Spatiotemporal Dynamics of Microplastics in Nakivubo Catchment: Implications for the Pollution of Lake Victoria
Abstract
1. Introduction
2. Methods
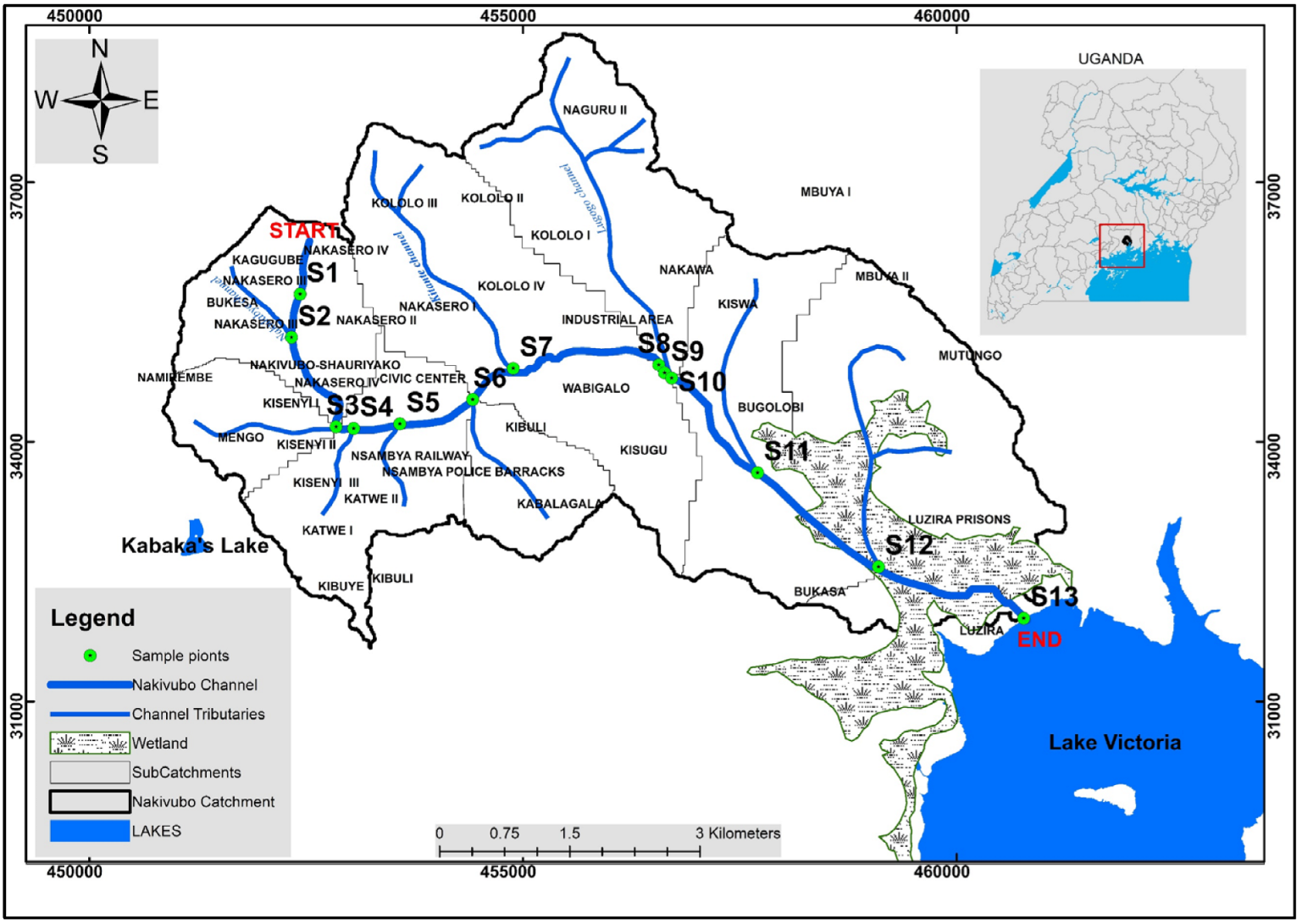
2.1. Study Area
2.2. Sampling Procedure
2.3. Microplastics Isolation
2.4. Stereomicroscopic Analysis
2.5. Fourier-Transform Infrared Analysis
2.6. Analytical Quality Assurance and Quality Control
2.7. Source Apportionment
2.8. Statistical Analysis
3. Results and Discussion
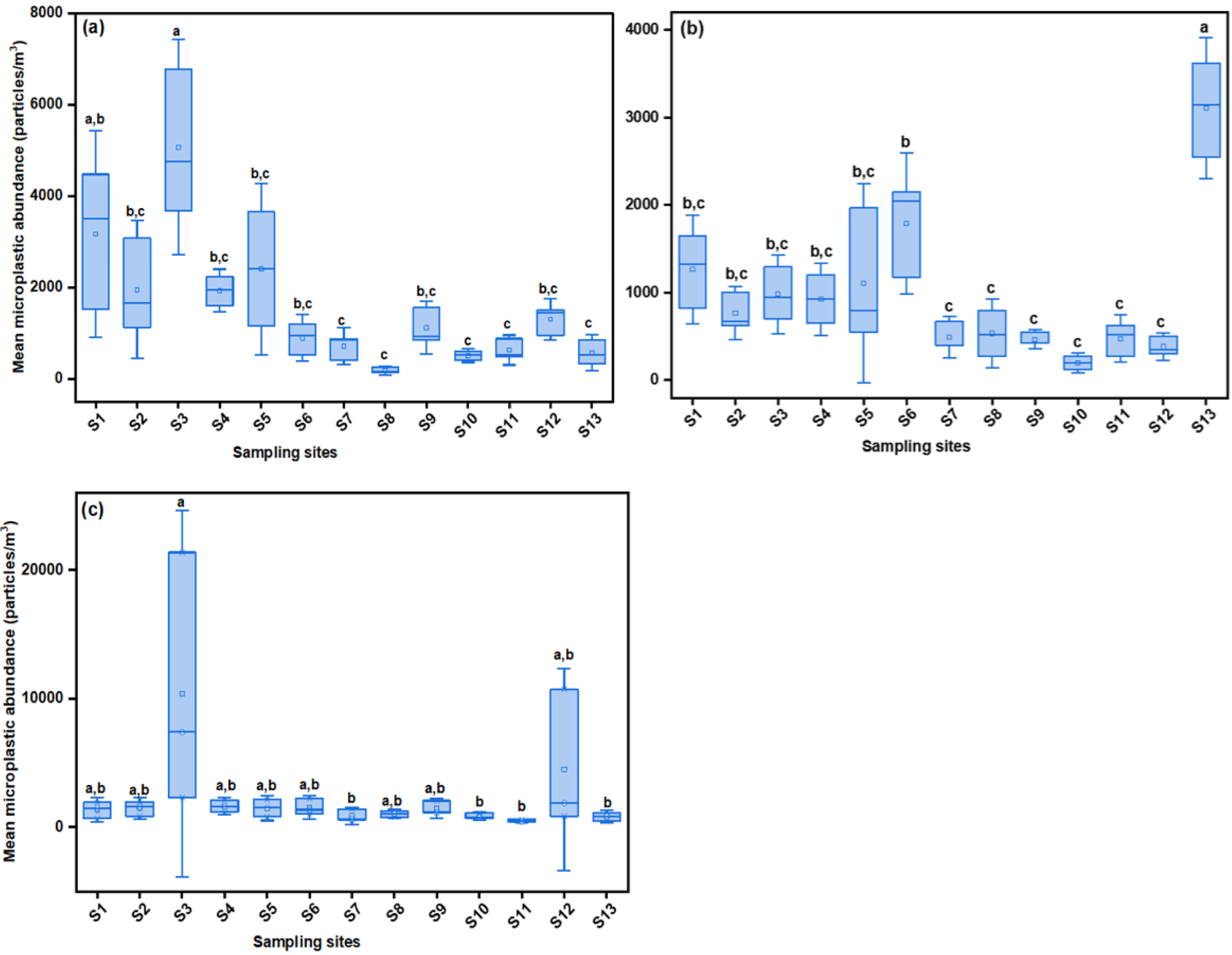
3.1. Abundance and Spatial Distribution of MPs in Nakivubo Catchment
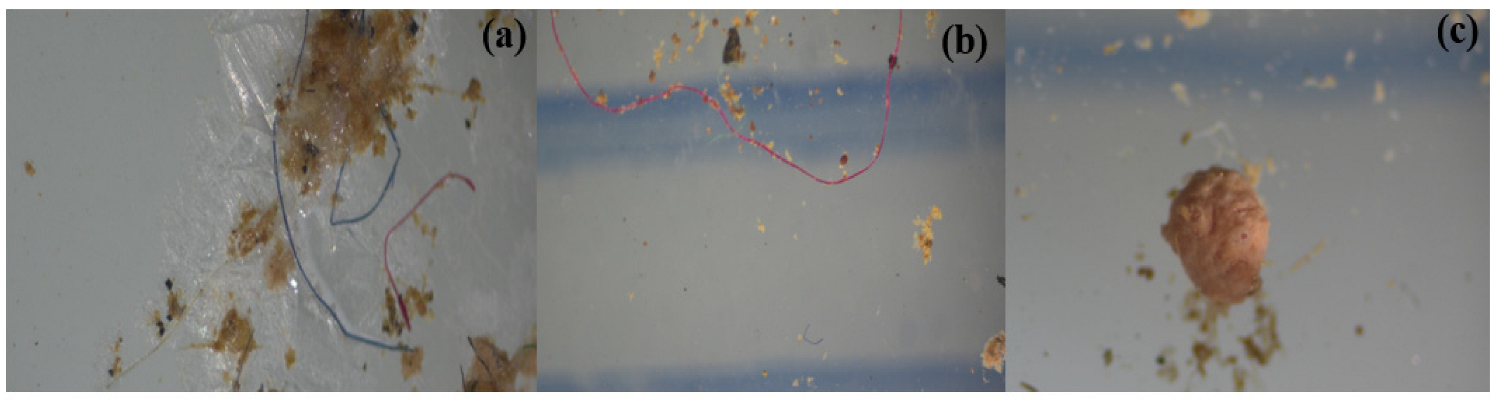
3.2. Morphology of MPs
3.3. Colour Diversity of MPs
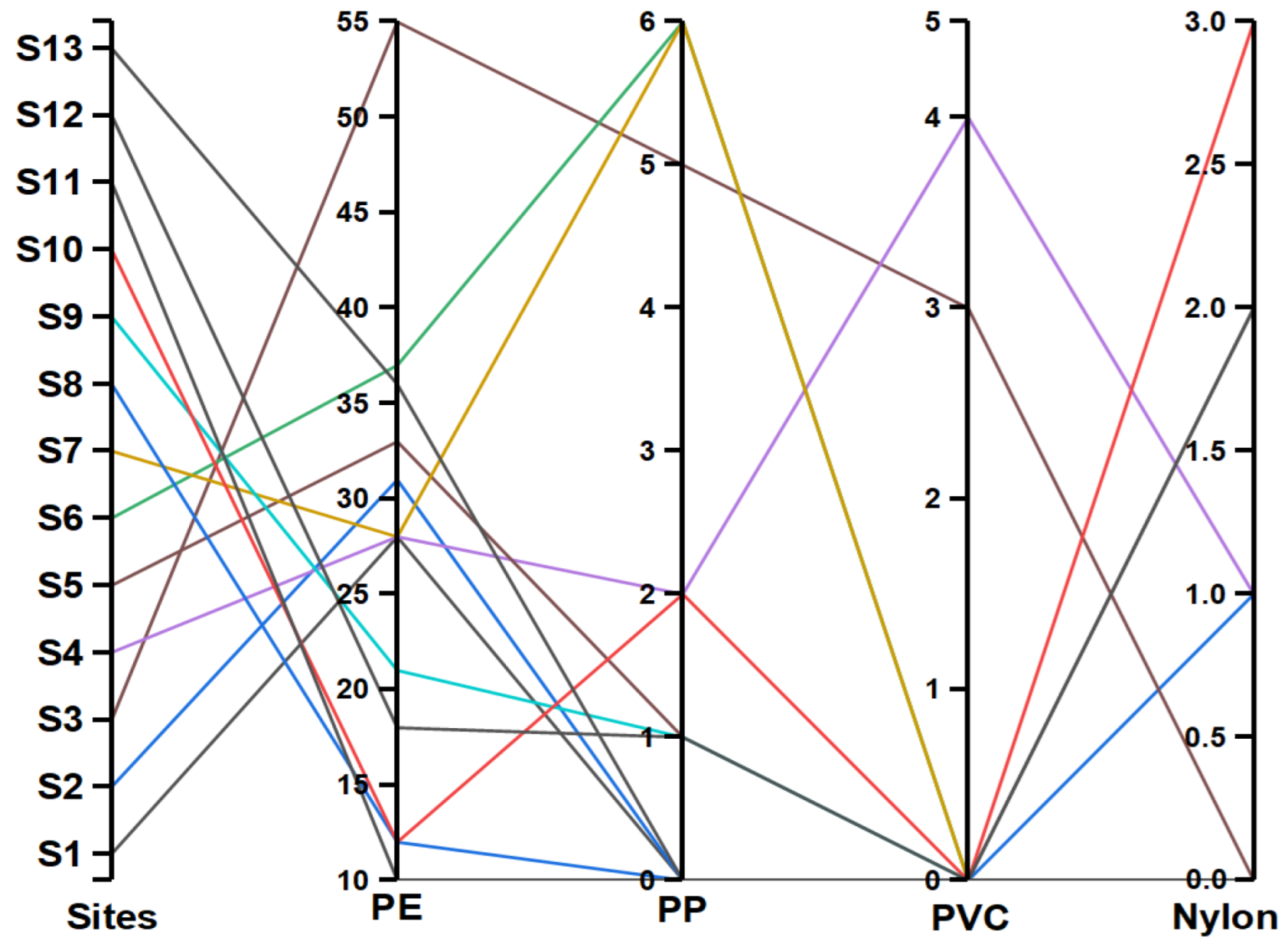
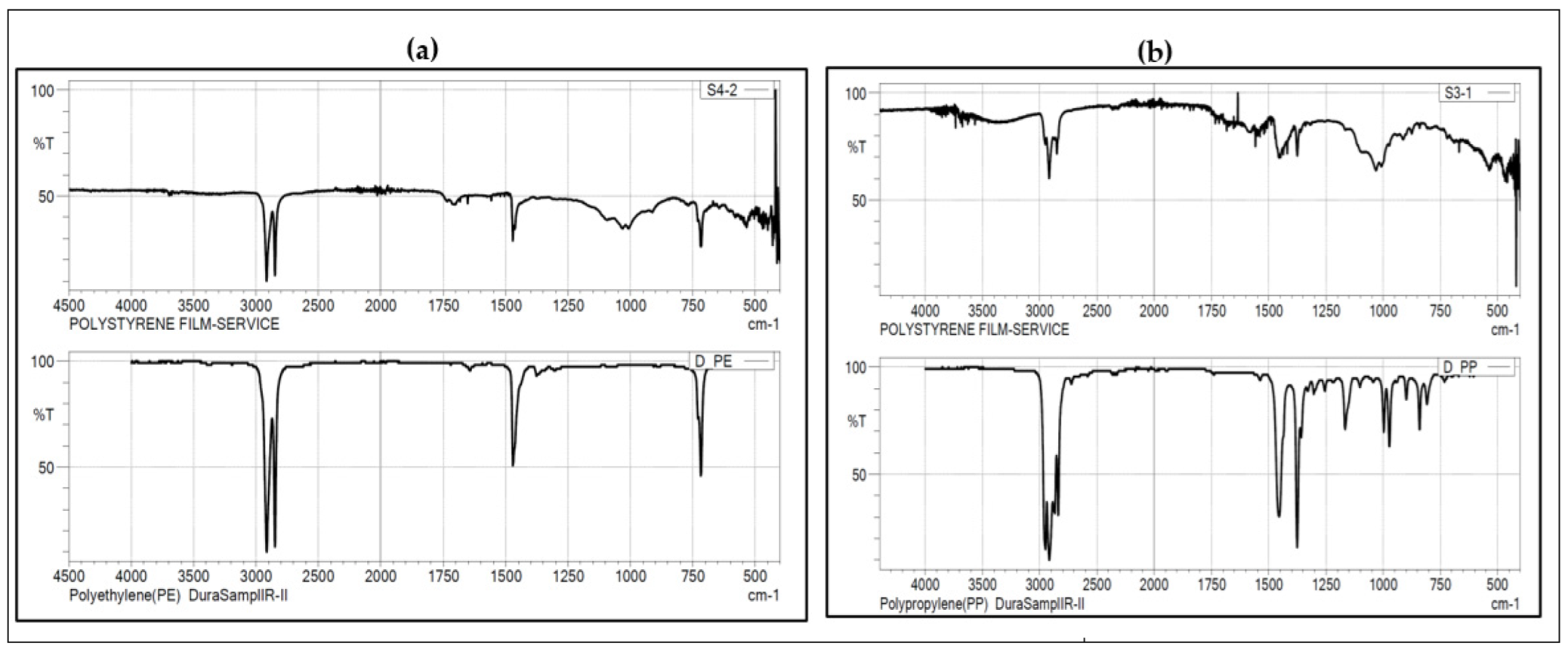
3.4. Polymer Composition of the Identified MPs
3.5. Implications for Lake Victoria’s Pollution
3.6. Study Limitations and Future Research Directions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Misra, A.; Zambrzycki, C.; Kloker, G.; Kotyrba, A.; Anjass, M.H.; Castillo, I.F.; Mitchell, S.G.; Güttel, R.; Streb, C. Water