Separation of Microplastics from Blood Samples Using Traveling Surface Acoustic Waves
Abstract
:1. Introduction
2. Materials and Methods
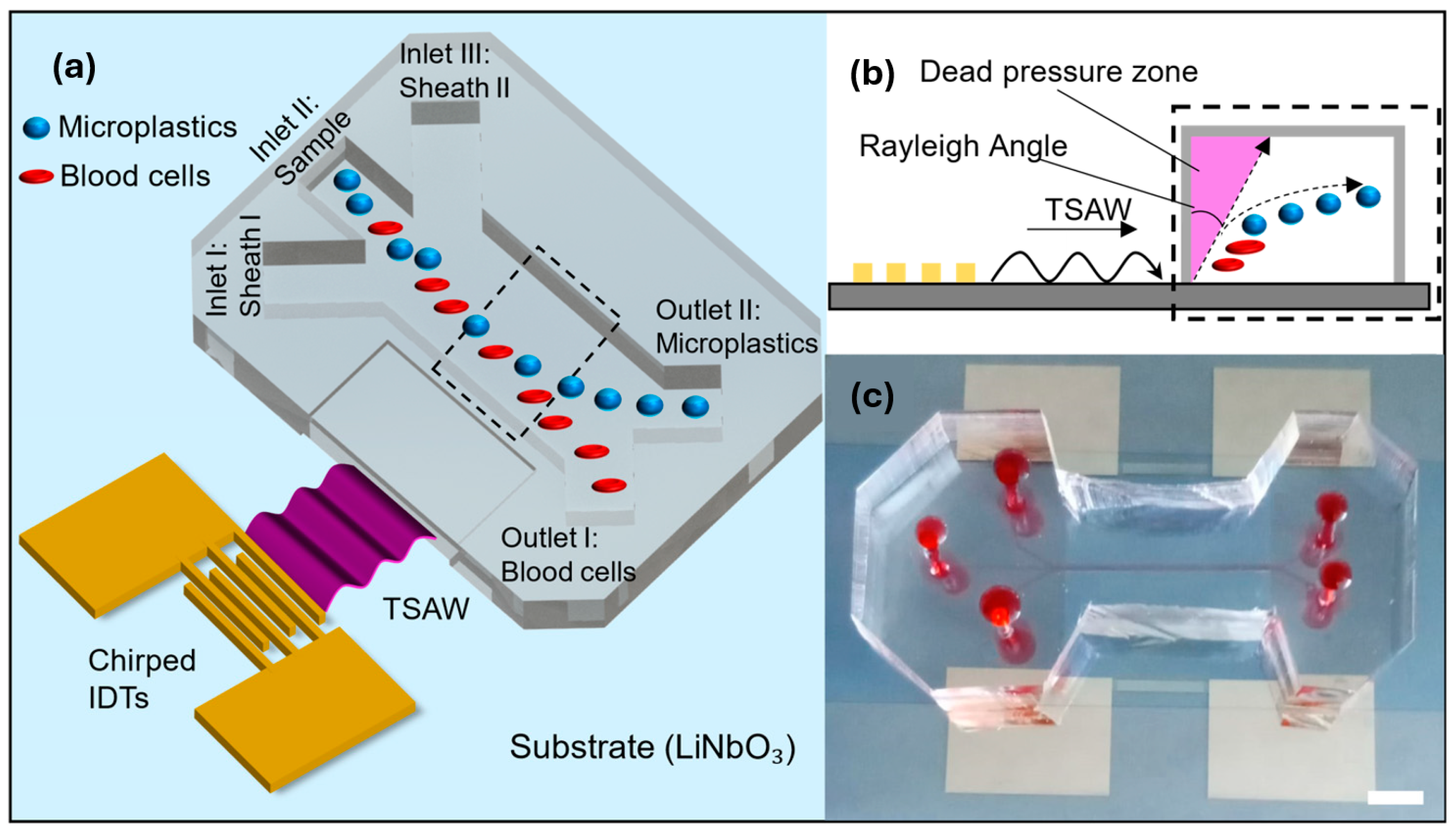
2.1. TSAW Microplastic Separation Mechanism
2.2. Fabrication of the Microfluidic Device
2.3. Microplastics and Blood Preparation
2.4. Separation Quantification and Data Analysis
3. Results and Discussion
3.1. Theoretical Acoustic Radiation Force
3.2. Experimental Determination of ARF

3.3. Separation of Microplastics from Blood Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental Exposure to Microplastics: An Overview on Possible Human Health Effects. Sci. Total Environ. 2020, 702, 134455. [Google Scholar] [CrossRef] [PubMed]
- Dick, V.A.; Juliette, L. Microplastics and Human Health. Science 2021, 371, 672–674. [Google Scholar] [CrossRef]
- Adyel, T.M. Accumulation of Plastic Waste during COVID-19. Science 2020, 369, 1314–1315. [Google Scholar] [CrossRef] [PubMed]
- MacLeod, M.; Arp, H.P.H.; Tekman, M.B.; Jahnke, A. The Global Threat from Plastic Pollution. Science 2021, 373, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Gasperi, J.; Wright, S.L.; Dris, R.; Collard, F.; Mandin, C.; Guerrouache, M.; Langlois, V.; Kelly, F.J.; Tassin, B. Microplastics in Air: Are We Breathing It In? Curr. Opin. Environ. Sci. Health 2018, 1, 1–5. [Google Scholar] [CrossRef]
- Law, K.L.; Thompson, R.C. Microplastics in the Seas. Science 2014, 345, 144–145. [Google Scholar] [CrossRef] [PubMed]
- de Souza Machado, A.A.; Lau, C.W.; Till, J.; Kloas, W.; Lehmann, A.; Becker, R.; Rillig, M.C. Impacts of Microplastics on the Soil Biophysical Environment. Environ. Sci. Technol. 2018, 52, 9656–9665. [Google Scholar] [CrossRef]
- Colmer, J.; Hardman, I.; Shimshack, J.; Voorheis, J. Disparities in PM2. 5 Air Pollution in the United States. Science 2020, 369, 575–578. [Google Scholar]
- Silva, A.L.P.; Prata, J.C.; Walker, T.R.; Duarte, A.C.; Ouyang, W.; Barcelò, D.; Rocha-Santos, T. Increased Plastic Pollution Due to COVID-19 Pandemic: Challenges and Recommendations. Chem. Eng. J. 2021, 405, 126683. [Google Scholar] [CrossRef]
- Rochman, C.M.; Hoellein, T. The Global Odyssey of Plastic Pollution. Science 2020, 368, 1184–1185. [Google Scholar] [CrossRef]
- Cózar, A.; Echevarría, F.; González-Gordillo, J.I.; Irigoien, X.; Úbeda, B.; Hernández-León, S.; Palma, Á.T.; Navarro, S.; García-de-Lomas, J.; Ruiz, A. Plastic Debris in the Open Ocean. Proc. Natl. Acad. Sci. USA 2014, 111, 10239–10244. [Google Scholar] [CrossRef]
- Fok, L.; Cheung, P.K.; Tang, G.; Li, W.C. Size Distribution of Stranded Small Plastic Debris on the Coast of Guangdong, South China. Environ. Pollut. 2017, 220, 407–412. [Google Scholar] [CrossRef]
