Detection of Secondary Microplastics in an Aquatic Mesocosm by Means of Object-Based Image Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Mechanical Degradation of Plastics
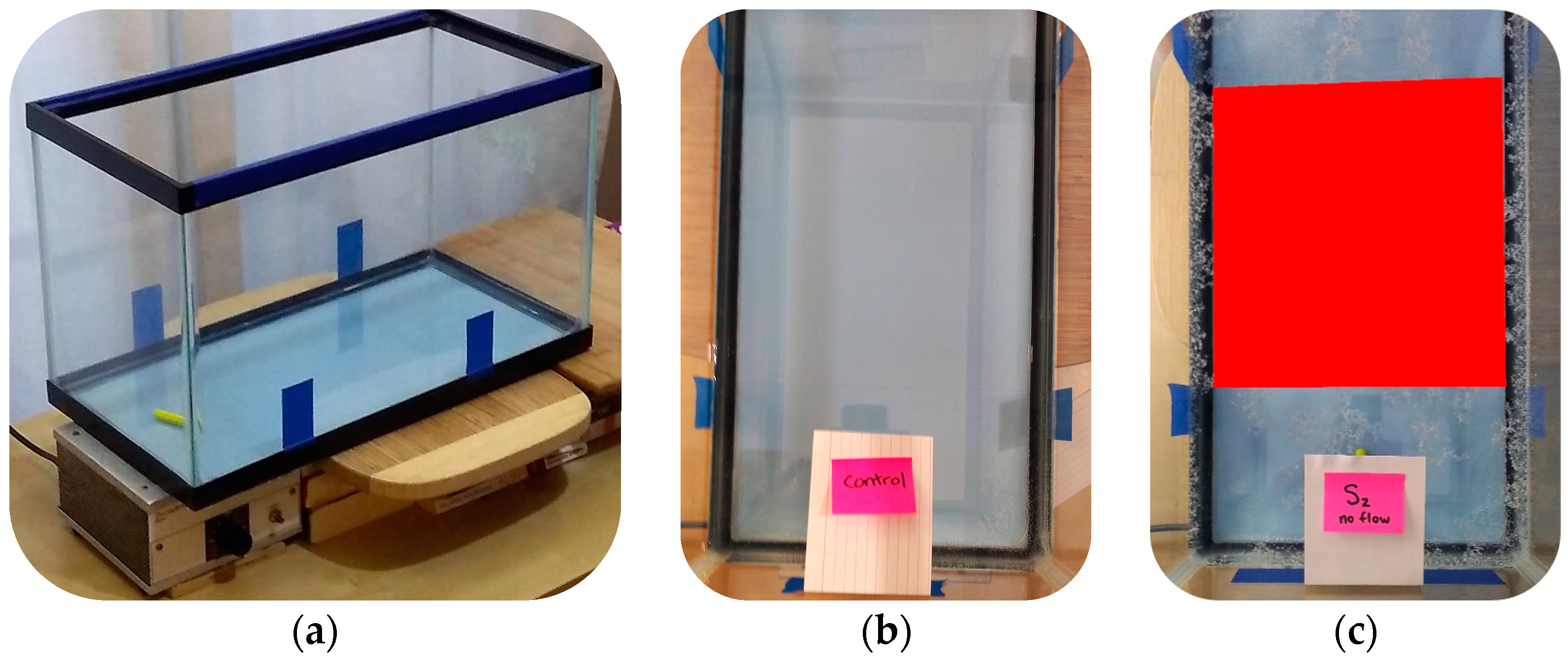
2.2. Controlled Aquatic Environment
2.3. Microplastic Treatments
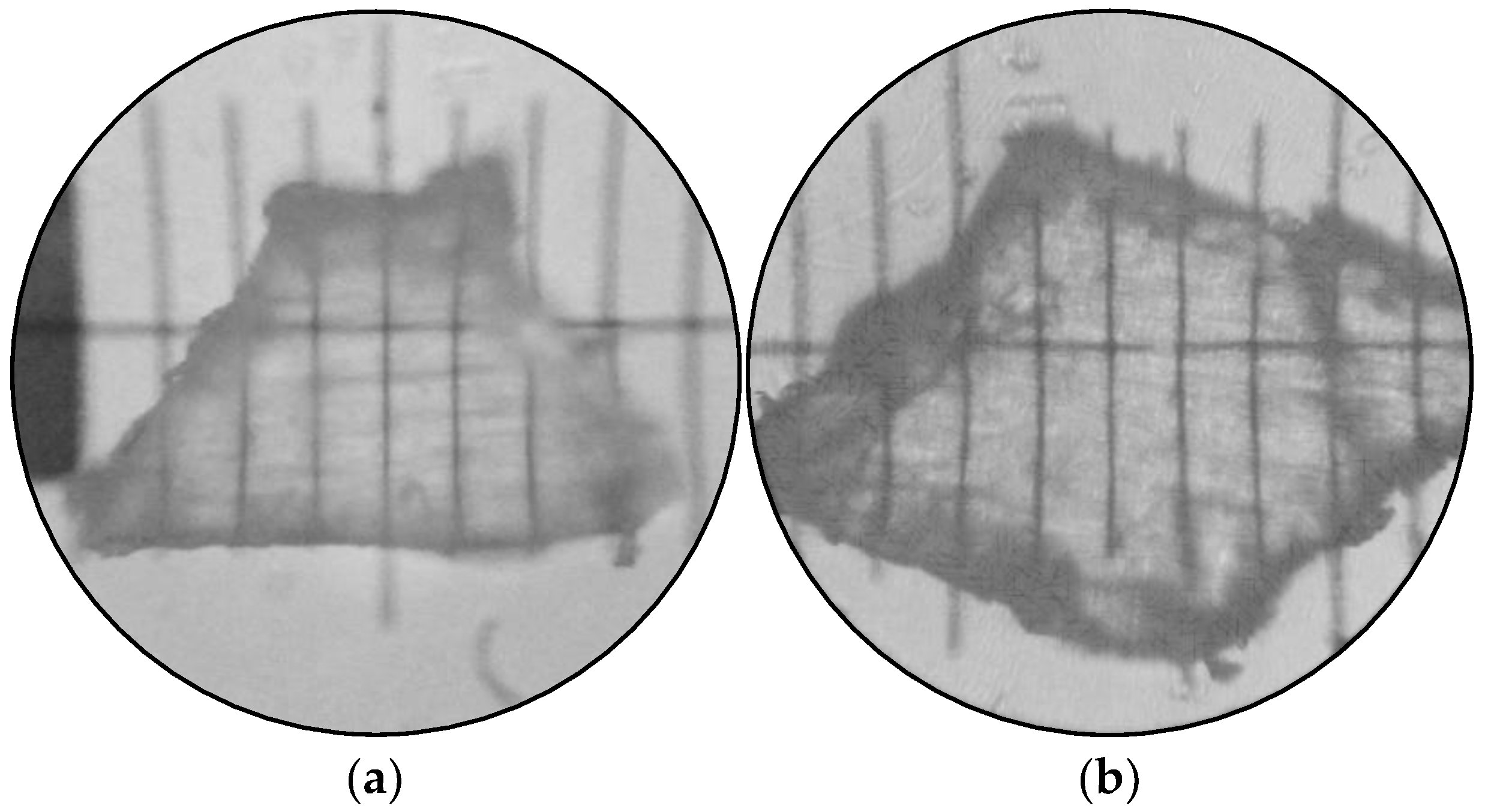
2.4. Digital Image Processing
2.5. Statistical Analyses
3. Results
3.1. Microplastic Size Classes
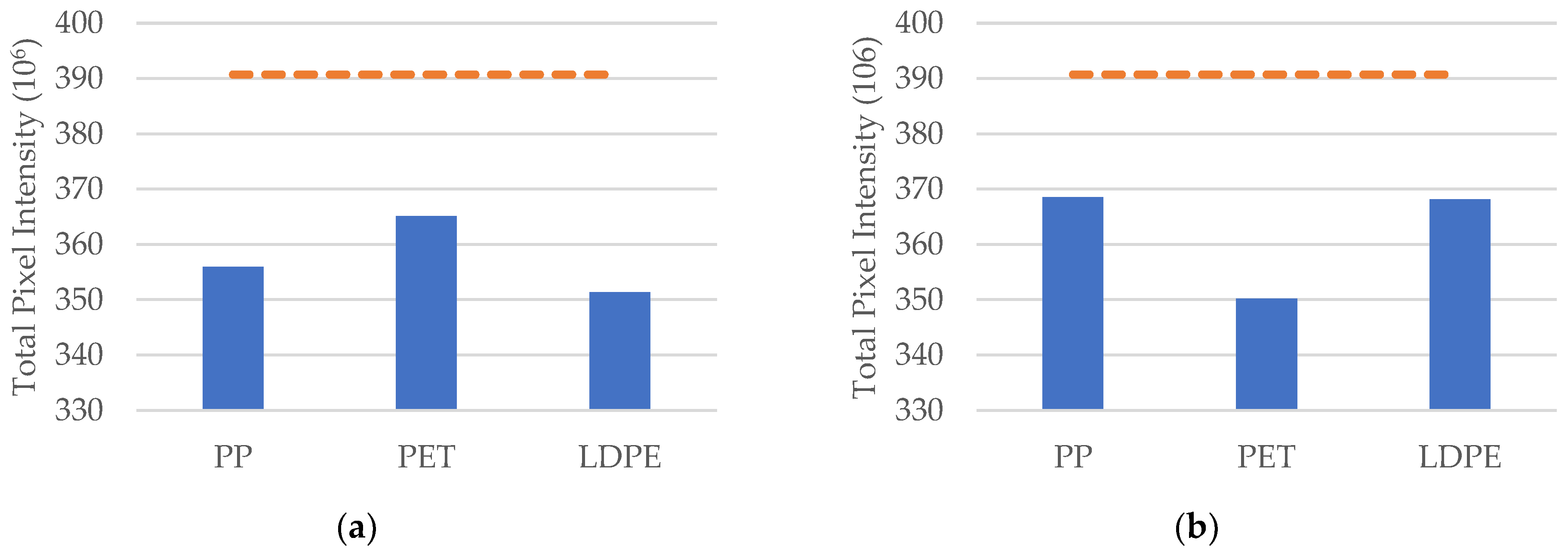
3.2. Single-Type Microplastics in No-Slip Aquatic Environments
3.3. Single-Type Microplastics in Turbulent Aquatic Environments
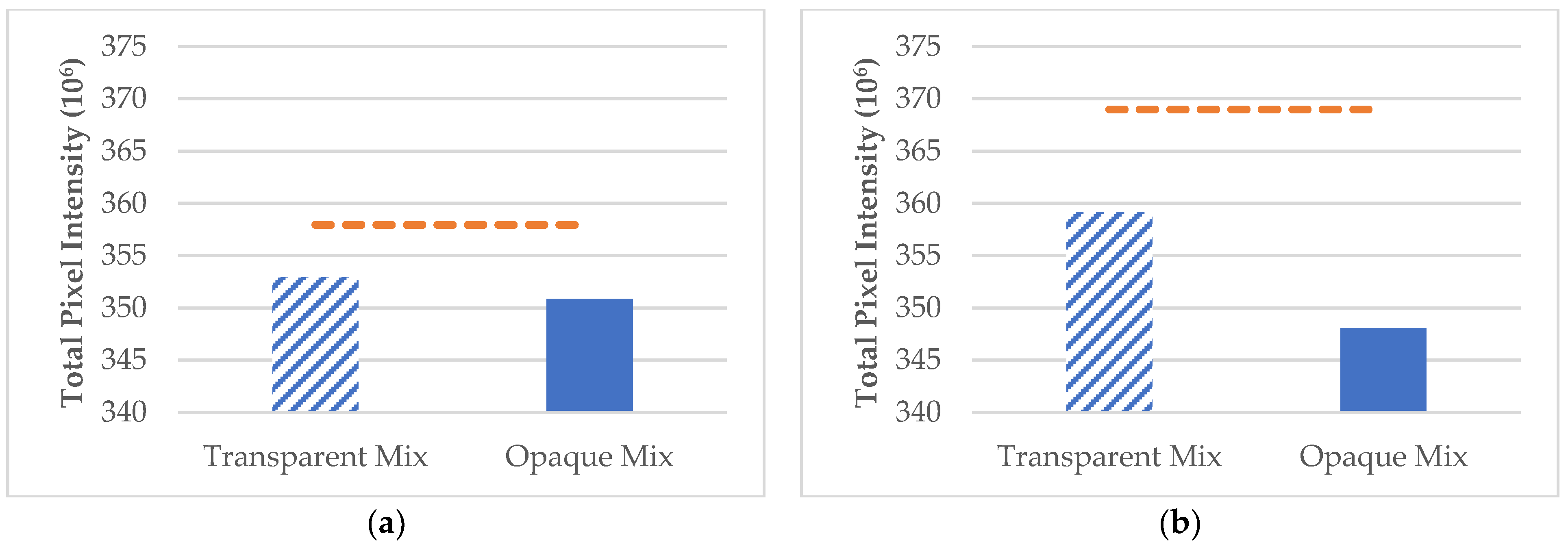
3.4. Mixed PET and LDPE Microplastics in Aquatic Environments
3.5. PET Concentration Gradient in Aquatic Environments
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Parkes, A. Improvements in the Manufacture of Parkesine or Compound of Pyroxyline, and also Solutions of Pyroxyline Known as Collodion. Patent No. 1313, 11 May 1857. [Google Scholar]
- Lau, W.W.Y.; Shiran, Y.; Bailey, R.M.; Cook, E.; Stuchtey, M.R.; Koskella, J.; Velis, C.A.; Godfrey, L.; Boucher, J.; Murphy, M.B.; et al. Evaluating scenarios toward zero plastic pollution. Science 2020, 369, 1455–1460. [Google Scholar] [CrossRef]
- Gibb, B.C. Plastics are forever. Nat. Chem. 2019, 11, 394–395. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Awasthi, A.K.; Wei, F.; Tan, Q.; Li, J. Single-use plastics: Production, usage, disposal, and adverse impacts. Sci. Total Environ. 2021, 752, 141772. [Google Scholar] [CrossRef]
- Tokiwa, Y.; Calabia, B.P.; Ugwu, C.U.; Aiba, S. Biodegradability of Plastics. Int. J. Mol. Sci. 2009, 10, 3722–3742. [Google Scholar] [CrossRef]
- Naidoo, T.; Rajkaran, A.; Sershen. Impacts of plastic debris on biota and implications for human health: A South African perspective. S. Afr. J. Sci. 2020, 116, 1–8. [Google Scholar] [CrossRef]
- Ward, C.P.; Reddy, C.M. We need better data about the environmental persistence of plastic goods. Proc. Natl. Acad. Sci. USA 2020, 117, 14618–14621. [Google Scholar] [CrossRef]