Purification and Microplastics Removal using Magnetic Polyoxometalate Supported-Ionic Liquid Phases (magPOMSILPs). Angew. Chem. 2019, 59, 1601–1605. [Google Scholar] [CrossRef] [PubMed]
- Joo, S.H.; Liang, Y.; Kim, M.; Byun, J.; Choi, H. Microplastics with adsorbed contaminants: Mechanisms and Treatment. Environ. Chall. 2021, 3, 100042. [Google Scholar] [CrossRef]
- Aragaw, T.A. Microplastic pollution in African countries’ water systems: A review on findings, applied methods, characteristics, impacts, and managements. SN Appl. Sci. 2021, 3, 629. [Google Scholar] [CrossRef]
- Tran-Nguyen, Q.A.; Vu, T.B.H.; Nguyen, Q.T.; Nguyen, H.N.Y.; Le, T.M.; Vo, V.M.; Trinh-Dang, M. Urban drainage channels as microplastics pollution hotspots in developing areas: A case study in Da Nang, Vietnam. Mar. Pollut. Bull. 2022, 175, 113323. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Wang, J. Current practices and future perspectives of microplastic pollution in freshwater ecosystems in China. Sci. Total Environ. 2019, 691, 697–712. [Google Scholar] [CrossRef]
- Tumwesigye, E.; Nnadozie, C.F.; Akamagwuna, F.C.; Noundou, X.S.; Nyakairu, G.W.; Odume, O.N. Microplastics as vectors of chemical contaminants and biological agents in freshwater ecosystems: Current knowledge status and future perspectives. Environ. Pollut. 2023, 330, 121829. [Google Scholar] [CrossRef]
- Liu, S.; Shi, J.; Wang, J.; Dai, Y.; Li, H.; Li, J.; Zhang, P. Interactions Between Microplastics and Heavy Metals in Aquatic Environments: A Review. Front. Microbiol. 2021, 12, 652520. [Google Scholar] [CrossRef]
- Benson, N.; Agboola, O.; Fred-Ahmadu, O.; De-la-Torre, G.; Oluwalana, A.; Williams, A. Micro(nano)plastics Prevalence, Food Web Interactions, and Toxicity Assessment in Aquatic Organisms: A Review. Front. Mar. Sci. 2022, 9, 851281. [Google Scholar] [CrossRef]
- Rojas-Luna, R.A.; Oquendo-Ruiz, L.; García-Alzate, C.A.; Arana, V.A.; García-Alzate, R.; Trilleras, J. Identification, Abundance, and Distribution of Microplastics in Surface Water Collected from Luruaco Lake, Low Basin Magdalena River, Colombia. Water Res. 2023, 15, 344. [Google Scholar] [CrossRef]
