Seasonal and Distributional Changes in the Composition and Flux of Anthropogenic Microparticles in the Surface Waters of the Charles River, Massachusetts, United States
Abstract
1. Introduction
- Determine the concentration and composition of anthropogenic microparticles (microplastics and microfibers) in the Charles River;
- Determine if the concentration or composition of the anthropogenic microparticles varied along the length of the river and between different sampling times, as well as if the variations were correlated to adjacent land use and impervious cover;
- Estimate the export flux of anthropogenic microparticles into the Boston Harbor via the Charles River.
2. Materials and Methods
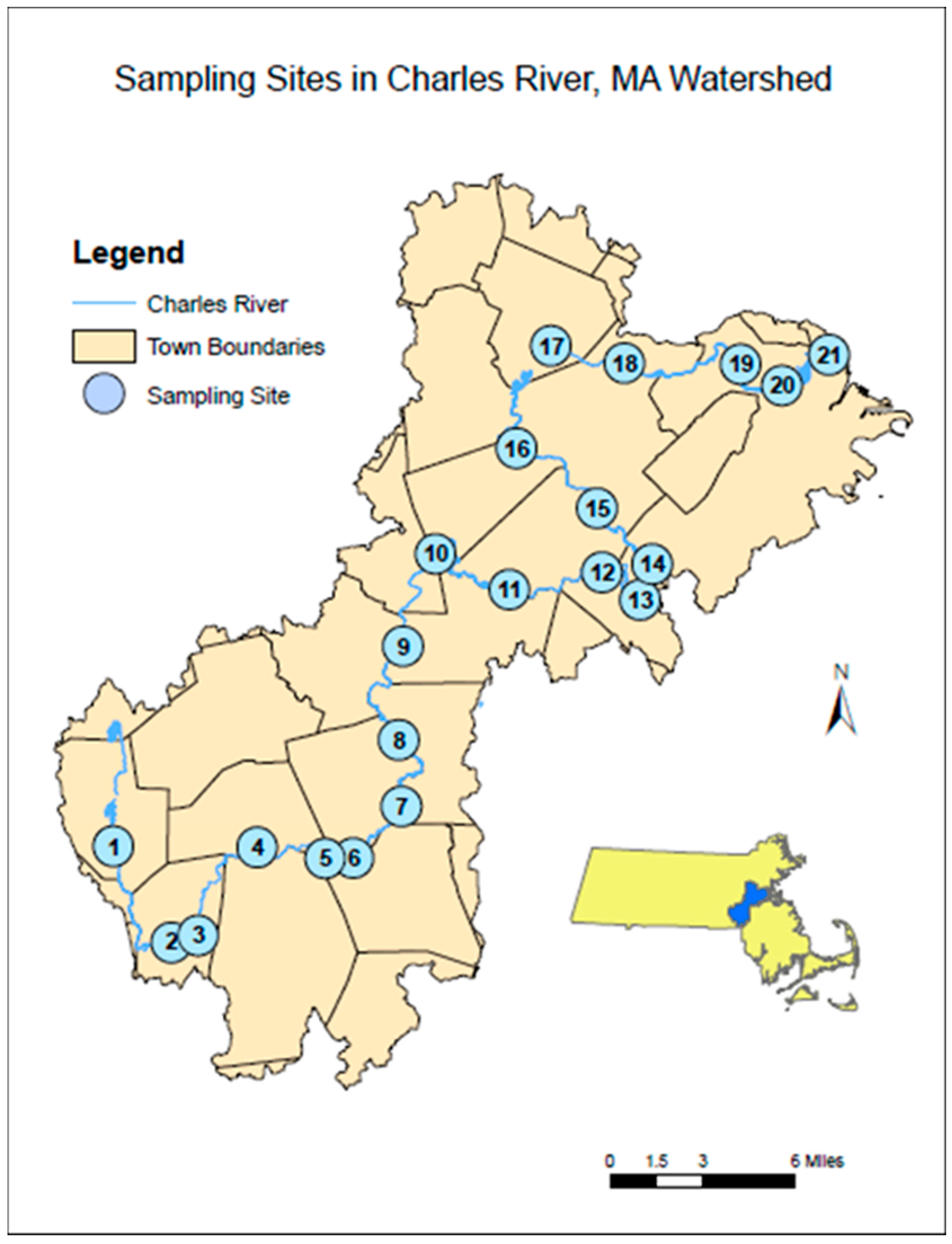
2.1. Charles River Sampling
2.2. Processing
2.3. Blanks
2.4. Verification
2.5. Impervious Cover and Land Use
2.6. Data Analysis
2.7. Estimation of Export Flux
3. Results
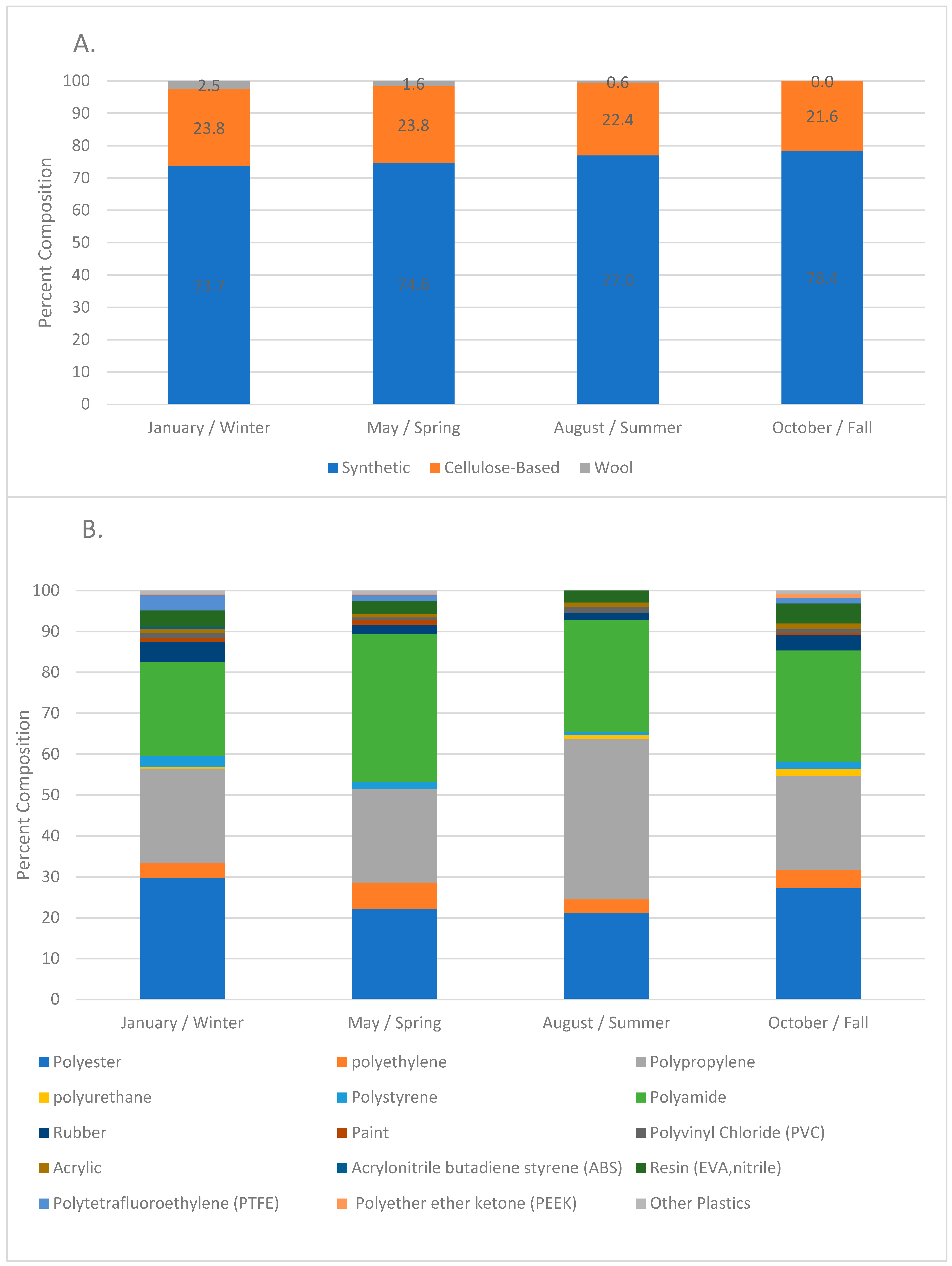
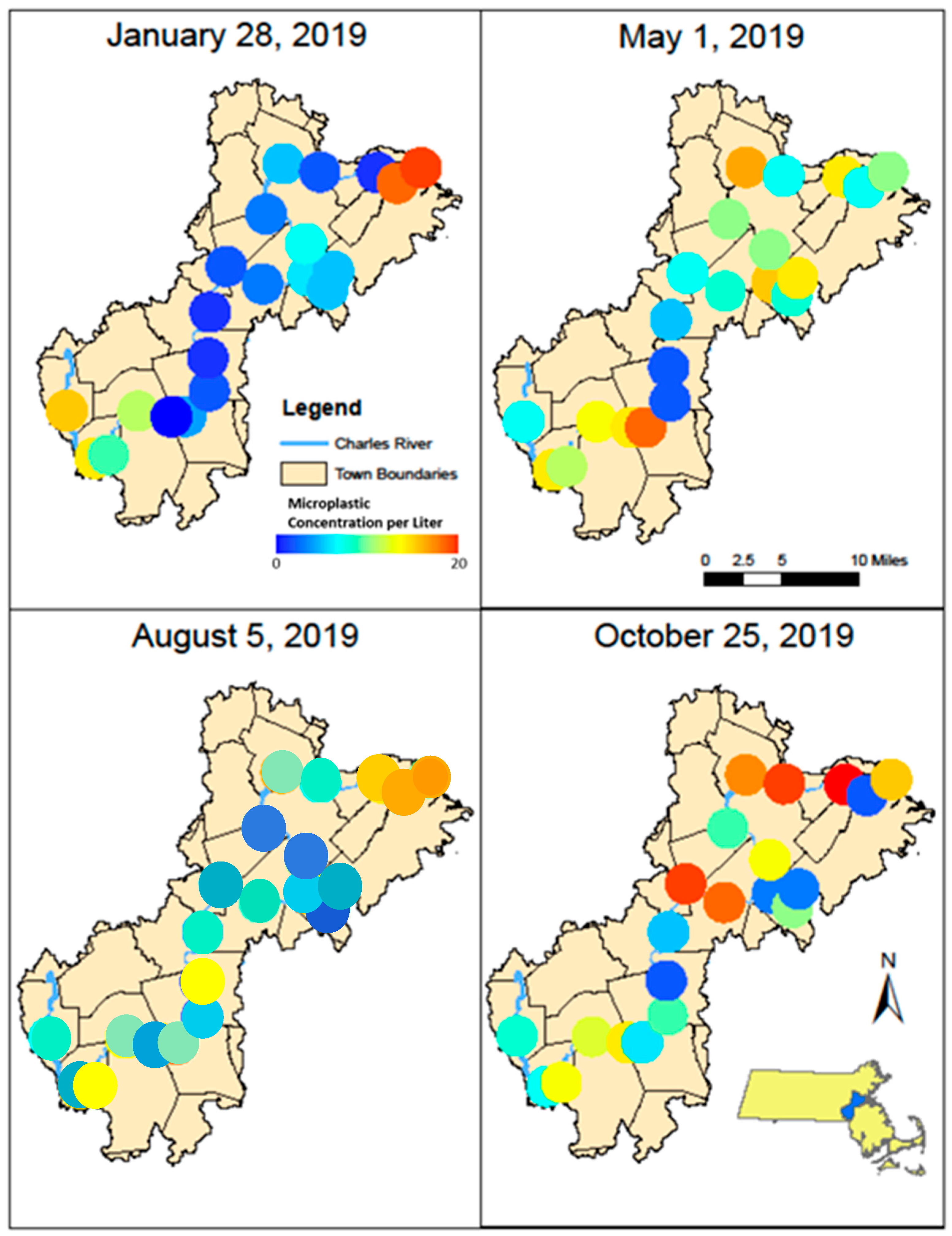
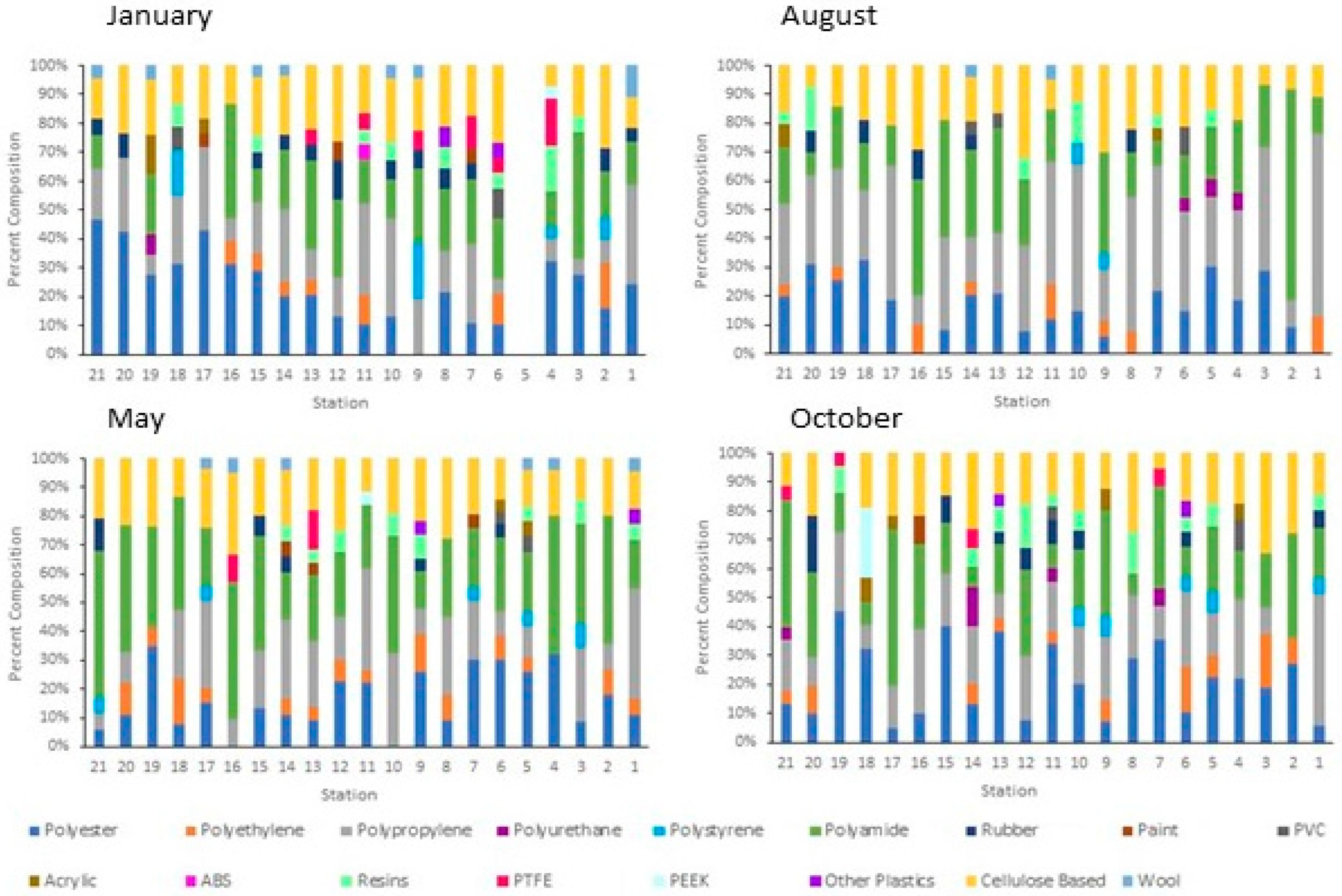
3.1. Anthropogenic Microparticle (MP) Abundance and Composition
3.2. Relationship to Impervious Coverage and Land-Use
3.3. Estimated Export Flux of Anthropogenic Microparticles into Boston Harbor
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| 28 January 2019 | 1 May 2019 | 5 August 2019 | 25 October 2019 | |||||
| Site # GPS Coordinates | Depth (cm) | Temp (°C) | Depth (cm) | Temp (°C) | Depth (cm) | Temp (°C) | Depth (cm) | Temp (°C) |
| 1 (−71.512, 42.139) | 64 | 5.3 | 48 | 13.3 | 25 | 27.3 | 30 | 14 |
| 2 (−71.476, 42.094) | 140 | 5.4 | 107 | 14 | 64 | 26.7 | 36 | 12.8 |
| 3 (−71.459, 42.097) | 112 | 5.8 | 102 | 14.6 | 79 | 25.3 | 86 | 13.2 |
| 4 (−71.422, 42.139) | 239 | 2.1 | 152 | 13.4 | 86 | 24.6 | 124 | 11.9 |
| 5 (−71.379, 42.133) | ND | ND | 89 | 14.7 | 109 | 29.1 | 84 | 13.6 |
| 6 (−71.362, 42.134) | 119 | 5.2 | 102 | 13.3 | 470 | 25.8 | 30 | 12.5 |
| 7 (−71.332, 42.158) | 267 | 4.2 | 264 | 13.3 | 155 | 25 | 163 | 11.7 |
| 8 (−71.333, 42.189) | 356 | 3.4 | 358 | 14.1 | 239 | 26.7 | 231 | 11.7 |
| 9 (−71.33, 42.233) | 290 | 3.5 | 310 | 13.8 | 173 | 28.2 | 163 | 12.7 |
| 10 (−71.31, 42.276) | 305 | 3.4 | 312 | 13.9 | 107 | 26.3 | 163 | 12 |
| 11 (−71.263, 42.259) | 330 | 3.7 | 351 | 13.8 | 267 | 26.7 | 320 | 12.2 |
| 12 (−71.205, 42.267) | 244 | 3.3 | 193 | 13.8 | 107 | 25.4 | 140 | 11.9 |
| 13 (−71.181, 42.254) | 229 | 3.3 | 226 | 13.9 | 147 | 26.2 | 137 | 11.9 |
| 14 (−71.173, 42.271) | 323 | 3.2 | 272 | 13.8 | 257 | 25.9 | 216 | 11.5 |
| 15 (−71.208, 42.297) | 272 | 2.9 | 251 | 13.3 | 191 | 26.7 | 185 | 11.8 |
| 16 (−71.259, 42.325) | 168 | 2.8 | 272 | 13.4 | 226 | 25.6 | 46 | 12.4 |