- Zhang, K.; Hamidian, A.H.; Tubic, A.; Zhang, Y.; Fang, J.K.H.; Wu, C.; Lam, P.K.S. Understanding plastic degradation and microplastic formation in the environment: A review. Environ. Pollut. 2021, 274, 116554. [Google Scholar] [CrossRef]
- Schnurr, R.E.J.; Alboiu, V.; Chaudhary, M.; Corbett, R.A.; Quanz, M.E.; Sankar, K.; Srain, H.S.; Thavarajah, V.; Xanthos, D.; Walker, T.R. Reducing marine pollution from single-use plastics (SUPs): A review. Mar. Pollut. Bull. 2018, 137, 157–171. [Google Scholar] [CrossRef]
- Morgana, S.; Casentini, B.; Amalfitano, S. Uncovering the release of micro/nanoplastics from disposable face masks at times of COVID-19. J. Hazard. Mater. 2021, 419, 126507. [Google Scholar] [CrossRef]
- Li, C.; Busquets, R.; Campos, L.C. Assessment of microplastics in freshwater systems: A review. Sci. Total Environ. 2020, 707, 135578. [Google Scholar] [CrossRef]
- Wu, W.-M.; Yang, J.; Criddle, C.S. Microplastics pollution and reduction strategies. Front. Environ. Sci. Eng. 2016, 11, 6. [Google Scholar] [CrossRef]
- Dissanayake, P.D.; Kim, S.; Sarkar, B.; Oleszczuk, P.; Sang, M.K.; Haque, M.N.; Ahn, J.H.; Bank, M.S.; Ok, Y.S. Effects of microplastics on the terrestrial environment: A critical review. Environ. Res. 2022, 209, 112734. [Google Scholar] [CrossRef]
- Luo, H.; Liu, C.; He, D.; Xu, J.; Sun, J.; Li, J.; Pan, X. Environmental behaviors of microplastics in aquatic systems: A systematic review on degradation, adsorption, toxicity and biofilm under aging conditions. J. Hazard. Mater. 2022, 423, 126915. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Song, B.; Liang, J.; Niu, Q.; Zeng, G.; Shen, M.; Deng, J.; Luo, Y.; Wen, X.; Zhang, Y. Microplastics and associated contaminants in the aquatic environment: A review on their ecotoxicological effects, trophic transfer, and potential impacts to human health. J. Hazard. Mater. 2021, 405, 124187. [Google Scholar] [CrossRef] [PubMed]
- Sandgaard, M.; Palmqvist, A.; Bour, A.; Grønlund, S.N.; Hooge, A.; Selck, H.; Thit, A.; Syberg, K. Sediment matters as a route of microplastic exposure: A call for more research on the benthic compartment. Front. Mar. Sci. 2023, 9, 1100567. [Google Scholar] [CrossRef]
