. Sci. Mar. 1992, 56, 69–74. [Google Scholar]
- Bellas, J.; Martínez-Armental, J.; Martínez-Cámara, A.; Besada, V.; Martínez-Gómez, C. Ingestion of microplastics by demersal fish from the Spanish Atlantic and Mediterranean coasts. Mar. Pollut. Bull. 2016, 109, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Rios-Fuster, B.; Alomar, C.; Compa, M.; Guijarro, B.; Deudero, S. Anthropogenic particles ingestion in fish species from two areas of the western Mediterranean Sea. Mar. Pollut. Bull. 2019, 144, 325–333. [Google Scholar] [CrossRef]
- Capone, A.; Petrillo, M.; Misic, C. Ingestion and elimination of anthropogenic fibres and microplastic fragments by the European anchovy (Engraulis encrasicolus) of the NW Mediterranean Sea. Mar. Biol. 2020, 167, 166. [Google Scholar] [CrossRef]
- Güven, O.; Gökdağ, K.; Jovanović, B.; Kıdeyş, A.E. Microplastic litter composition of the Turkish territorial waters of the Mediterranean Sea, and its occurrence in the gastrointestinal tract of fish. Environ. Pollut. 2017, 223, 286–294. [Google Scholar] [CrossRef]
- Anastasopoulou, A.; Mytilineou, C.; Smith, C.J.; Papadopoulou, K.N. Plastic debris ingested by deep-water fish of the Ionian Sea (Eastern Mediterranean). Deep Sea Res. Part I Oceanogr. Res. Pap. 2013, 74, 11–13. [Google Scholar] [CrossRef]
- Digka, N.; Tsangaris, C.; Torre, M.; Anastasopoulou, A.; Zeri, C. Microplastics in mussels and fish from the Northern Ionian Sea. Mar. Pollut. Bull. 2018, 135, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, A.M.; Morrison, L.; Croot, P.L.; Allcock, A.L.; MacLoughlin, E.; Savard, O.; Brownlow, H.; Doyle, T.K. Frequency of microplastics in mesopelagic fishes from the Northwest Atlantic. Front. Mar. Sci. 2018, 5, 39. [Google Scholar] [CrossRef] [Green Version]
- Bernal, A.; Toresen, R.; Riera, R. Mesopelagic fish composition and diets of three myctophid species with potential incidence of microplastics, across the southern tropical gyre. Deep Sea Res. Part II Top. Stud. Oceanogr. 2020, 179, 104706. [Google Scholar] [CrossRef]
- Romeo, T.; Pedà, C.; Fossi, M.C.; Andaloro, F.; Battaglia, P. First record of plastic debris in the stomach of Mediterranean lanternfishes. Acta Adriat. 2016, 57, 113–122. [Google Scholar]
- Bernal, A.; Olivar, M.P.; Maynou, F.; de Puelles, M.L.F. Diet and feeding strategies of mesopelagic fishes in the western Mediterranean. Prog. Oceanogr. 2015, 135, 1–17. [Google Scholar] [CrossRef]
- de Haan, W.P.; Sanchez-Vidal, A.; Canals, M.; Party, N.S.S. Floating microplastics and aggregate formation in the Western Mediterranean Sea. Mar. Pollut. Bul. 2019, 140, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Fagiano, V.; Alomar, C.; Compa, M.; Soto-Navarro, J.; Jordá, G.; Deudero, S. Neustonic microplastics and zooplankton in coastal waters of Cabrera marine protected area (Western Mediterranean Sea). Sci. Tot. Environ. 2022, 804, 150120. [Google Scholar] [CrossRef] [PubMed]
- Woodall, L.C.; Sanchez-Vidal, A.; Canals, M.; Paterson, G.L.; Coppock, R.; Sleight, V.; Calafat, A.; Rogers, A.D.; Narayanaswamy, B.E.; Thompson, R.C. The deep sea is a major sink for microplastic debris. R. Soc. Open Sci. 2014, 1, 140317. [Google Scholar] [CrossRef] [Green Version]
- Lusher, A.L.; Burke, A.; O’Connor, I.; Officer, R. Microplastic pollution in the Northeast Atlantic Ocean: Validated and opportunistic sampling. Mar. Pollut. Bull. 2014, 88, 325–333. [Google Scholar] [CrossRef]