| 17 (−71.237, 42.373) | 284 | 2.6 | 216 | 13.6 | 244 | 26.7 | 244 | 12.6 |
| 18 (−71.19, 42.365) | 142 | 2.5 | 160 | 13.5 | 74 | 25.2 | 66 | 12.1 |
| 19 (−71.117, 42.364) | 384 | 2.5 | 480 | 13.5 | 508 | 26.1 | 508 | 12.6 |
| 20 (−71.091, 42.354) | 137 | 2.5 | 178 | 14 | 224 | 25.5 | 198 | 12.6 |
| 21 (−71.061, 42.369) | 800 | 2.2 | 742 | 13.9 | 762 | 25.7 | 777 | 12.6 |
References
- Barnes, D.K.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1985–1998. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cera, A.; Cesarini, G.; Scalici, M. Microplastics in Freshwater: What Is the News from the World? Diversity 2020, 12, 276. [Google Scholar] [CrossRef]
- Li, C.; Busquets, R.; Campos, L.C. Assessment of microplastics in freshwater systems: A review. Sci. Total Environ. 2020, 707, 135578. [Google Scholar] [CrossRef] [PubMed]
- Talbot, R.; Chang, H. Microplastics in freshwater: A global review of factors affecting spatial and temporal variations. Environ. Pollut. 2022, 292, 118393. [Google Scholar] [CrossRef]
- Wu, P.; Huang, J.; Zheng, Y.; Yang, Y.; Zhang, Y.; He, F.; Chen, H.; Quan, G.; Yan, J.; Li, T.; et al. Environmental occurrences, fate, and impacts of microplastics. Ecotoxicol. Environ. Saf. 2019, 184, 109612. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhenyi, J.; Zheng, G.; Chen, L.; Zhang, Q.; Su, B.; Zhou, S. Microplastics in agricultural soils on the coastal plain: Spatial characteristics, influencing factors and sources. Sci. Total Environ. 2023, 901, 165948. [Google Scholar] [CrossRef]
- Mendrik, F.; Houseago, R.C.; Hackney, C.R.; Parsons, D.R. Microplastic trapping efficiency and hydrodynamics in model coral reefs: A physical experimental investigation. Environ. Pollut. 2023, 342, 123094. [Google Scholar] [CrossRef] [PubMed]
- Van Emmerik, T.; Strady, E.; Kieu-Le, T.C.; Nguyen, L.; Gratiot, N. Seasonality of riverine macroplastic transport. Sci. Rep. 2019, 9, 13549. [Google Scholar] [CrossRef]
- Van Emmerik, T.; Mellink, Y.; Hauk, R.; Waldschläger, K.; Schreyers, L. Rivers as plastic reservoirs. Front. Water 2022, 3, 212. [Google Scholar] [CrossRef]
- Strokal, M.; Vriend, P.; Bak, M.P.; Kroeze, C.; van Wijnen, J.; van Emmerik, T. River export of macro- and microplastics to seas by sources worldwide. Nat. Commun. 2023, 14, 4842. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lebreton, L.C.M.; van der Zwet, J.; Damsteeg, J.-W.; Slat, B.; Andrady, A.; Reisser, J. River plastic emissions to the world’s oceans. Nat. Commun. 2017, 8, 15611. [Google Scholar] [CrossRef]
- Van der Wal, M.; van der Meulen, M.; Tweehuijsen, G.; Peterlin, M.; Palatinus, A.; Kovac Virsek, M.; Coscia, L.; Krzan, A. SFRA0025: Identification and Assessment of Riverine Input of (Marine) Litter. Final Report for the European Commission DG Environment; Ifremer: Plouzané, France, 2015. [Google Scholar]