- Migwi, F.K.; Ogunah, J.A.; Kiratu, J.M. Occurrence and Spatial Distribution of Microplastics in the Surface Waters of Lake Naivasha, Kenya. Environ. Toxicol. Chem. 2020, 39, 765–774. [Google Scholar] [CrossRef]
- Ross, M.S.; Loutan, A.; Groeneveld, T.; Molenaar, D.; Kroetch, K.; Bujaczek, T.; Kolter, S.; Moon, S.; Huynh, A.; Khayam, R.; et al. Estimated discharge of microplastics via urban stormwater during individual rain events. Front. Environ. Sci. 2023, 11, 1090267. [Google Scholar] [CrossRef]
- Egessa, R.; Nankabirwa, A.; Ocaya, H.; Pabire, W.G. Microplastic pollution in surface water of Lake Victoria. Sci. Total Environ. 2020, 741, 140201. [Google Scholar] [CrossRef]
- Egessa, R.; Nankabirwa, A.; Basooma, R.; Nabwire, R. Occurrence, distribution and size relationships of plastic debris along shores and sediment of northern Lake Victoria. Environ. Pollut. 2020, 257, 113442. [Google Scholar] [CrossRef] [PubMed]
- Kakooza, B. Assessment of the Occurrence, Abundance and Characteristics of microplastics in Nakivubo Channel and the Murchison Bay of Lake Victoria. Master’s Thesis, Makerere University, Kampala, Uganda, 2022. [Google Scholar]
- Biginagwa, J.F.; Mayoma, S.B.; Shashoua, Y.; Syberg, K.; Khan, F.R. First evidence of microplastics in the African great lakes: Recovery from Lake Victoria Nile perch and Nile tilapia. J. Great Lakes Res. 2016, 42, 146–149. [Google Scholar] [CrossRef]
- Gathu, S.W.; Kitaka, N.K.; Sitoki, L.M.; Otachi, E.O. Levels and Classification of Microplastics and Their Impact on the Wellbeing of Selected Commercially Important Fish Species in Kisumu Bay, Lake Victoria. Bull. Environ. Contam. Toxicol. 2024, 113, 65. [Google Scholar] [CrossRef]
- Onyango, J.; Huang, J.; Babu, K.; Njuguna, S.; Wanzala, W.; Yan, X. Evaluating the Interaction of Plant Communities with Microplastics Found in Water and Soil Sediments of Lake Victoria. Research Square. Available online: https://doi.org/10.21203/rs.3.rs-4513150/v1 (accessed on 13 March 2025).