- Pedrotti, M.L.; Petit, S.; Elineau, A.; Bruzaud, S.; Crebassa, J.-C.; Dumontet, B.; Martí, E.; Gorsky, G.; Cózar, A. Changes in the Floating Plastic Pollution of the Mediterranean Sea in Relation to the Distance to Land. PLoS ONE 2016, 11, e0161581. [Google Scholar] [CrossRef]
- Rahman, A.; Sarkar, A.; Yadav, O.P.; Achari, G.; Slobodnik, J. Potential Human Health Risks Due to Environmental Exposure to Nano- and Microplastics and Knowledge Gaps: A Scoping Review. Sci. Total Environ. 2021, 757, 143872. [Google Scholar] [CrossRef]
- Rillig, M.C.; Lehmann, A. Microplastic in Terrestrial Ecosystems. Science 2020, 368, 1430–1431. [Google Scholar] [CrossRef]
- Cox, K.D.; Covernton, G.A.; Davies, H.L.; Dower, J.F.; Juanes, F.; Dudas, S.E. Human Consumption of Microplastics. Environ. Sci. Technol. 2019, 53, 7068–7074. [Google Scholar] [CrossRef]
- Liebezeit, G.; Liebezeit, E. Non-Pollen Particulates in Honey and Sugar. Food Addit. Contam. Part A 2013, 30, 2136–2140. [Google Scholar] [CrossRef]
- Neves, D.; Sobral, P.; Ferreira, J.L.; Pereira, T. Ingestion of Microplastics by Commercial Fish off the Portuguese Coast. Mar. Pollut. Bull. 2015, 101, 119–126. [Google Scholar] [CrossRef]
- Karami, A.; Golieskardi, A.; Keong Choo, C.; Larat, V.; Galloway, T.S.; Salamatinia, B. The Presence of Microplastics in Commercial Salts from Different Countries. Sci. Rep. 2017, 7, 46173. [Google Scholar] [CrossRef]
- Schwabl, P. Microplastics in Hot Water. Nat. Food 2020, 1, 671–672. [Google Scholar] [CrossRef]
- Oßmann, B.E.; Sarau, G.; Holtmannspötter, H.; Pischetsrieder, M.; Christiansen, S.H.; Dicke, W. Small-Sized Microplastics and Pigmented Particles in Bottled Mineral Water. Water Res. 2018, 141, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C. Airborne Microplastics: Consequences to Human Health? Environ. Pollut. 2018, 234, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Vianello, A.; Jensen, R.L.; Liu, L.; Vollertsen, J. Simulating Human Exposure to Indoor Airborne Microplastics Using a Breathing Thermal Manikin. Sci. Rep. 2019, 9, 8670. [Google Scholar] [CrossRef] [PubMed]
- Schwabl, P.; Köppel, S.; Königshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of Various Microplastics in Human Stool. Ann. Intern. Med. 2019, 171, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First Evidence of Microplastics in Human Placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef] [PubMed]
- Horvatits, T.; Tamminga, M.; Liu, B.; Sebode, M.; Carambia, A.; Fischer, L.; Püschel, K.; Huber, S.; Fischer, E.K. Microplastics Detected in Cirrhotic Liver Tissue. EBioMedicine 2022, 82, 104147. [Google Scholar] [CrossRef]