- Weideman, E.A.; Perold, V.; Ryan, P.G. Limited long-distance transport of plastic pollution by the Orange-Vaal River system, South Africa. Sci. Total Environ. 2020, 727, 138653. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.Z.; Watts, A.J.R.; Winslow, B.O.; Galloway, T.S.; Barrows, A.P.W. Mountains to the sea: River study of plastic and non-plastic microfiber pollution in the northeast USA. Mar. Pollut. Bull. 2017, 124, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Vermaire, J.C.; Pomeroy, C.; Herczegh, S.M.; Haggart, O.; Murphy, M. Microplastic abundance and distribution in the open water and sediment of the Ottawa River, Canada, and its tributaries. Facets 2017, 2, 301–314. [Google Scholar] [CrossRef]
- Baldwin, A.K.; Corsi, S.R.; Mason, S.A. Plastic Debris in 29 Great Lakes Tributaries: Relations to Watershed Attributes and Hydrology. Environ. Sci. Technol. 2016, 50, 10377–10385. [Google Scholar] [CrossRef]
- Kunz, A.; Schneider, F.; Anthony, N.; Lin, H.T. Microplastics in rivers along an urban-rural gradient in an urban agglomeration: Correlation with land use, potential sources and pathways. Environ. Pollut. 2023, 321, 121096. [Google Scholar] [CrossRef]
- He, B.; Goonetilleke, A.; Ayoko, G.A.; Rintoul, L. Abundance, distribution patterns, and identification of microplastics in Brisbane River sediments, Australia. Sci. Total Environ. 2020, 700, 134467. [Google Scholar] [CrossRef]
- Townsend, K.R.; Lu, H.-C.; Sharley, D.J.; Pettigrove, V. Associations between microplastic pollution and land use in urban wetland sediments. Environ. Sci. Pollut. Res. 2019, 26, 22551–22561. [Google Scholar] [CrossRef]
- Yonkos, L.T.; Friedel, E.A.; Perez-Reyes, A.C.; Ghosal, S.; Arthur, C.D. Microplastics in Four Estuarine Rivers in the Chesapeake Bay, U.S.A. Environ. Sci. Technol. 2014, 48, 14195–14202. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.; Tan, Q.; Qin, H.; Wang, D.; Cai, Y.; Zhang, J. Sequestration and export of microplastics in urban river sediments. Environ. Int. 2023, 181, 108265. [Google Scholar] [CrossRef] [PubMed]
- Mani, T.; Burkhardt-Holm, P. Seasonal microplastics variation in nival and pluvial stretches of the Rhine River—From the Swiss catchment towards the North Sea. Sci. Total Environ. 2020, 707, 135579. [Google Scholar] [CrossRef] [PubMed]
- Kye, H.; Kim, J.; Ju, S.; Lee, J.; Lim, C.; Yoon, Y. Microplastics in water systems: A review of their impacts on the environment and their potential hazards. Heliyon 2023, 9, e14359. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kummu, M.; de Moel, H.; Ward, P.J.; Varis, O. How Close Do We Live to Water? A Global Analysis of Population Distance to Freshwater Bodies. PLoS ONE 2011, 6, e20578. [Google Scholar] [CrossRef]