- Neves, D.; Sobral, P.; Ferreira, J.L.; Pereira, T. Ingestion of microplastics by commercial fish off the Portuguese coast. Mar. Pollut. Bull. 2015, 101, 119–126. [Google Scholar] [CrossRef]
- Hernandez-Gonzalez, A.; Saavedra, C.; Gago, J.; Covelo, P.; Santos, M.B.; Pierce, G.J. Microplastics in the stomach contents of common dolphin (Delphinus delphis) stranded on the Galician coasts (NW Spain, 2005–2010). Mar. Pollut. Bull. 2018, 137, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Barboza, L.G.A.; Lopes, C.; Oliveira, P.; Bessa, F.; Otero, V.; Henriques, B.; Raimundo, J.; Caetano, M.; Vale, C.; Guilhermino, L. Microplastics in wild fish from North East Atlantic Ocean and its potential for causing neurotoxic effects, lipid oxidative damage, and human health risks associated with ingestion exposure. Sci. Total Environ. 2020, 717, 134625. [Google Scholar] [CrossRef] [PubMed]
- Froese, R.; Pauly, D. (Eds.) FishBase 2000, Concepts, Design and Data Sources; ICLARM Contrib. No.1594; International Center for Living Aquatic Resources Management (ICLARM): Los Banos, Philippines, 2000; p. 344. ISBN 971-8709-99-1. [Google Scholar]
- Valls, M.; Olivar, M.P.; Fernández de Puelles, M.L.; Molí, B.; Bernal, A.; Sweeting, C.J. Trophic structure of mesopelagic fishes in the western Mediterranean based on stable isotopes of carbon and nitrogen. J. Mar. Syst. 2014, 138, 160–170. [Google Scholar] [CrossRef]
- Bozzano, A.; Pankhurst, P.M.; Sabatés, A. Early development of eye and retina in lanternfish larvae. Vis. Neurosci. 2007, 24, 423–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alava, J.J. Modeling the Bioaccumulation and Biomagnification Potential of Microplastics in a Cetacean Foodweb of the Northeastern Pacific: A Prospective Tool to Assess the Risk Exposure to Plastic Particles. Front. Mar. Sci. 2020, 7, 566101. [Google Scholar] [CrossRef]
- Filgueiras, A.V.; Preciado, I.; Cartón, A.; Gago, J. Microplastic ingestion by pelagic and benthic fish and diet composition: A case study in the NW Iberian shelf. Mar. Pollut. Bull. 2020, 160, 111623. [Google Scholar] [CrossRef]
- Savoca, S.; Capillo, G.; Mancuso, M.; Bottari, T.; Crupi, R.; Branca, C.; Romano, V.; Faggio, C.; D’angelo, G.; Spanò, N. Microplastics occurrence in the Tyrrhenian waters and in the gastrointestinal tract of two congener species of seabreams. Environ. Toxicol. Pharmacol. 2019, 67, 35–41. [Google Scholar] [CrossRef]
- Alomar, C.; Sureda, A.; Capó, X.; Guijarro, B.; Tejada, S.; Deudero, S. Microplastic ingestion by Mullus surmuletus Linnaeus, 1758 fish and its potential for causing oxidative stress. Environ. Res. 2017, 159, 135–142. [Google Scholar] [CrossRef]
- Alomar, C.; Deudero, S. Evidence of microplastic ingestion in the shark Galeus melastomus Rafinesque, 1810 in the continental shelf off the western Mediterranean Sea. Environ. Pollut. 2017, 223, 223–229. [Google Scholar] [CrossRef]
- Garcia-Garin, O.; Vighi, M.; Aguilar, A.; Tsangaris, C.; Digka, N.; Kaberi, H.; Borrell, A. Boops boops as a bioindicator of microplastic pollution along the Spanish Catalan coast. Mar. Pollut. Bull. 2019, 149, 110648. [Google Scholar] [CrossRef]
- Giani, D.; Baini, M.; Galli, M.; Casini, S.; Fossi, M.C. Microplastics occurrence in edible fish species (Mullus barbatus and Merluccius merluccius) collected in three different geographical sub-areas of the Mediterranean Sea. Mar. Pollut. Bull. 2019, 140, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Sayed, A.E.D.H.; Hamed, M.; Badrey, A.E.; Ismail, R.F.; Osman, Y.A.; Osman, A.G.; Soliman, H.A. Microplastic distribution, abundance, and composition in the sediments, water, and fishes of the Red and Mediterranean seas, Egypt. Mar. Pollut. Bull. 2021, 173, 112966. [Google Scholar] [CrossRef]