- Huang, S.; Huang, X.; Bi, R.; Guo, Q.; Yu, X.; Zeng, Q.; Huang, Z.; Liu, T.; Wu, H.; Chen, Y. Detection and Analysis of Microplastics in Human Sputum. Environ. Sci. Technol. 2022, 56, 2476–2486. [Google Scholar] [CrossRef]
- Schirinzi, G.F.; Pérez-Pomeda, I.; Sanchís, J.; Rossini, C.; Farré, M.; Barceló, D. Cytotoxic Effects of Commonly Used Nanomaterials and Microplastics on Cerebral and Epithelial Human Cells. Environ. Res. 2017, 159, 579–587. [Google Scholar] [CrossRef]
- Wu, B.; Wu, X.; Liu, S.; Wang, Z.; Chen, L. Size-Dependent Effects of Polystyrene Microplastics on Cytotoxicity and Efflux Pump Inhibition in Human Caco-2 Cells. Chemosphere 2019, 221, 333–341. [Google Scholar] [CrossRef]
- Prüst, M.; Meijer, J.; Westerink, R.H.S. The Plastic Brain: Neurotoxicity of Micro- and Nanoplastics. Part. Fibre Toxicol. 2020, 17, 24. [Google Scholar] [CrossRef]
- Ribeiro, F.; Garcia, A.R.; Pereira, B.P.; Fonseca, M.; Mestre, N.C.; Fonseca, T.G.; Ilharco, L.M.; Bebianno, M.J. Microplastics Effects in Scrobicularia Plana. Mar. Pollut. Bull. 2017, 122, 379–391. [Google Scholar] [CrossRef]
- Rubio, L.; Barguilla, I.; Domenech, J.; Marcos, R.; Hernández, A. Biological Effects, Including Oxidative Stress and Genotoxic Damage, of Polystyrene Nanoparticles in Different Human Hematopoietic Cell Lines. J. Hazard. Mater. 2020, 398, 122900. [Google Scholar] [CrossRef]
- Sun, T.; Zhan, J.; Li, F.; Ji, C.; Wu, H. Evidence-Based Meta-Analysis of the Genotoxicity Induced by Microplastics in Aquatic Organisms at Environmentally Relevant Concentrations. Sci. Total Environ. 2021, 783, 147076. [Google Scholar] [CrossRef]
- Wei, Y.; Zhou, Y.; Long, C.; Wu, H.; Hong, Y.; Fu, Y.; Wang, J.; Wu, Y.; Shen, L.; Wei, G. Polystyrene Microplastics Disrupt the Blood-Testis Barrier Integrity through ROS-Mediated Imbalance of MTORC1 and MTORC2. Environ. Pollut. 2021, 289, 117904. [Google Scholar] [CrossRef]
- Leslie, H.A.; van Velzen, M.J.M.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and Quantification of Plastic Particle Pollution in Human Blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef]
- Vethaak, A.D.; Leslie, H.A. Plastic Debris Is a Human Health Issue. Environ. Sci. Technol. 2016, 50, 6825–6826. [Google Scholar] [CrossRef]
- Kutralam-Muniasamy, G.; Shruti, V.C.; Pérez-Guevara, F.; Roy, P.D. Microplastic Diagnostics in Humans:“The 3Ps” Progress, Problems, and Prospects. Sci. Total Environ. 2022, 159164. [Google Scholar] [CrossRef]
- Fu, W.; Min, J.; Jiang, W.; Li, Y.; Zhang, W. Separation, Characterization and Identification of Microplastics and Nanoplastics in the Environment. Sci. Total Environ. 2020, 721, 137561. [Google Scholar] [CrossRef]
- Li, S.; Ma, F.; Bachman, H.; Cameron, C.E.; Zeng, X.; Huang, T.J. Acoustofluidic Bacteria Separation. J. Micromech. Microeng. 2016, 27, 15031. [Google Scholar] [CrossRef]