- Anderson, E.P.; Jackson, S.; Tharme, R.E.; Douglas, M.; Flotemersch, J.E.; Zwarteveen, M.; Lokgariwar, C.; Montoya, M.; Wali, A.; Tipa, G.T.; et al. Understanding rivers and their social relations: A critical step to advance environmental water management. WIREs Water 2019, 6, e1381. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Jawitz, J.W. The evolution of human population distance to water in the USA from 1790 to 2010. Nat. Commun. 2019, 10, 430. [Google Scholar] [CrossRef]
- Barrows, A.P.W.; Cathey, S.E.; Petersen, C.W. Marine environment microfiber contamination: Global patterns and the diversity of microparticle origins. Environ. Pollut. 2018, 237, 275–284. [Google Scholar] [CrossRef]
- Wiggin, K.J.; Holland, E.B. Validation and application of cost and time effective methods for the detection of 3–500 μm sized microplastics in the urban marine and estuarine environments surrounding Long Beach, California. Mar. Pollut. Bull. 2019, 143, 152–162. [Google Scholar] [CrossRef]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the Marine Environment: A Review of the Methods Used for Identification and Quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef] [PubMed]
- Sierra, I.; Chialanza, M.R.; Faccio, R.; Carrizo, D.; Fornaro, L.; Pérez-Parada, A. Identification of microplastics in wastewater samples by means of polarized light optical microscopy. Environ. Sci. Pollut. Res. 2020, 27, 7409–7419. [Google Scholar] [CrossRef]
- Cowger, W.; Steinmetz, Z.; Gray, A.; Munno, K.; Lynch, J.; Hapich, H.; Primpke, S.; De Frond, H.; Rochman, C.; Herodotou, O. Microplastic Spectral Classification Needs an Open Source Community: Open Specy to the Rescue! Anal. Chem. 2021, 93, 7543–7548. [Google Scholar] [CrossRef] [PubMed]
- Allan, J. Landscapes and riverscapes: The influence of land use on stream ecosystems. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 257–284. [Google Scholar] [CrossRef]
- Dris, R.; Imhof, H.; Sanchez, W.; Gasperi, J.; Galgani, F.; Tassin, B.; Laforsch, C. Beyond the ocean: Contamination of freshwater ecosystems with (micro-)plastic particles. Environ. Chem. 2015, 12, 539–550. [Google Scholar] [CrossRef]
- Kowalczyk, N.; Blake, N.; Charko, F.; Quek, Y. Microplastics in the maribyrnong and yarra rivers, melbourne, Australia. Port Phillip EcoCentre Clean Bay Bluepr. 2017, 1–36. [Google Scholar]
- Kapp, K.J.; Yeatman, E. Microplastic hotspots in the Snake and Lower Columbia rivers: A journey from the Greater Yellowstone Ecosystem to the Pacific Ocean. Environ. Pollut. 2018, 241, 1082–1090. [Google Scholar] [CrossRef] [PubMed]