- Kayima, J.; Kyakula, M.; Komakech, W.; Echimu, S.P. A study of the degree of Pollution in Nakivubo Channel, Kampala, Uganda. J. Appl. Sci. Environ. Manag. 2008, 12, 93–98. [Google Scholar] [CrossRef][Green Version]
- Fuhrimann, S.; Stalder, M.; Winkler, M.S.; Niwagaba, C.B.; Babu, M.; Masaba, G.; Kabatereine, N.B.; Halage, A.A.; Schneeberger, P.H.H.; Utzinger, J.; et al. Microbial and chemical contamination of water, sediment and soil in the Nakivubo wetland area in Kampala, Uganda. Environ. Monit. Assess. 2015, 18, 475. [Google Scholar] [CrossRef]
- Mbabazi, J.; Kwetegyeka, J.; Ntale, M.; Wasswa, J. Ineffectiveness of Nakivubo wetland in filtering out heavy metals from untreated Kampala urban effluent prior to discharge into Lake Victoria, Uganda. Afr. J. Agric. Res. 2010, 5, 3431–3439. [Google Scholar]
- John, P.H.; Johnson, F.A.; Sutcliffe, P. Integrating Float Method of Discharge Measurement. Proc. Inst. Civ. Eng. 1978, 65, 569–588. [Google Scholar] [CrossRef]
- Collicutt, B.; Juanes, F.; Dudas, S.E. Microplastics in juvenile Chinook salmon and their nearshore environments on the east coast of Vancouver Island. Environ. Pollut. 2019, 244, 135–142. [Google Scholar] [CrossRef]
- Masura, J.; Baker, J.; Foster, G.; Arthur, C.; Herring, C. Laboratory Methods for the Analysis of Microplastics in the Marine Environment: Recommendations for Quantifying Synthetic Particles in Waters and Sediments; NOAA Technical Memorandum NOSOR&R-48; NOAA Marine Debris Division: Silver Spring, MD, USA, 2015. [Google Scholar]
- Nuelle, M.; Dekiff, J.H.; Remy, D.; Fries, E. A new analytical approach for monitoring microplastics in marine sediments. Environ. Pollut. 2014, 184, 161–169. [Google Scholar] [CrossRef]
- Viršek, M.; Palatinus, A.; Koren, Š.; Peterlin, M.; Horvat, P.; Kržan, A. Protocol for microplastics sampling on the sea surface and sample analysis. J. Vis. Exp. 2016, 118, e55161. [Google Scholar]
- Hendrickson, E.; Minor, E.C.; Schreiner, K. Microplastic Abundance and Composition in Western Lake Superior As Determined via Microscopy, Pyr-GC/MS, and FTIR. Environ. Sci. Technol. 2018, 52, 1787–1796. [Google Scholar] [CrossRef] [PubMed]
- Akhbarizadeh, R.; Moore, F.; Keshavarzi, B. Investigating a probable relationship between microplastics and potentially toxic elements in fish muscles from northeast of Persian Gulf. Environ. Pollut. 2018, 232, 154–163. [Google Scholar] [CrossRef]
- Daana, L.; Kanhai, K.; Officer, R.; Lyashevska, O.; Thompson, R.C.; Connor, I.O. Microplastic abundance, distribution and composition along a latitudinal gradient in the Atlantic Ocean. Mar. Pollut. Bull. 2017, 115, 307–314. [Google Scholar]
- Akhbarizadeh, R.; Moore, F.; Keshavarzi, B. Investigating microplastics bioaccumulation and biomagnification in seafood from the Persian Gulf: A threat to human health? Food Add. Contam. 2019, 36, 1696–1708. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, J.; Li, J. The removal of microplastics in the wastewater treatment process and their potential impact on anaerobic digestion due to pollutants association. Chemosphere 2020, 251, 126360. [Google Scholar] [CrossRef]
- Treilles, R.; Gasperi, J.; Gallard, A.; Saad, M.; Dris, R.; Partibane, C.; Breton, J.; Tassin, B. Microplastics and microfibers in urban runoff from a suburban catchment of Greater Paris. Environ. Pollut. 2021, 287, 117352. [Google Scholar] [CrossRef]
- Hitchcock, J.N. Storm events as key moments of microplastic contamination in aquatic ecosystems. Sci. Total Environ. 2020, 734, 139436. [Google Scholar] [CrossRef]