- Antfolk, M.; Magnusson, C.; Augustsson, P.; Lilja, H.; Laurell, T. Acoustofluidic, Label-Free Separation and Simultaneous Concentration of Rare Tumor Cells from White Blood Cells. Anal. Chem. 2015, 87, 9322–9328. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, X.; Ren, J.; Lin, F.; Wu, J. Recent Advances in Acoustofluidic Separation Technology in Biology. Microsyst. Nanoeng. 2022, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Ozcelik, A.; Rufo, J.; Wang, Z.; Fang, R.; Jun Huang, T. Acoustofluidic Separation of Cells and Particles. Microsyst. Nanoeng. 2019, 5, 32. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wu, M.; Lin, Y.; Xu, J. Acoustic Microfluidic Separation Techniques and Bioapplications: A Review. Micromachines 2020, 11, 921. [Google Scholar] [CrossRef] [PubMed]
- Destgeer, G.; Lee, K.H.; Jung, J.H.; Alazzam, A.; Sung, H.J. Continuous Separation of Particles in a PDMS Microfluidic Channel via Travelling Surface Acoustic Waves (TSAW). Lab Chip 2013, 13, 4210–4216. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Wu, M.; Yang, S.; Wu, Y.; Gu, Y.; Chen, C.; Ye, J.; Xie, Z.; Tian, Z.; Bachman, H. A Disposable Acoustofluidic Chip for Nano/Microparticle Separation Using Unidirectional Acoustic Transducers. Lab Chip 2020, 20, 1298–1308. [Google Scholar] [CrossRef]
- Devendran, C.; Gunasekara, N.R.; Collins, D.J.; Neild, A. Batch Process Particle Separation Using Surface Acoustic Waves (SAW): Integration of Travelling and Standing SAW. RSC Adv. 2016, 6, 5856–5864. [Google Scholar] [CrossRef]
- Ma, Z.; Collins, D.J.; Ai, Y. Detachable Acoustofluidic System for Particle Separation via a Traveling Surface Acoustic Wave. Anal. Chem. 2016, 88, 5316–5323. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Collins, D.J.; Guo, J.; Ai, Y. Mechanical Properties Based Particle Separation via Traveling Surface Acoustic Wave. Anal. Chem. 2016, 88, 11844–11851. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Li, P.; Lin, S.-C.S.; Stratton, Z.S.; Nama, N.; Guo, F.; Slotcavage, D.; Mao, X.; Shi, J.; Costanzo, F. Surface Acoustic Wave Microfluidics. Lab Chip 2013, 13, 3626–3649. [Google Scholar] [CrossRef]
- Destgeer, G.; Ha, B.H.; Jung, J.H.; Sung, H.J. Submicron Separation of Microspheres via Travelling Surface Acoustic Waves. Lab Chip 2014, 14, 4665–4672. [Google Scholar] [CrossRef]
- Fakhfouri, A.; Devendran, C.; Ahmed, A.; Soria, J.; Neild, A. The Size Dependant Behaviour of Particles Driven by a Travelling Surface Acoustic Wave (TSAW). Lab Chip 2018, 18, 3926–3938. [Google Scholar] [CrossRef] [PubMed]
- Weng, H.; Duan, F.L.; Xie, Z.; Liu, S.; Ji, Z.; Zhang, Y. LiNbO 3-Based SAW Sensors Capable to Measure up to 1100° C High Temperature. IEEE Sens. J. 2020, 20, 12679–12683. [Google Scholar] [CrossRef]
