Developments in Non-Invasive Imaging to Guide Diagnosis and Treatment of Proliferative Diabetic Retinopathy: A Systematic Review
Abstract
:1. Introduction
2. Methods: Systematic Literature Search
2.1. Selection of Studies
2.2. Eligibility Criteria and Study Content
3. Invasive Fundus Imaging Modalities to Guide Diagnosis of PDR
3.1. Fundus Fluorescein Angiography (FFA)
3.2. Indocyanine Green Angiography (ICG-A)
3.3. Ophthalmic B-Scan Ultrasonography
4. Non-Invasive Fundus Imaging Modalities to Guide Diagnosis and Treatment of PDR
4.1. Colour Fundus Imaging
4.2. Optical Coherence Tomography (OCT)
4.3. OCTA
5. Future Directions
5.1. AI Approaches
5.2. Development of Other Non-Invasive Imaging Modalities
5.3. Training, Education, and Equipment Maintenance
5.4. Validation in Prospective Observational Studies and Randomised Clinical Trials
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Keel, S.; Xie, J.; Foreman, J.; van Wijngaarden, P.; Taylor, H.R.; Dirani, M. The Prevalence of Diabetic Retinopathy in Australian Adults with Self-Reported Diabetes: The National Eye Health Survey. Ophthalmology 2017, 124, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Mathur, R.; Bhaskaran, K.; Edwards, E.; Lee, H.; Chaturvedi, N.; Smeeth, L.; Douglas, I. Population trends in the 10-year incidence and prevalence of diabetic retinopathy in the UK: A cohort study in the Clinical Practice Research Datalink 2004–2014. BMJ Open 2017, 7, e014444. [Google Scholar] [CrossRef]
- Amoaku, W.M.; Ghanchi, F.; Bailey, C.; Banerjee, S.; Banerjee, S.; Downey, L.; Gale, R.; Hamilton, R.; Khunti, K.; Posner, E.; et al. Diabetic retinopathy and diabetic macular oedema pathways and management: UK Consensus Working Group. Eye 2020, 34, 1–51. [Google Scholar] [CrossRef]
- Ramchandran, R.; Bawany, M.H.; Ding, L.; Sharma, G.; Wykoff, C.C.; Kuriyan, A.E. Automated vessel density detection in fluorescein angiography images correlates with vision in proliferative diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2020, 61, 5312. [Google Scholar]
- Chhablani, J.; Sharma, A.; Goud, A.; Peguda, H.K.; Rao, H.L.; Begum, V.U.; Barteselli, G. Neurodegeneration in type 2 diabetes: Evidence from spectral-domain optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6333–6338. [Google Scholar] [CrossRef] [Green Version]
- Duh, E.J.; Sun, J.; Stitt, A.W. Diabetic retinopathy: Current understanding, mechanisms, and treatment strategies. JCI Insight 2017, 2, e93751. [Google Scholar] [CrossRef]
- Fan, W.; Nittala, M.G.; Velaga, S.B.; Hirano, T.; Wykoff, C.C.; Ip, M.; Lampen, S.I.; van Hemert, J.; Fleming, A.; Verhoek, M.; et al. Distribution of Nonperfusion and Neovascularization on Ultrawide-Field Fluorescein Angiography in Proliferative Diabetic Retinopathy (RECOVERY Study): Report 1. Am. J. Ophthalmol. 2019, 206, 154–160. [Google Scholar] [CrossRef]
- Pan, J.; Chen, D.; Yang, X.; Zou, R.; Zhao, K.; Cheng, D.; Huang, S.; Zhou, T.; Yang, Y.; Chen, F. Characteristics of Neovascularization in Early Stages of Proliferative Diabetic Retinopathy by Optical Coherence Tomography Angiography. Am. J. Ophthalmol. 2018, 192, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Rush, R.B.; Del Valle Penella, A.; Reinauer, R.M.; Rush, S.W.; Bastar, P.G. Internal Limiting Membrane Peeling during Vitrectomy for Diabetic Vitreous Hemorrhage: A Randomized Clinical Trial. RETINA 2020, 41, 1118–1126. [Google Scholar] [CrossRef] [PubMed]