- Setala, O.; Fleming-Lehtinen, V.; Lehtiniemi, M. Ingestion and transfer of microplastics in the planktonic food web. Environ. Pollut. 2014, 185, 77–83. [Google Scholar] [CrossRef]
- Martin, C.; Baalkhuyur, F.; Valluzzi, L.; Saderne, V.; Cusack, M.; Almahasheer, H.; Krishnakumar, P.K.; Rabaoui, L.; Qurban, M.A.; Arias-Ortiz, A.; et al. Exponential increase of plastic burial in mangrove sediments as a major plastic sink. Sci. Adv. 2020, 6, eaaz5593. [Google Scholar] [CrossRef]
- Kane, I.; Clare, M. Dispersion, Accumulation and the Ultimate Fate of Microplastics in Deep-Marine Environments: A Review and Future Directions. Front. Earth Sci. 2019, 7, 80. [Google Scholar] [CrossRef]
- Yadav, S.; Gupta, E.; Patel, A.; Srivastava, S.; Mishra, V.K.; Singh, P.C.; Srivastava, P.K.; Barik, S.K. Unravelling the emerging threats of microplastics to agroecosystems. Rev. Environ. Sci. Bio/Technol. 2022, 21, 771–798. [Google Scholar] [CrossRef]
- Padervand, M.; Lichtfouse, E.; Robert, D.; Wang, C. Removal of microplastics from the environment. A review. Environ. Chem. Lett. 2020, 18, 807–828. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Bakir, A.; Burton, G.A.; Janssen, C.R. Microplastic as a Vector for Chemicals in the Aquatic Environment: Critical Review and Model-Supported Reinterpretation of Empirical Studies. Environ. Sci. Technol. 2016, 50, 3315–3326. [Google Scholar] [CrossRef]
- Mak, C.W.; Yeung, K.C.-F.; Chan, K.M. Acute toxic effects of polyethylene microplastic on adult zebrafish. Ecotoxicol. Environ. Saf. 2019, 182, 109442. [Google Scholar] [CrossRef]
- Bowley, J.; Baker-Austin, C.; Porter, A.; Hartnell, R.; Lewis, C. Oceanic Hitchhikers–Assessing Pathogen Risks from Marine Microplastic. Trends Microbiol. 2021, 29, 107–116. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Besseling, E.; Wegner, A.; Foekema, E.M. Plastic as a Carrier of POPs to Aquatic Organisms: A Model Analysis. Environ. Sci. Technol. 2013, 47, 7812–7820. [Google Scholar] [CrossRef]