- Duan, F.L.; Xie, Z.; Ji, Z. Breakthrough of Upper Limit of Temperature Measurement of SAW Sensors for Wireless Passive Sensing inside Propulsion System. In Proceedings of the AIAA Propulsion and Energy 2020 Forum, Online, 24–28 August 2020; p. 3512. [Google Scholar]
- Freudenberg, J.; Von Schickfus, M.; Hunklinger, S. A SAW Immunosensor for Operation in Liquid Using a SiO2 Protective Layer. Sens. Actuators B Chem. 2001, 76, 147–151. [Google Scholar] [CrossRef]
- Xia, Y.; Whitesides, G.M. Soft Lithography. Annu. Rev. Mater. Sci. 1998, 28, 153–184. [Google Scholar] [CrossRef]
- Amin, R.; Knowlton, S.; Hart, A.; Yenilmez, B.; Ghaderinezhad, F.; Katebifar, S.; Messina, M.; Khademhosseini, A.; Tasoglu, S. 3D-Printed Microfluidic Devices. Biofabrication 2016, 8, 22001. [Google Scholar] [CrossRef] [PubMed]
- Bourquin, Y.; Reboud, J.; Wilson, R.; Cooper, J.M. Tuneable Surface Acoustic Waves for Fluid and Particle Manipulations on Disposable Chips. Lab Chip 2010, 10, 1898–1901. [Google Scholar] [CrossRef] [PubMed]
- Reboud, J.; Bourquin, Y.; Wilson, R.; Pall, G.S.; Jiwaji, M.; Pitt, A.R.; Graham, A.; Waters, A.P.; Cooper, J.M. Shaping Acoustic Fields as a Toolset for Microfluidic Manipulations in Diagnostic Technologies. Proc. Natl. Acad. Sci. USA 2012, 109, 15162–15167. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, R.K.; Elder, J.B.; Tuchin, V. V Effect of Dextran-Induced Changes in Refractive Index and Aggregation on Optical Properties of Whole Blood. Phys. Med. Biol. 2003, 48, 1205. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Begum, H.; Lee, J.E.-Y. Acoustofluidic Localization of Sparse Particles on a Piezoelectric Resonant Sensor for Nanogram-Scale Mass Measurements. Microsyst. Nanoeng. 2021, 7, 61. [Google Scholar] [CrossRef]
- Wang, K.; Zhou, W.; Lin, Z.; Cai, F.; Li, F.; Wu, J.; Meng, L.; Niu, L.; Zheng, H. Sorting of Tumour Cells in a Microfluidic Device by Multi-Stage Surface Acoustic Waves. Sens. Actuators B Chem. 2018, 258, 1174–1183. [Google Scholar] [CrossRef]
- Hasegawa, T.; Yosioka, K. Acoustic-radiation Force on a Solid Elastic Sphere. J. Acoust. Soc. Am. 1969, 46, 1139–1143. [Google Scholar] [CrossRef]
- Sinha, M.; Buckley, D.J. Acoustic Properties of Polymers. In Physical Properties of Polymers Handbook; Springer: Berlin/Heidelberg, Germany, 2007; pp. 1021–1031. [Google Scholar]
- Carvalho, M.R.; Barata, D.; Teixeira, L.M.; Giselbrecht, S.; Reis, R.L.; Oliveira, J.M.; Truckenmüller, R.; Habibovic, P. Colorectal Tumor-on-a-Chip System: A 3D Tool for Precision Onco-Nanomedicine. Sci. Adv. 2019, 5, eaaw1317. [Google Scholar] [CrossRef] [PubMed]