- Kurki-Fox, J.J.; Doll, B.A.; Monteleone, B.; West, K.; Putnam, G.; Kelleher, L.; Krause, S.; Schneidewind, U. Microplastic distribution and characteristics across a large river basin: Insights from the Neuse River in North Carolina, USA. Sci. Total Environ. 2023, 878, 162940. [Google Scholar] [CrossRef] [PubMed]
- Van Thi, K.L.; van Emmerik, T.H.; Vermeulen, B.; Pham, N.Q.; Hoitink, A.T. Division and retention of floating plastic at river bifurcations. Environ. Pollut. 2024, 345, 123490. [Google Scholar] [CrossRef]
- Soltani, N.; Keshavarzi, B.; Moore, F.; Busquets, R.; Nematollahi, M.J.; Javid, R.; Gobert, S. Effect of land use on microplastic pollution in a major boundary waterway: The Arvand River. Sci. Total Environ. 2022, 830, 154728. [Google Scholar] [CrossRef]
- Nyberg, B.; Harris, P.T.; Kane, I.; Maes, T. Leaving a plastic legacy: Current and future scenarios for mismanaged plastic waste in rivers. Sci. Total Environ. 2023, 869, 161821. [Google Scholar] [CrossRef]
- Drummond, J.D.; Schneidewind, U.; Li, A.; Hoellein, T.J.; Krause, S.; Packman, A.I. Microplastic accumulation in riverbed sediment via hyporheic exchange from headwaters to mainstems. Sci. Adv. 2022, 8, 9305. [Google Scholar] [CrossRef]
- Watkins, L.; McGrattan, S.; Sullivan, P.J.; Walter, M.T. The effect of dams on river transport of microplastic pollution. Sci. Total Environ. 2019, 664, 834–840. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, A.R.; Garcia, F.; Riem-Galliano, L.; Tudesque, L.; Albignac, M.; ter Halle, A.; Cucherousset, J. Urbanization and hydrological conditions drive the spatial and temporal variability of microplastic pollution in the Garonne River. Sci. Total Environ. 2021, 769, 144479. [Google Scholar] [CrossRef] [PubMed]
- Stanton, T.; Johnson, M.; Nathanail, P.; MacNaughtan, W.; Gomes, R.L. Freshwater microplastic concentrations vary through both space and time. Environ. Pollut. 2020, 263, 114481. [Google Scholar] [CrossRef]
- Silva, P.M.; Nanny, M.A. Impact of Microplastic Fibers from the Degradation of Nonwoven Synthetic Textiles to the Magdalena River Water Column and River Sediments by the City of Neiva, Huila (Colombia). Water 2020, 12, 1210. [Google Scholar] [CrossRef]
- Kataoka, T.; Nihei, Y.; Kudou, K.; Hinata, H. Assessment of the sources and inflow processes of microplastics in the river environments of Japan. Environ. Pollut. 2019, 244, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Birch, Q.T.; Potter, P.M.; Pinto, P.X.; Dionysiou, D.D.; Al-Abed, S.R. Sources, transport, measurement and impact of nano and microplastics in urban watersheds. Rev. Environ. Sci. Bio/Technol. 2020, 19, 275–336. [Google Scholar] [CrossRef]