- Schwabl, P.; Koeppel, S.; Koenigshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of Various Microplastics in Human Stool A Prospective Case Series. Ann. Intern. Med. 2019, 171, 453–457. [Google Scholar] [CrossRef]
- Wang, W.; Gao, H.; Jin, S.; Li, R.; Na, G. The ecotoxicological effects of microplastics on aquatic food web, from primary producer to human: A review. Ecotoxicol. Environ. Saf. 2019, 173, 110–117. [Google Scholar] [CrossRef]
- Mai, L.; Bao, L.-J.; Shi, L.; Wong, C.S.; Zeng, E.Y. A review of methods for measuring microplastics in aquatic environments. Environ. Sci. Pollut. Res. Int. 2018, 25, 11319–11332. [Google Scholar] [CrossRef]
- Loeder, M.G.J.; Kuczera, M.; Mintenig, S.; Lorenz, C.; Gerdts, G. Focal plane array detector-based micro-Fourier-transform infrared imaging for the analysis of microplastics in environmental samples. Environ. Chem. 2015, 12, 563–581. [Google Scholar] [CrossRef]
- Lenz, R.; Enders, K.; Stedmon, C.A.; Mackenzie, D.M.A.; Nielsen, T.G. A critical assessment of visual identification of marine microplastic using Raman spectroscopy for analysis improvement. Mar. Pollut. Bull. 2015, 100, 82–91. [Google Scholar] [CrossRef]
- Claessens, M.; Van Cauwenberghe, L.; Vandegehuchte, M.B.; Janssen, C.R. New techniques for the detection of microplastics in sediments and field collected organisms. Mar. Pollut. Bull. 2013, 70, 227–233. [Google Scholar] [CrossRef]
- Petersen, J.E.; Cornwell, J.C.; Kemp, W.M. Implicit Scaling in the Design of Experimental Aquatic Ecosystems. Oikos 1999, 85, 3–18. [Google Scholar] [CrossRef]
- Boots, B.; Russell, C.W.; Green, D.S. Effects of Microplastics in Soil Ecosystems: Above and Below Ground. Environ. Sci. Technol. 2019, 53, 11496–11506. [Google Scholar] [CrossRef]
- Sendra, M.; Sparaventi, E.; Novoa, B.; Figueras, A. An overview of the internalization and effects of microplastics and nanoplastics as pollutants of emerging concern in bivalves. Sci. Total Environ. 2021, 753, 142024. [Google Scholar] [CrossRef]