- Ochoa, L.; Chan, J.; Auguste, C.; Arbuckle-Keil, G.; Fahrenfeld, N.L. Stormwater runoff microplastics: Polymer types, particle size, and factors controlling loading rates. Sci. Total Environ. 2024, 929, 172485. [Google Scholar] [CrossRef]
- Boni, W.; Arbuckle-Keil, G.; Fahrenfeld, N.L. Inter-storm variation in microplastic concentration and polymer type at stormwater outfalls and a bioretention basin. Sci. Total Environ. 2022, 809, 151104. [Google Scholar] [CrossRef] [PubMed]
- Monira, S.; Roychand, R.; Bhuiyan, M.A.; Hai, F.I.; Pramanik, B.K. Identification, classification and quantification of microplastics in road dust and stormwater. Chemosphere 2022, 299, 134389. [Google Scholar] [CrossRef] [PubMed]
- Bbosa, N.B.; Oyoo, W.S.; Vicent, M.O.; Atieno, A.D. Physicochemical parameters and magnetic speciation of Iron in Nakivubo Channel and Lake Victoria waters. Lakes Reserv. 2009, 14, 127–137. [Google Scholar] [CrossRef]
- Mugume, S.N.; Gomez, D.E.; Fu, G.; Farmani, R.; Butler, D. A global analysis approach for investigating structural resilience in urban drainage systems. Water Res. 2015, 81, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Omara, T.; Benetková, B.M.; Rosenau, T.; Nagawa, C.B.; Böhmdorfer, S. Microplastic analysis by complementary analytical techniques: μ-FTIR and pyrolysis GC-MS. In Proceedings of the ANAKON 2025, Leipzig, Germany, 10–13 March 2025; Leipzig University: Leipzig, Germany. [Google Scholar]
- Primus, A.; Azman, S. Quantification and Characterisation of Microplastics in Fish and Surface Water at Melayu River, Johor. IOP Conf. Ser. Mat. Sci. Eng. 2022, 1229, 012014. [Google Scholar] [CrossRef]
- Gago, J.; Carretero, O.; Filgueiras, A.V.; Viñas, L. Synthetic microfibers in the marine environment: A review on their occurrence in seawater and sediments. Mar. Pollut. Bull. 2018, 127, 365–376. [Google Scholar] [CrossRef]
- Yusuf, M.A.; Yaqin, K.; Wicaksono, E.A.; Tahir, A. Abundance and characteristics of microplastics in Lake Towuti, East Luwu, South Sulawesi. AACL Bioflux. 2022, 15, 621–631. [Google Scholar]
- Courtene-Jones, W.; Quinn, B.; Gary, S.F.; Mogg, A.O.M.; Narayanaswamy, B.E. Microplastic pollution identified in deep-sea water and ingested by benthic invertebrates in the Rockall Trough, North Atlantic Ocean. Environ. Pollut. 2017, 231, 271–280. [Google Scholar] [CrossRef]
- Koros, V.; Kitetu, J.; Kebenei, S. Influence of Polythene Bag Alternatives on Compliance to Environmental Legislation on Polythene Bag Ban in Rongai Sub-County, Nakuru County, Kenya. OALib J. 2019, 6, 1–10. [Google Scholar] [CrossRef]
- Behuria, P. The Comparative Political Economy of Plastic Bag Bans in East Africa: Why Implementation Has Varied in Rwanda, Kenya and Uganda; GDI Working Paper 2019-037; The University of Manchester: Manchester, UK, 2019. [Google Scholar]
- Albignac, M.; de Oliveira, T.; Landebrit, L.; Miquel, S.; Auguin, B.; Leroy, E.; Emmanuelle, M.; Mingotaud, A.F.; Halle, A. Tandem mass spectrometry enhances the performances of pyrolysis-gas chromatography-mass spectrometry for microplastic quantification. J. Anal. Appl. Pyrol. 2023, 172, 105993. [Google Scholar] [CrossRef]
- Gomiero, A.; Øysæd, K.B.; Palmas, L.; Skogerbø, G. Application of GCMS-pyrolysis to estimate the levels of microplastics in a drinking water supply system. J. Hazard. Mat. 2021, 416, 125708. [Google Scholar] [CrossRef] [PubMed]
- Fernández-González, V.; Andrade-Garda, J.M.; López-Mahía, P.; Muniategui-Lorenzo, S. Misidentification of PVC microplastics in marine environmental samples. TrAC 2022, 153, 116649. [Google Scholar] [CrossRef]