- Siegfried, M.; Koelmans, A.A.; Besseling, E.; Kroeze, C. Export of microplastics from land to sea. A modelling approach. Water Res. 2019, 127, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Shim, W.J.; Ha, S.Y.; Han, G.M.; Jang, M.; Hong, S.H. Microplastic emission characteristics of stormwater runoff in an urban area: Intra-event variability and influencing factors. Sci. Total Environ. 2023, 866, 161318. [Google Scholar] [CrossRef] [PubMed]
- Ross, M.S.; Loutan, A.; Groeneveld, T.; Molenaar, D.; Kroetch, K.; Bujaczek, T.; Ruecker, N.J. Estimated discharge of microplastics via urban stormwater during individual rain events. Front. Environ. Sci. 2023, 11, 1090267. [Google Scholar] [CrossRef]
- Werbowski, L.M.; Gilbreath, A.N.; Munno, K.; Zhu, X.; Grbic, J.; Wu, T.; Sutton, R.; Sedlak, M.D.; Deshpande, A.D.; Rochman, C.M. Urban stormwater runoff: A major pathway for anthropogenic particles, black rubbery fragments, and other types of microplastics to urban receiving waters. ACS EST Water 2021, 16, 1420–1428. [Google Scholar] [CrossRef]
- Kukkola, A.; Runkel, R.L.; Schneidewind, U.; Murphy, S.F.; Kelleher, L.; Sambrook Smith, G.H.; Nel, H.A.; Lynch, I.; Krause, S. Prevailing impacts of river management on microplastic transport in contrasting US streams: Rethinking global microplastic flux estimations. Water Res. 2023, 240, 120112. [Google Scholar] [CrossRef] [PubMed]
- Van Calcar, C.J.; van Emmerik, T.H.M. Abundance of plastic debris across European and Asian rivers. Environ. Res. Lett. 2019, 14, 124051. [Google Scholar] [CrossRef]
- McCormick, A.R.; Hoellein, T.J.; London, M.G.; Hittie, J.; Scott, J.W.; Kelly, J.J. Microplastic in surface waters of urban rivers: Concentration, sources, and associated bacterial assemblages. Ecosphere 2016, 7, e01556. [Google Scholar] [CrossRef]
- Tobin, C.; Urban-Rich, J. A Localized Perspective—Are Microfibers a Problem in Boston Harbor? Environ. Sci. Pollut. Res. 2024; submitted. [Google Scholar]
- Van Cappellen, P.; Maavara, T. Rivers in the Anthropocene: Global scale modifications of riverine nutrient fluxes by damming. Ecohydrol. Hydrobiol. 2016, 16, 106–111. [Google Scholar] [CrossRef]
- Picard, C.J.; Winter, J.M.; Cockburn, C.; Hanrahan, J.; Teale, N.G.; Clemins, P.J.; Beckage, B. Twenty-first century increases in total and extreme precipitation across the Northeastern USA. Clim. Chang. 2023, 176, 72. [Google Scholar] [CrossRef]
- Douglas, E.M.; Fairbank, C.A. Is precipitation in northern New England becoming more extreme? Statistical analysis of extreme rainfall in Massachusetts, New Hampshire, and Maine and updated estimates of the 100-year storm. J. Hydrol. Eng. 2011, 16, 203–217. [Google Scholar] [CrossRef]