- Yan, J.; Lin, L.; Zhou, W.; Ma, K.; Pickett, S.T.A. A novel approach for quantifying particulate matter distribution on leaf surface by combining SEM and object-based image analysis. Remote Sens. Environ. 2016, 173, 156–161. [Google Scholar] [CrossRef]
- Zhang, T.; Huang, B.; Yan, Y.; Lin, Y.; Wong, H.; Wong, S.Y.-s.; Chung, R.Y.-N. Street-view and traditional greenness metrics with adults’ sitting time in high-density living in Hong Kong: Comparing associations, air pollution and noise roles, and population heterogeneity. Sci. Total Environ. 2023, 870, 161778. [Google Scholar] [CrossRef]
- He, H.; Cheng, X.; Li, X.; Zhu, R.; Hui, F.; Wu, W.; Zhao, T.; Kang, J.; Tang, J. Aerial photography based census of Adelie Penguin and its application in CH4 and N2O budget estimation in Victoria Land, Antarctic. Sci. Rep. 2017, 7, 12942. [Google Scholar] [CrossRef]
- Rizeei, H.M.; Azeez, O.S.; Pradhan, B.; Khamees, H.H. Assessment of groundwater nitrate contamination hazard in a semi-arid region by using integrated parametric IPNOA and data-driven logistic regression models. Environ. Monit. Assess. 2018, 190, 633. [Google Scholar] [CrossRef]
- McCallum, J.B. SigmaScan Pro 5.0. (Brief Article). Science 2000, 289, 412–413. [Google Scholar] [CrossRef]
- Statistica (Data Analysis Software System), Version 13; TIBCO Software Inc.: Palo Alto, CA, USA, 2017.
- Koelmans, A.A.; Mohamed Nor, N.H.; Hermsen, E.; Kooi, M.; Mintenig, S.M.; De France, J. Microplastics in freshwaters and drinking water: Critical review and assessment of data quality. Water Res. 2019, 155, 410–422. [Google Scholar] [CrossRef]
- Ikenoue, T.; Nakajima, R.; Fujiwara, A.; Onodera, J.; Itoh, M.; Toyoshima, J.; Watanabe, E.; Murata, A.; Nishino, S.; Kikuchi, T. Horizontal distribution of surface microplastic concentrations and water-column microplastic inventories in the Chukchi Sea, western Arctic Ocean. Sci. Total Environ. 2023, 855, 159564. [Google Scholar] [CrossRef]
- Naidoo, T.; Glassom, D. Sea-surface microplastic concentrations along the coastal shelf of KwaZulu–Natal, South Africa. Mar. Pollut. Bull. 2019, 149, 110514. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Rocher, V.; Tassin, B. Synthetic and non-synthetic anthropogenic fibers in a river under the impact of Paris Megacity: Sampling methodological aspects and flux estimations. Sci. Total Environ. 2018, 618, 157–164. [Google Scholar] [CrossRef]