- Marcus, D.M.; Singh, H.; Farooq, A.; Starnes, D.; Walia, H. Endolaserless vitrectomy with intravitreal aflibercept injection (IAI) for proliferative diabetic retinopathy (PDR)-related vitreous hemorrhage (LASER LESS TRIAL). Investig. Ophthalmol. Vis. Sci. 2017, 58, 5036. [Google Scholar]
- Curtis, T.M.; Gardiner, T.A.; Stitt, A.W. Microvascular lesions of diabetic retinopathy: Clues towards understanding pathogenesis? Eye 2009, 23, 1496–1508. [Google Scholar] [CrossRef] [Green Version]
- Tan, B.; Chua, J.; Lin, E.; Cheng, J.; Gan, A.; Yao, X.; Wong, D.W.; Sabanayagam, C.; Wong, D.; Chan, C.M.; et al. Quantitative Microvascular Analysis with Wide-Field Optical Coherence Tomography Angiography in Eyes with Diabetic Retinopathy. JAMA Netw. Open 2020, 3, e1919469. [Google Scholar] [CrossRef]
- Early Treatment Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs—An extension of the modified Airlie House classification: ETDRS report number 10. Ophthalmology 1991, 98, 786–806. [Google Scholar] [CrossRef]
- The Diabetic Retinopathy Vitrectomy Study Research Group. Early vitrectomy for severe vitreous hemorrhage in diabetic retinopathy: Two-year results of a randomized trial—Diabetic Retinopathy Vitrectomy Study report 2. Arch. Ophthalmol. 1985, 103, 1644–1652. [Google Scholar] [CrossRef]
- Silva, P.S.; Cruz, A.J.D.; Ledesma, M.G.; van Hemert, J.; Radwan, A.; Cavallerano, J.; Aiello, L.M.; Sun, J.K. Diabetic Retinopathy Severity and Peripheral Lesions Are Associated with Nonperfusion on Ultrawide Field Angiography. Ophthalmology 2015, 122, 2465–2472. [Google Scholar] [CrossRef]
- Wilkinson, C.; Ferris, F.; Klein, R.; Lee, P.; Agardh, C.D.; Davis, M.; Dills, D.; Kampik, A.; Pararajasegaram, R.; Verdaguer, J.T. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003, 110, 1677–1682. [Google Scholar] [CrossRef]
- Gross, J.G.; Glassman, A.R.; Liu, D.; Sun, J.K.; Antoszyk, A.N.; Baker, C.W.; Bressler, N.M.; Elman, M.J.; Ferris, F.L.; Gardner, T.W.; et al. Five-Year Outcomes of Panretinal Photocoagulation vs Intravitreous Ranibizumab for Proliferative Diabetic Retinopathy. JAMA Ophthalmol. 2018, 136, 1138–1148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preti, R.C.; Vasquez Ramirez, L.M.; Ribeiro Monteiro, M.L.; Pelayes, D.E.; Takahashi, W.Y. Structural and functional assessment of macula in patients with high-risk proliferative diabetic retinopathy submitted to panretinal photocoagulation and associated intravitreal bevacizumab injections: A comparative, randomised, controlled trial. Ophthalmologica 2013, 230, 1–8. [Google Scholar] [CrossRef]
- Nikkhah, H.; Ghazi, H.; Razzaghi, M.R.; Karimi, S.; Ramezani, A.; Soheilian, M. Extended targeted retinal photocoagulation versus conventional pan-retinal photocoagulation for proliferative diabetic retinopathy in a randomized clinical trial. Int. Ophthalmol. 2017, 38, 313–321. [Google Scholar] [CrossRef]