- Chenet, T.; Mancia, A.; Bono, G.; Falsone, F.; Scannella, D.; Vaccaro, C.; Baldi, A.; Catani, M.; Cavazzini, A.; Pasti, L. Plastic ingestion by Atlantic horse mackerel (Trachurus trachurus) from central Mediterranean Sea: A potential cause for endocrine disruption. Environ. Pollut. 2021, 284, 117449. [Google Scholar] [CrossRef] [PubMed]
- Bayo, J.; Rojo, D.; Martínez-Baños, P.; López-Castellanos, J.; Olmos, S. Commercial gilthead seabream (Sparus aurata L.) from the Mar Menor Coastal Lagoon as hotspots of microplastic accumulation in the digestive system. Int. J. Environ. Res. Public Health 2021, 18, 6844. [Google Scholar] [CrossRef] [PubMed]
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.; Tonkin, A.; Galloway, T.; Thompson, R. Accumulation of microplastic on shorelines woldwide: Sources and sinks. Environ. Sci. Technol. 2011, 45, 9175–9179. [Google Scholar] [CrossRef]
- Montarsolo, A.; Mossotti, R.; Patrucco, A.; Caringella, R.; Zoccola, M.; Pozzo, P.D.; Tonin, C. Study on the microplastics release from fishing nets. Eur. Phys. J. Plus. 2018, 133, 494. [Google Scholar] [CrossRef]
- Reissner, J.; Slat, B.; Noble, K.; du Plessis, K.; Epp, M.; Proietti, M.; de Sonneville, J.; Becker, T.; Pattiaratchi, C. The vertical distribution of buoyant plastics at sea: An observational study in the North Atlantic Gyre. Biogeosciences 2015, 12, 1249–1256. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H. Transport of microplastics in coastal seas. Estuar. Coast. Shelf Sci. 2017, 99, 74–86. [Google Scholar] [CrossRef]
- Kazour, M.; Jemaa, S.; Issa, C.; Khalaf, G.; Amara, R. Microplastics pollution along the Lebanese coast (Eastern Mediterranean Basin): Occurrence in surface water, sediments and biota samples. Sci. Total Environ. 2019, 696, 133933. [Google Scholar] [CrossRef]
- Galafassi, S.; Campanale, C.; Massarelli, C.; Uricchio, V.F.; Volta, P. Do Freshwater Fish Eat Microplastics? A Review with A Focus on Effects on Fish Health and Predictive Traits of MPs Ingestion. Water J. 2021, 13, 2214. [Google Scholar] [CrossRef]
- Compa, M.; Ventero, A.; Iglesias, M.; Deudero, S. Ingestion of microplastics and natural fibres in Sardina pilchardus (Walbaum, 1792) and Engraulis encrasicolus (Linnaeus, 1758) along the Spanish Mediterranean coast. Mar. Pollut. Bull. 2018, 128, 89–96. [Google Scholar] [CrossRef]
- Rochman, C.M.; Lewison, R.L.; Eriksen, M.; Allen, H.; Cook, A.-M.; Teh, S.J. Polybrominated diphenyl ethers (PBDEs) in fish tissue may be an indicator of plastic contamination in marine habitats. Sci. Total Environ. 2014, 476–477, 622–633. [Google Scholar] [CrossRef] [PubMed]
- Herzke, D.; Anker-Nilssen, T.; Nøst, T.H.; Götsch, A.; Christensen-Dalsgaard, S.; Langset, M.; Fangel, K.; Koelmans, A.A. Negligible impact of ingested microplastics on tissue concentrations of persistent organic pollutants in northern fulmars off coastal Norway. Environ. Sci. Technol. 2016, 50, 1924–1933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koelmans, A.A.; Bakir, A.; Burton, G.A.; Janssen, C.R. Microplastic as a vector for chemicals in the aquatic environment: Critical review and model-supported reinterpretation of empirical studies. Environ. Sci. Technol. 2016, 50, 3315–3326. [Google Scholar] [CrossRef]