- Dahms, H.T.J.; van Rensburg, G.J.; Greenfield, R. The microplastic profile of an urban African stream. Sci. Total Environ. 2020, 731, 138893. [Google Scholar] [CrossRef]
- Talvitie, J.; Heinonen, M.; Pääkkönen, J.P.; Vahtera, E.; Mikola, A.; Setälä, O.; Vahala, R. Do wastewater treatment plants act as a potential point source of microplastics? Preliminary study in the coastal Gulf of Finland, Baltic Sea. Water Sci. Technol. 2015, 72, 1495–1504. [Google Scholar] [CrossRef]
- Harris, P.T. The fate of microplastic in marine sedimentary environments: A review and synthesis. Mar. Pollut. Bull. 2020, 158, 111398. [Google Scholar] [CrossRef]
- Fischer, E.K.; Paglialonga, L.; Czech, E.; Tamminga, M. Microplastic pollution in lakes and lake shoreline sediments—A case study on Lake Bolsena and Lake Chiusi (central Italy). Environ. Pollut. 2016, 213, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, T.; Cabigliera, S.B.; Martellini, T.; Laurati, M.; Chelazzi, D.; Galassi, D.M.P.; Cincinelli, A. Ingestion of microplastics and textile cellulose particles by some meiofaunal taxa of an urban stream. Chemosphere 2023, 310, 136830. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, K.; Zhang, J.; Yan, C.; Lock, T.R.; Kallenbach, R.L.; Yuan, Z. Fractional coverage rather than green chromatic coordinate is a robust indicator to track grassland phenology using smartphone photography. Ecol. Inform. 2022, 68, 101544. [Google Scholar] [CrossRef]
- Cai, F.; Lu, W.; Shi, W.; He, S. A mobile device-based imaging spectrometer for environmental monitoring by attaching a lightweight small module to a commercial digital camera. Sci. Rep. 2017, 7, 15602. [Google Scholar] [CrossRef] [PubMed]
- Cuthbert, I.D.; del Giorgio, P. Toward a standard method of measuring color in freshwater. Limnol. Oceanogr. 1992, 37, 1319–1326. [Google Scholar] [CrossRef]