- The Diabetic Retinopathy Study Research Group. Photocoagulation Treatment of Proliferative Diabetic Retinopathy: Clinical application of Diabetic Retinopathy Study (DRS) findings, DRS Report Number 8. Ophthalmology 1981, 88, 583–600. [Google Scholar] [CrossRef]
- Flynn, H.W.; Chew, E.Y.; Simons, B.D.; Barton, F.B.; Remaley, N.A.; Ferris, F.L. Pars Plana Vitrectomy in the Early Treatment Diabetic Retinopathy Study. Ophthalmology 1992, 99, 1351–1357. [Google Scholar] [CrossRef]
- Silva, P.S.; Cavallerano, J.; Haddad, N.M.N.; Kwak, H.; Dyer, K.H.; Omar, A.F.; Shikari, H.; Aiello, L.M.; Sun, J.K. Peripheral Lesions Identified on Ultrawide Field Imaging Predict Increased Risk of Diabetic Retinopathy Progression over 4 Years. Ophthalmology 2015, 122, 949–956. [Google Scholar] [CrossRef]
- Elnahry, A.G.; Ramsey, D.J. Automated Image Alignment for Comparing Microvascular Changes Detected by Fluorescein Angiography and Optical Coherence Tomography Angiography in Diabetic Retinopathy. Semin. Ophthalmol. 2021, 36, 757–764. [Google Scholar] [CrossRef]
- Ishibazawa, A.; Nagaoka, T.; Yokota, H.; Takahashi, A.; Omae, T.; Song, Y.S.; Takahashi, T.; Yoshida, A. Characteristics of retinal neovascularization in proliferative diabetic retinopathy imaged by optical coherence tomography angiography. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6247–6255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, A.A.; Morse, L.; Handa, J.T.; Morales, R.B.; Tucker, R.; Hjelmeland, L.; A Yannuzzi, L. Histologic localization of indocyanine green dye in aging primate and human ocular tissues with clinical angiographic correlation. Ophthalmology 1998, 105, 1060–1068. [Google Scholar] [CrossRef]
- Shiragami, C.; Shiraga, F.; Matsuo, T.; Tsuchida, Y.; Ohtsuki, H. Risk factors for diabetic choroidopathy in patients with diabetic retinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2002, 240, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.A.; Calucci, D.; Orefice, J.L. Indocyanine Green Angiography for The Detection Of Macular “Treatable Lesions” In Patients with Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2011, 52, 583. [Google Scholar]
- Mohamed, I.E.; Mohamed, M.A.; Yousef, M.; Mahmoud, M.Z.; Alonazi, B. Use of ophthalmic B-scan ultrasonography in determining the causes of low vision in patients with diabetic retinopathy. Eur. J. Radiol. Open 2018, 5, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Diabetic Retinopathy Vitrectomy Study Research Group. Early Vitrectomy for Severe Vitreous Hemorrhage in Diabetic Retinopathy. Four-year results of a randomized trial. Diabetic Retinopathy Study report 5. Arch. Ophthalmol. 1990, 108, 958–964. [Google Scholar] [CrossRef]
- Babiuch, A.; Wykoff, C.C.; Hach, J.; Srivastava, S.; E Talcott, K.; Yu, H.J.; Nittala, M.; Sadda, S.; Ip, M.S.; Le, T.; et al. Longitudinal panretinal microaneurysm dynamics on ultra-widefield fluorescein angiography in eyes treated with intravitreal aflibercept for proliferative diabetic retinopathy in the recovery study. Br. J. Ophthalmol. 2020, 105, 1111–1115. [Google Scholar] [CrossRef] [PubMed]