- Schütze, B.; Thomas, D.; Kraft, M.; Brunotte, J.; Kreuzig, R. Comparison of different salt solutions for density separation of conventional and biodegradable microplastic from solid sample matrices. Environ. Sci. Pollut. Res. Int. 2022, 29, 81452–81467. [Google Scholar] [CrossRef] [PubMed]
- Mutesi, S. Microplastic Pollution of Water and Fish from Lake Victoria and the Potential Water Decontamination Methods. Master’s Thesis, Makerere University, Kampala, Uganda, 2023. [Google Scholar]
- Omara, T.; Benetková, B.M.; Sumerskii, I.; Rosenau, T.; Nagawa, C.B.; Böhmdorfer, S. Spatiotemporal dynamics and trophic transfer of microplastics in some endemic fish species of Lake Victoria. In Proceedings of the MICROPLASTICdays, Ljubljana, Slovenia, 25–27 March 2025; University of Ljubljana: Ljubljana, Slovenia, 2025. [Google Scholar]

| Area (Country) | Abundance (Particles/m3) a | Authors |
|---|---|---|
| Nakivubo Catchment (Uganda) | 1569–2140 | This study |
| Calgary City, Canada | 700–2,004,000 | Ross et al. [11] |
| New Jersey (USA) | 410–990 | Ochoa et al. [33] |
| New Jersey (USA) | 300–800 | Boni et al. [34] |
| Nakivubo Channel (Uganda) | 688 | Kakooza [14] |
| Wetlands (Australia) | 26,000–17,000 | Monira et al. [35] |
| Phu Loc Channel (Vietnam) | 630–3840 | Tran-Nguyen et al. [4] |
| Sucy-en-Brie Catchment (France) | 3000–129,000 | Treilles et al. [31] |
| Sampling Sites | Mean Flow Rate (m3/s) | Mean Flux (Million Particles/Day) |
|---|---|---|
| S1 | 0.39 ± 0.02 | 64.443 ± 3.331 |
| S2 | 0.23 ± 0.08 | 28.004 ± 9.657 |
| S3 | 0.13 ± 0.09 | 59.990 ± 42.509 |
| S4 | 0.31 ± 0.25 | 39.735 ± 32.280 |
| S5 | 0.21 ± 0.12 | 29.570 ± 17.251 |
| S6 | 1.11 ± 1.46 | 135.067 ± 177.302 |
| S7 | 0.82 ± 0.70 | 48.999 ± 41.664 |
| S8 | 0.56 ± 0.31 | 28.258 ± 15.550 |
| S10 | 0.58 ± 0.34 | 26.053 ± 15.340 |
| S11 | 0.49 ± 0.10 | 22.481 ± 4.584 |
| S12 | 0.67 ± 0.51 | 118.306 ± 90.576 |
| S13 | 2.27 ± 1.35 | 293.957 ± 174.636 |
| Month | Precipitation (mm) |
|---|---|
| January | 8.65 |
| February | 60.72 |
| March | 144.72 |
| April | 156.61 |
| May | 166.66 |
| June | 74.79 |
| July | 21.60 |
| August | 142.19 |
| September | 277.44 |
| October | 111.97 |
| November | 233.36 |
| December | 180.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ocakacon, S.; Nyenje, P.M.; Kalibbala, H.M.; Kulabako, R.N.; Nagawa, C.B.; Omara, T.; Kyarimpa, C.; Lugasi, S.O.; Ssebugere, P. Spatiotemporal Dynamics of Microplastics in Nakivubo Catchment: Implications for the Pollution of Lake Victoria. Microplastics 2025, 4, 21. https://doi.org/10.3390/microplastics4020021
Ocakacon S, Nyenje PM, Kalibbala HM, Kulabako RN, Nagawa CB, Omara T, Kyarimpa C, Lugasi SO, Ssebugere P. Spatiotemporal Dynamics of Microplastics in Nakivubo Catchment: Implications for the Pollution of Lake Victoria. Microplastics. 2025; 4(2):21. https://doi.org/10.3390/microplastics4020021
Chicago/Turabian StyleOcakacon, Simon, Philip Mayanja Nyenje, Herbert Mpagi Kalibbala, Robinah Nakawunde Kulabako, Christine Betty Nagawa, Timothy Omara, Christine Kyarimpa, Solomon Omwoma Lugasi, and Patrick Ssebugere. 2025. "Spatiotemporal Dynamics of Microplastics in Nakivubo Catchment: Implications for the Pollution of Lake Victoria" Microplastics 4, no. 2: 21. https://doi.org/10.3390/microplastics4020021
APA StyleOcakacon, S., Nyenje, P. M., Kalibbala, H. M., Kulabako, R. N., Nagawa, C. B., Omara, T., Kyarimpa, C., Lugasi, S. O., & Ssebugere, P. (2025). Spatiotemporal Dynamics of Microplastics in Nakivubo Catchment: Implications for the Pollution of Lake Victoria. Microplastics, 4(2), 21. https://doi.org/10.3390/microplastics4020021