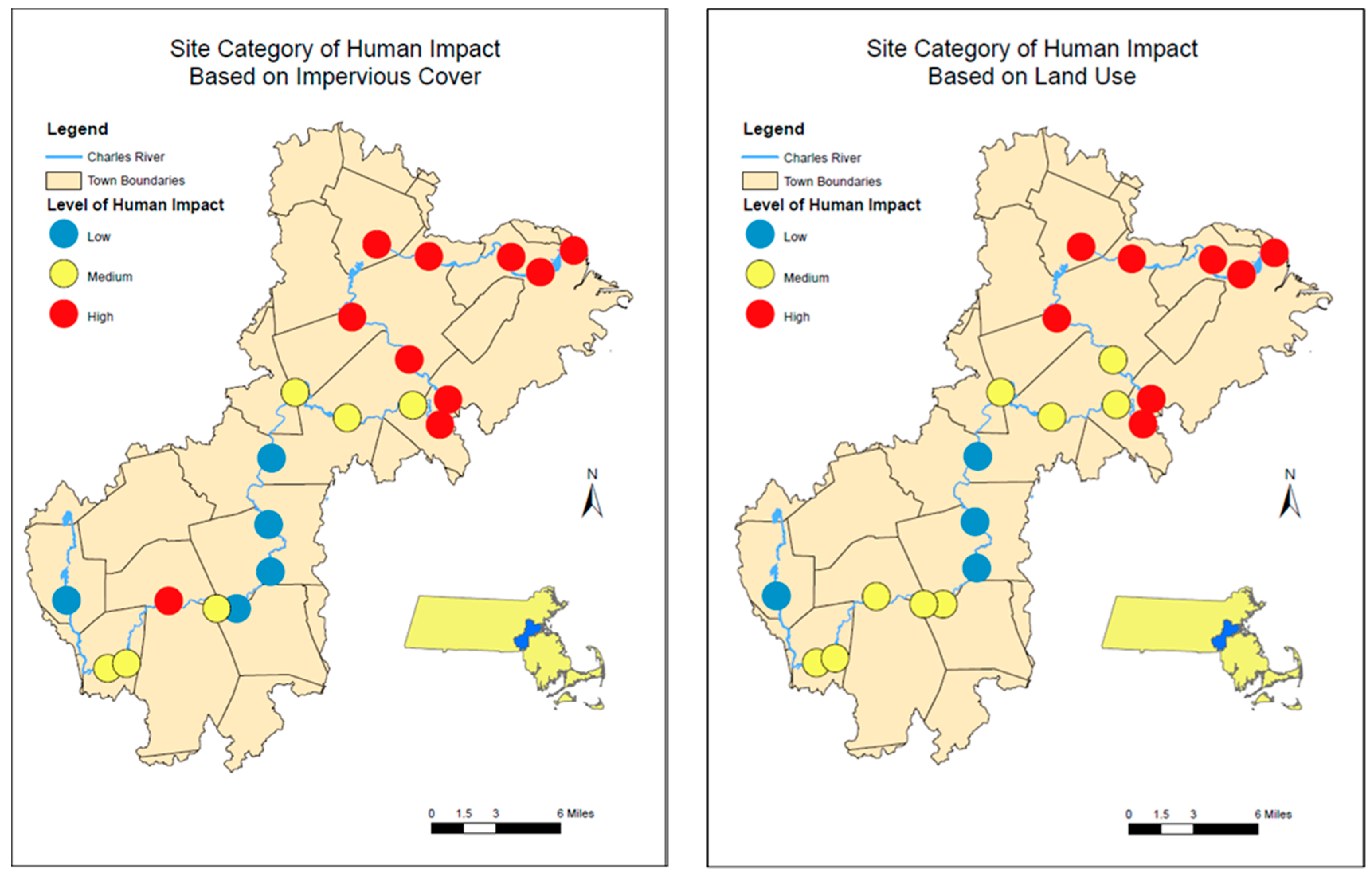



| Low | Medium | High | |
|---|---|---|---|
| Land Use | Open Land | Residential Multi-Family | Mixed Use, Primarily residential |
| Land Use | Tax Exempt | Residential Single-Family | Mixed Use, Other |
| Unknown | Commercial | ||
| Right-of-Way | Industrial | ||
| Impervious Cover | 0% to 33% | 34% to 66% | 67% to 100% |
| Site # | January | May | August | October | Average |
|---|---|---|---|---|---|
| 1 | 15 ± 1 | 7 ± 2 | 8 ± 1 | 8 ± 2 | 9 ± 4 |
| 2 | 13 ± 2 | 14 ± 5 | 5 ± 3 | 7 ± 1 | 10 ± 5 |
| 3 | 9 ± 1 | 11 ± 4 | 12 ± 0 | 13 ± 1 | 11 ± 2 |
| 4 | 10 ± 3 | 12 ± 4 | 10 ± 2 | 12 ± 3 | 11 ± 3 |
| 5 | ND | 14 ± 6 | 4 ± 1 | 14 ± 3 | 11 ± 6 |
| 6 | 4 ± 1 | 18 ± 0 | 10 ± 3 | 6 ± 2 | 9 ± 6 |
| 7 | 2 ± 1 | 2 ± 1 | 6 ± 3 | 9 ± 1 | 5 ± 3 |
| 8 | 1 ± 2 | 2 ± 1 | 12 ± 2 | 2 ± 1 | 4 ± 5 |
| 9 | 1 ± 0 | 5 ± 2 | 8 ± 4 | 5 ± 3 | 5 ± 3 |
| 10 | 2 ± 2 | 7 ± 3 | 5 ± 2 | 19 ± 3 | 8 ± 7 |
| 11 | 3 ± 3 | 8 ± 7 | 7 ± 2 | 18 ± 4 | 9 ± 7 |
| 12 | 6 ± 2 | 15 ± 4 | 6 ± 3 | 3 ± 1 | 7 ± 5 |
| 13 | 5 ± 1 | 8 ± 1 | 2 ± 2 | 10 ± 1 | 6 ± 3 |
| 14 | 5 ± 1 | 14 ± 3 | 5 ± 1 | 3 ± 1 | 7 ± 5 |
| 15 | 7 ± 1 | 10 ± 3 | 3 ± 2 | 13 ± 1 | 8 ± 4 |
| 16 | 3 ± 1 | 10 ± 4 | 3 ± 2 | 9 ± 2 | 6 ± 4 |
| 17 | 5 ±2 | 16 ± 6 | 10 ± 3 | 17 ± 2 | 12 ± 6 |
| 18 | 2 ± 0 | 7 ± 5 | 9 ± 1 | 19 ± 12 | 9 ± 9 |
| 19 | 1 ± 1 | 13 ± 2 | 11 ± 4 | 20 ± 5 | 11 ± 8 |
| 20 | 18 ± 2 | 7 ± 1 | 15 ± 2 | 2 ± 2 | 11 ± 7 |
| 21 | 19 ± 2 | 10 ± 1 | 16 ± 2 | 15 ± 5 | 15 ± 4 |
| Average | 7 ± 6 | 10 ± 5 | 8 ± 4 | 11 ± 7 | 9 ± 4 |
| Site | January | May | August | October |
|---|---|---|---|---|
| 1 | 98/2 | 86/14 | 27/73 | 20/80 |
| 2 | 87/13 | 90/10 | 53/47 | 9/91 |
| 3 | 95/5 | 92/8 | 86/14 | 95/5 |
| 4 | 85/15 | 92/8 | 54/46 | 98/2 |
| 5 | ND | 53/47 | 53/47 | 70/30 |
| 6 | 66/34 | 93/3 | 46/54 | 18/82 |
| 7 | 77/23 | 0/100 | 68/32 | 92/8 |
| 8 | 100/0 | 30/70 | 36/64 | 91/9 |
| 9 | 0/100 | 78/22 | 52/48 | 99/1 |
| 10 | 85/15 | 90/10 | 33/67 | 90/10 |
| 11 | 81/19 | 90/10 | 82/18 | 80/20 |
| 12 | 79/21 | 95/5 | 53/47 | 78/22 |
| 13 | 80/20 | 90/10 | 53/47 | 85/15 |
| 14 | 81/19 | 92/8 | 6238 | 78/22 |
| 15 | 72/28 | 90/10 | 38/62 | 88/12 |
| 16 | 32/68 | 91/9 | 57/43 | 97/3 |
| 17 | 95/5 | 98/2 | 53/47 | 95/5 |
| 18 | 55/45 | 87/13 | 60/40 | 96/4 |
| 19 | 75/25 | 97/3 | 56/44 | 90/10 |
| 20 | 90/10 | 92/8 | 8/92 | 73/27 |
| 21 | 92/8 | 85/15 | 50/50 | 80/20 |
| Average | 76/24 | 82/18 | 51/49 | 77/23 |
| A. Annual | Lowest (MP/L = 1) | Medium (MP/L = 8) | 3rd Quartile (MP/L = 13) | ||||
| Gage Site | Flow (L/s) | Total Depth (cm) (Dt) | Depth Proportion (Dp) | Flow w/Dp (L/s) | Low (MP/s) | Med (MP/s) | 3rd Q (MP/s) |
| Medway | 3837 | 151 | 0.12 | 459 | 459 | 3672 | 5966 |