- Shimura, M.; Kitano, S.; Muramatsu, D.; Fukushima, H.; Takamura, Y.; Matsumoto, M.; Kokado, M.; Kogo, J.; Sasaki, M.; Morizane, Y.; et al. Real-world management of treatment-naïve diabetic macular oedema: 2-year visual outcome focusing on the starting year of intervention from STREAT-DMO study. Br. J. Ophthalmol. 2020, 104, 1755–1761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gross, J.G.; Glassman, A.R.; Jampol, L.M.; Inusah, S.; Aiello, L.P.; Antoszyk, A.N.; Baker, C.W.; Berger, B.B.; Bressler, N.M.; Browning, D.; et al. Panretinal Photocoagulation vs Intravitreous Ranibizumab for Proliferative Diabetic Retinopathy: A randomized clinical trial. JAMA 2015, 314, 2137–2146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, C.C.; Yang, C.H.; Yeh, P.T.; Yang, C.M. Macular tractional retinoschisis in proliferative diabetic retinopathy: Clinical characteristics and surgical outcome. Ophthalmologica 2013, 231, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Inigo, Y.J.; Acaba, L.A.; Berrocal, M.H. Surgical management of retinal diseases: Proliferative diabetic retinopathy and traction retinal detachment. Dev. Ophthalmol. 2014, 54, 196–203. [Google Scholar]
- Ophir, A.; Martinez, M.R.; Mosqueda, P.; Trevino, A. Vitreous traction and epiretinal membranes in diabetic macular oedema using spectral-domain optical coherence tomography. Eye 2010, 24, 1545–1553. [Google Scholar] [CrossRef]
- Chhablani, J.K.; Kim, J.S.; Cheng, L.; Kozak, I.; Freeman, W. External limiting membrane as a predictor of visual improvement in diabetic macular edema after pars plana vitrectomy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2012, 250, 1415–1420. [Google Scholar] [CrossRef]
- Mishra, D.K.; Shanmugam, M.P.; Ramanjulu, R.; Sagar, P. Comparison of standard and “innovative wide-field” optical coherence tomography images in assessment of vitreoretinal interface in proliferative diabetic retinopathy: A pilot study. Indian J. Ophthalmol. 2020, 69, 99–102. [Google Scholar] [CrossRef]
- Um, T.; Seo, E.J.; Kim, Y.J.; Yoon, Y.H. Optical coherence tomography angiography findings of type 1 diabetic patients with diabetic retinopathy, in comparison with type 2 patients. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 258, 281–288. [Google Scholar] [CrossRef]
- Vaz-Pereira, S.; Monteiro-Grillo, M.; Engelbert, M. Near-infrared reflectance imaging of neovascularization in proliferative diabetic retinopathy. Int. J. Retin. Vitr. 2020, 6, 59. [Google Scholar] [CrossRef]
- Schwartz, R.; Khalid, H.; Sivaprasad, S.; Nicholson, L.; Anikina, E.; Sullivan, P.; Patel, P.J.; Balaskas, K.; Keane, P.A. Objective Evaluation of Proliferative Diabetic Retinopathy Using OCT. Ophthalmol. Retin. 2020, 4, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Levine, E.S.; Arya, M.; Chaudhari, J.; Greig, E.C.; Alibhai, A.Y.; Baumal, C.R.; Witkin, A.J.; Duker, J.S.; Waheed, N.K. Repeatability and reproducibility of vessel density measurements on optical coherence tomography angiography in diabetic retinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 258, 1687–1695. [Google Scholar] [CrossRef] [PubMed]
- Kase, S.; Endo, H.; Takahashi, M.; Saito, M.; Yokoi, M.; Ito, Y.; Katsuta, S.; Sonoda, S.; Sakamoto, T.; Ishida, S.; et al. Alteration of choroidal vascular structure in diabetic retinopathy. Br. J. Ophthalmol. 2020, 104, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Hoshiyama, K.; Hirabayashi, K.; Wakabayashi, M.; Toriyama, Y.; Tokimitsu, M.; Murata, T. Vitreoretinal Interface Slab in OCT Angiography for Detecting Diabetic Retinal Neovascularization. Ophthalmol. Retin. 2020, 4, 588–594. [Google Scholar] [CrossRef]