- Cushing, K.W.; Garofalo, F.; Magnusson, C.; Ekblad, L.; Bruus, H.; Laurell, T. Ultrasound Characterization of Microbead and Cell Suspensions by Speed of Sound Measurements of Neutrally Buoyant Samples. Anal. Chem. 2017, 89, 8917–8923. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Pye, S.D.; Browne, J.E.; Janeczko, A.; Ellis, B.; Butler, M.B.; Sboros, V.; Thomson, A.J.W.; Brewin, M.P.; Earnshaw, C.H. The Speed of Sound and Attenuation of an IEC Agar-Based Tissue-Mimicking Material for High Frequency Ultrasound Applications. Ultrasound Med. Biol. 2012, 38, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- Fung, Y.-C.; Fung, Y.-C. Bioviscoelastic Solids. In Biomechanics: Mechanical Properties of Living Tissues; Springer Science & Business Media: Berlin, Germany, 1993; pp. 242–320. [Google Scholar]
- King, L.V. On the Acoustic Radiation Pressure on Spheres. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1934, 147, 212–240. [Google Scholar]
- Leung, E.; Lee, C.P.; Jacobi, N.; Wang, T.G. Resonance Frequency Shift of an Acoustic Chamber Containing a Rigid Sphere. J. Acoust. Soc. Am. 1982, 72, 615–620. [Google Scholar] [CrossRef]
- Chanaud, R.C. Effects of Geometry on the Resonance Frequency of Helmholtz Resonators. J. Sound. Vib. 1994, 178, 337–348. [Google Scholar] [CrossRef]


| Acronym | Poly- | Density (kg/m3) | (m/s) | (m/s) |
|---|---|---|---|---|
| ABS | Acrylonitrile-butadiene-styrene | 1041 | 2160 | 930 |
| Epoxy | DGEBA/PDA | 1184 | 2890 | 1290 |
| Nylon | Hexamethylene adipamide | 1147 | 2710 | 1120 |
| PC | Carbonate | 1194 | 2220 | 909 |
| PE | Ethylene | 957 | 2430 | 950 |
| PMMA | Methyl methacrylate | 1191 | 2690 | 1340 |
| PP | Propylene | 913 | 2650 | 1300 |
| PS | Styrene | 1052 | 2400 | 1150 |
| PVC | Vinyl chloride | 1386 | 2330 | 1070 |
| Teflon® | Tetrafluoroethylene | 2180 | 1410 | 730 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mesquita, P.; Lin, Y.; Gong, L.; Schwartz, D. Separation of Microplastics from Blood Samples Using Traveling Surface Acoustic Waves. Microplastics 2024, 3, 449-462. https://doi.org/10.3390/microplastics3030028
Mesquita P, Lin Y, Gong L, Schwartz D. Separation of Microplastics from Blood Samples Using Traveling Surface Acoustic Waves. Microplastics. 2024; 3(3):449-462. https://doi.org/10.3390/microplastics3030028
Chicago/Turabian StyleMesquita, Pedro, Yang Lin, Liyuan Gong, and Daniel Schwartz. 2024. "Separation of Microplastics from Blood Samples Using Traveling Surface Acoustic Waves" Microplastics 3, no. 3: 449-462. https://doi.org/10.3390/microplastics3030028
APA StyleMesquita, P., Lin, Y., Gong, L., & Schwartz, D. (2024). Separation of Microplastics from Blood Samples Using Traveling Surface Acoustic Waves. Microplastics, 3(3), 449-462. https://doi.org/10.3390/microplastics3030028