- Barrows, A.P.W.; Cathey, S.E.; Petersen, C.W. Marine environment microfiber contamination: Global patterns and the diversity of microparticle origins. Environ. Pollut. 2018, 237, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Balan, K.; Santora, M.; Faied, M.; Carmona, V. A Survey of Computational Intelligence Techniques in Ecology and Sustainability. Mich. Acad. 2021, 47, 36. [Google Scholar]

| Pixel Intensity (PI) | No-Slip Conditions | Turbulence Flow Conditions | ||||||
|---|---|---|---|---|---|---|---|---|
| β (PI/ppm) | R2 | b (PI) | p | β (PI/ppm) | R2 | b (PI) | p | |
| Average Red (R) | −0.0074 | 0.04 | 143 | >0.05 | 0.0080 | 0.05 | 141 | >0.05 |
| Average Green (G) | −0.0090 | 0.09 | 145 | >0.05 | 0.0060 | 0.07 | 142 | >0.05 |
| Average Blue (B) | −0.0051 | 0.01 | 159 | >0.05 | 0.0071 | 0.04 | 157 | >0.05 |
| Average RGB | −0.0070 | 0.07 | 150 | >0.05 | 0.0070 | 0.12 | 148 | >0.05 |
| Total RGB | −88,726.9 | 0.29 | 396,370,873 | >0.05 | 35,937.4 | 0.15 | 369,510,089 | >0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carmona-Valdivieso, D.E.; Valdivieso, T.; Carmona-Galindo, V.D. Detection of Secondary Microplastics in an Aquatic Mesocosm by Means of Object-Based Image Analysis. Microplastics 2023, 2, 268-277. https://doi.org/10.3390/microplastics2030022
Carmona-Valdivieso DE, Valdivieso T, Carmona-Galindo VD. Detection of Secondary Microplastics in an Aquatic Mesocosm by Means of Object-Based Image Analysis. Microplastics. 2023; 2(3):268-277. https://doi.org/10.3390/microplastics2030022
Chicago/Turabian StyleCarmona-Valdivieso, Dahlia E., Tizziana Valdivieso, and Víctor D. Carmona-Galindo. 2023. "Detection of Secondary Microplastics in an Aquatic Mesocosm by Means of Object-Based Image Analysis" Microplastics 2, no. 3: 268-277. https://doi.org/10.3390/microplastics2030022
APA StyleCarmona-Valdivieso, D. E., Valdivieso, T., & Carmona-Galindo, V. D. (2023). Detection of Secondary Microplastics in an Aquatic Mesocosm by Means of Object-Based Image Analysis. Microplastics, 2(3), 268-277. https://doi.org/10.3390/microplastics2030022