- Ashraf, M.; Sampani, K.; Clermont, A.; Abu-Qamar, O.; Rhee, J.; Silva, P.S.; Aiello, L.P.; Sun, J.K. Vascular Density of Deep, Intermediate and Superficial Vascular Plexuses Are Differentially Affected by Diabetic Retinopathy Severity. Investig. Opthalmology Vis. Sci. 2020, 61, 53. [Google Scholar] [CrossRef]
- Wang, H.; Tao, Y. Choroidal structural changes correlate with severity of diabetic retinopathy in diabetes mellitus. BMC Ophthalmol. 2019, 19, 186. [Google Scholar] [CrossRef] [Green Version]
- Motulsky, E.H.; Liu, G.; Shi, Y.; Zheng, F.; Flynn Jr, H.W.; Gregori, G.; Rosenfeld, P.J. Widefield swept-source optical coherence tomography angiography of proliferative diabetic retinopathy. Ophthalmic Surg. Lasers Imaging Retin. 2019, 50, 474–484. [Google Scholar] [CrossRef]
- La Mantia, A.; Kurt, R.A.; Mejor, S.; Egan, C.; Tufail, A.; Keane, P.A.; Sim, D.A. Comparing fundus fluorescein angiography and swept-source optical coherence tomography angiography in the evaluation of diabetic macular perfusion. Retina 2019, 39, 926–937. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, C.C.; Hsu, H.M.; Yang, C.M.; Yang, C.H. Correlation of retinal vascular perfusion density with dark adaptation in diabetic retinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 257, 1401–1410. [Google Scholar] [CrossRef]
- Hirano, T.; Kitahara, J.; Toriyama, Y.; Kasamatsu, H.; Murata, T.; Sadda, S. Quantifying vascular density and morphology using different swept-source optical coherence tomography angiographic scan patterns in diabetic retinopathy. Br. J. Ophthalmol. 2019, 103, 216–221. [Google Scholar] [CrossRef]
- Cui, Y.; Zhu, Y.; Wang, J.C.; Lu, Y.; Zeng, R.; Katz, R.; Wu, D.M.; Vavvas, D.G.; Husain, D.; Miller, J.W.; et al. Imaging artifacts and segmentation errors with wide-field swept-source optical coherence tomography angiography in diabetic retinopathy. Transl. Vis. Sci. Technol. 2019, 8, 18. [Google Scholar] [CrossRef] [Green Version]
- Plasencia, M.A.; Abreu-Gonzalez, R.; Culebras, M.A.G. Structure–Function Correlation Using OCT Angiography And Microperimetry In Diabetic Retinopathy. Clin. Ophthalmol. 2019, 13, 2181–2188. [Google Scholar] [CrossRef] [PubMed]
- Savastano, M.C.; Federici, M.; Falsini, B.; Caporossi, A.; Minnella, A.M. Detecting papillary neovascularization in proliferative diabetic retinopathy using optical coherence tomography angiography. Acta Ophthalmol. 2018, 96, 321–323. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, E.S.; Yu, S.Y. Longitudinal changes in retinal microvasculature after panretinal photocoagulation in diabetic retinopathy using swept-source OCT angiography. Sci. Rep. 2021, 11, 216. [Google Scholar] [CrossRef] [PubMed]
- Vergmann, A.S.; Sorensen, K.T.; Torp, T.L.; Kawasaki, R.; Wong, T.; Peto, T.; Grauslund, J. Optical coherence tomography angiography measured area of retinal neovascularization is predictive of treatment response and progression of disease in patients with proliferative diabetic retinopathy. Int. J. Retin. Vitr. 2020, 6, 49. [Google Scholar] [CrossRef]