- Lohmann, R. Microplastics are not important for the cycling and bioaccumulation of organic pollutants in the oceans—But should microplastics be considered POPs themselves? Integr. Environ. Assess. Manag. 2017, 13, 460–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, C.; Erzini, K.; Teodósio, M.A.; Pousão-Ferreira, P.; Baptista, V.; Ekau, W. Assessing microplastic uptake and impact on omnivorous juvenile white seabream Diplodus sargus (Linnaeus, 1758) under laboratory conditions. Mar. Pollut. Bull. 2020, 157, 111162. [Google Scholar] [CrossRef] [PubMed]
- Choy, C.A.; Robison, B.H.; Gagne, T.O.; Erwin, B.; Firl, E.; Halden, R.U.; Hamilton, J.A.; Katija, K.; Lisin, S.E.; Rolsky, C.; et al. The vertical distribution and biological transport of marine microplastics across the epipelagic and mesopelagic water column. Sci. Rep. 2019, 9, 7843. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, N.B.; Rist, S.; Bodin, J.; Jensen, L.H.; Schmidt, S.N.; Mayer, P.; Meibom, A.; Baun, A. Microplastics as vectors for environmental contaminants: Exploring sorption, desorption, and transfer to biota. Integr. Environ. Assess. Manag. 2017, 13, 488–493. [Google Scholar] [CrossRef] [Green Version]
- Roman, J.; McCarthy, J.J. The whale pump: Marine mammals enhance primary productivity in a coastal basin. PLoS ONE 2010, 5, e13255. [Google Scholar] [CrossRef] [Green Version]
- Novillo, O.; Raga, J.A.; Tomás, J. Evaluating the presence of microplastics in striped dolphins (Stenella coeruleoalba) stranded in the Western Mediterranean Sea. Mar. Pollut. Bull. 2020, 160, 111557. [Google Scholar] [CrossRef]
- Cincinelli, A.; Martellini, T.; Guerranti, C.; Scopetani, C.; Chelazzi, D.; Giarrizzo, T. A potpourri of microplastics in the sea surface and water column of the Mediterranean Sea. TrAC 2019, 110, 321–326. [Google Scholar] [CrossRef]



| All Myctophids | Myctophids with Microplastics | |
|---|---|---|
| Mean (MPs/fish) | 1.907 | 4.744 |
| 95% CI for the mean | 1.26–2.92 | 3.44–6.87 |
| Median (MPs/fish) | 0 | 3 |
| 95% CI for the median | 1.27–2.97 | 3.46–6.8 |
| Range (MPs/fish) | 0–23 | 1–23 |
| Mean Fulton’s K | 0.5268 | 0.5097 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novillo-Sanjuan, O.; Gallén, S.; Raga, J.A.; Tomás, J. Microplastics in Lampanyctus crocodilus (Risso 1810, Myctophidae), a Common Lanternfish Species from the Ibiza Channel (Western Mediterranean). Microplastics 2023, 2, 242-254. https://doi.org/10.3390/microplastics2030020
Novillo-Sanjuan O, Gallén S, Raga JA, Tomás J. Microplastics in Lampanyctus crocodilus (Risso 1810, Myctophidae), a Common Lanternfish Species from the Ibiza Channel (Western Mediterranean). Microplastics. 2023; 2(3):242-254. https://doi.org/10.3390/microplastics2030020
Chicago/Turabian StyleNovillo-Sanjuan, Olga, Sergio Gallén, Juan Antonio Raga, and Jesús Tomás. 2023. "Microplastics in Lampanyctus crocodilus (Risso 1810, Myctophidae), a Common Lanternfish Species from the Ibiza Channel (Western Mediterranean)" Microplastics 2, no. 3: 242-254. https://doi.org/10.3390/microplastics2030020
APA StyleNovillo-Sanjuan, O., Gallén, S., Raga, J. A., & Tomás, J. (2023). Microplastics in Lampanyctus crocodilus (Risso 1810, Myctophidae), a Common Lanternfish Species from the Ibiza Channel (Western Mediterranean). Microplastics, 2(3), 242-254. https://doi.org/10.3390/microplastics2030020