| Dover | 11,479 | 317 | 0.06 | 652 | 652 | 5217 | 8477 |
| Wellesley | 10,272 | 225 | 0.08 | 823 | 823 | 6580 | 10,693 |
| Waltham | 11,709 | 247 | 0.07 | 853 | 853 | 6826 | 11,092 |
| B. January | Lowest (MP/L = 1) | Medium (MP/L = 5) | 3rd Quartile (MP/L = 9) | ||||
| Medway | 11,236 | 239 | 0.08 | 847 | 847 | 4235 | 7623 |
| Dover | 34,971 | 330 | 0.05 | 1906 | 1906 | 9532 | 17,157 |
| Wellesley | 27,086 | 272 | 0.07 | 1794 | 1794 | 8969 | 16,145 |
| Waltham | 29,845 | 284 | 0.06 | 1888 | 1888 | 9442 | 16,995 |
| C. May | Lowest (MP/L = 2) | Medium (MP/L = 10) | 3rd Quartile (MP/L = 14) | ||||
| Medway | 8394 | 152 | 0.12 | 991 | 1983 | 9914 | 13,880 |
| Dover | 34,508 | 351 | 0.05 | 1772 | 3544 | 17,721 | 24,809 |
| Wellesley | 24,452 | 251 | 0.07 | 1750 | 3501 | 17,503 | 24,504 |
| Waltham | 27,753 | 216 | 0.08 | 2314 | 4628 | 23,138 | 32,394 |
| D. August | Lowest (MP/L = 2) | Medium (MP/L = 8) | 3rd Quartile (MP/L = 10) | ||||
| Medway | 869 | 86 | 0.21 | 181 | 362 | 1449 | 1812 |
| Dover | 3494 | 267 | 0.07 | 236 | 472 | 1886 | 2358 |
| Wellesley | 3294 | 191 | 0.09 | 311 | 623 | 2490 | 3113 |
| Waltham | 3763 | 244 | 0.07 | 278 | 556 | 2222 | 2778 |
| E. October | Lowest (MP/L = 2) | Medium (MP/L = 10) | 3rd Quartile (MP/L = 15) | ||||
| Medway | 1346 | 124 | 0.14 | 195 | 389 | 1946 | 2919 |
| Dover | 4261 | 320 | 0.06 | 240 | 479 | 2396 | 3595 |
| Wellesley | 5217 | 185 | 0.10 | 506 | 1013 | 5065 | 7597 |
| Waltham | 4818 | 244 | 0.07 | 356 | 711 | 3556 | 5335 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mabry, L.; Urban-Rich, J. Seasonal and Distributional Changes in the Composition and Flux of Anthropogenic Microparticles in the Surface Waters of the Charles River, Massachusetts, United States. Microplastics 2024, 3, 539-558. https://doi.org/10.3390/microplastics3040034
Mabry L, Urban-Rich J. Seasonal and Distributional Changes in the Composition and Flux of Anthropogenic Microparticles in the Surface Waters of the Charles River, Massachusetts, United States. Microplastics. 2024; 3(4):539-558. https://doi.org/10.3390/microplastics3040034
Chicago/Turabian StyleMabry, Lee, and Juanita Urban-Rich. 2024. "Seasonal and Distributional Changes in the Composition and Flux of Anthropogenic Microparticles in the Surface Waters of the Charles River, Massachusetts, United States" Microplastics 3, no. 4: 539-558. https://doi.org/10.3390/microplastics3040034
APA StyleMabry, L., & Urban-Rich, J. (2024). Seasonal and Distributional Changes in the Composition and Flux of Anthropogenic Microparticles in the Surface Waters of the Charles River, Massachusetts, United States. Microplastics, 3(4), 539-558. https://doi.org/10.3390/microplastics3040034