- Russell, J.F.; Al-Khersan, H.; Shi, Y.; Scott, N.L.; Hinkle, J.W.; Fan, K.C.; Lyu, C.; Feuer, W.J.; Gregori, G.; Rosenfeld, P.J. Retinal Nonperfusion in Proliferative Diabetic Retinopathy Before and After Panretinal Photocoagulation Assessed by Widefield OCT Angiography. Am. J. Ophthalmol. 2020, 213, 177–185. [Google Scholar] [CrossRef]
- Lupidi, M.; Gujar, R.; Cerquaglia, A.; Chhablani, J.; Fruttini, D.; Muzi, A.; Corbucci, R.; Fiore, T.; Coscas, F.; Coscas, G.; et al. OCT-Angiography as a reliable prognostic tool in laser-treated proliferative diabetic retinopathy: The RENOCTA Study. Eur. J. Ophthalmol. 2020. Online ahead of print. [Google Scholar] [CrossRef]
- Zacharias, L.C.; Azevedo BM, S.; de Araujo, R.B.; Ciongoli, M.R.; Hatanaka, M.; Preti, R.C.; Monteiro, M.L.R. Effect of panretinal photocoagulation on the peripapillary retinal nerve fiber layer in diabetic retinopathy patients. Clinics 2019, 74, e1163. [Google Scholar] [CrossRef] [Green Version]
- Mirshahi, A.; Ghassemi, F.; Fadakar, K.; Mirshahi, R.; Bazvand, F.; Riazi-Esfahani, H. Effects of panretinal photocoagulation on retinal vasculature and foveal avascular zone in diabetic retinopathy using optical coherence tomography angiography: A pilot study. J. Curr. Ophthalmol. 2019, 31, 287–291. [Google Scholar] [CrossRef]
- Lorusso, M.; Milano, V.; Nikolopoulou, E.; Ferrari, L.M.; Cicinelli, M.V.; Querques, G.; Ferrari, T.M. Panretinal Photocoagulation Does Not Change Macular Perfusion in Eyes With Proliferative Diabetic Retinopathy. Ophthalmic Surg. Lasers Imaging Retin. 2019, 50, 174–178. [Google Scholar] [CrossRef]
- Choi, W.; Kang, H.G.; Choi, E.Y.; Kim, S.S.; Koh, H.J.; Kim, M. Effect of intravitreal bevacizumab injection before panretinal photocoagulation on the prevention of macular edema aggravation in proliferative diabetic retinopathy. J. Clin. Med. 2020, 9, 3772. [Google Scholar] [CrossRef] [PubMed]
- Chatziralli, I.; Dimitriou, E.; Theodossiadis, G.; Kazantzis, D.; Theodossiadis, P. Intravitreal ranibizumab alone or in combination with panretinal photocoagulation for the treatment of proliferative diabetic retinopathy with coexistent macular edema: Long-term outcomes of a prospective study. Acta Diabetol. 2020, 57, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Bressler, N.M.; Beaulieu, W.T.; Bressler, S.B.; Glassman, A.R.; Melia, B.M.; Jampol, L.M.; Jhaveri, C.D.; Salehi-Had, H.; Velez, G.; Sun, J.K.; et al. Anti–vascular endothelial growth factor therapy and risk of traction retinal detachment in eyes with proliferative diabetic retinopathy: Pooled Analysis of Five DRCR Retina Network Randomized Clinical Trials. Retina 2020, 40, 1021–1028. [Google Scholar] [CrossRef]
- Arevalo, J.F.; Lasave, A.F.; Kozak, I.; Al Rashaed, S.; Al Kahtani, E.; Maia, M.; Farah, M.E.; Cutolo, C.; Brito, M.; Osorio, C.; et al. Preoperative Bevacizumab for Tractional Retinal Detachment in Proliferative Diabetic Retinopathy: A Prospective Randomized Clinical Trial. Am. J. Ophthalmol. 2019, 207, 279–287. [Google Scholar] [CrossRef]
- Pan, J.; Chen, F.; Chen, D.; Yang, X.; Wang, J.; Chen, Z.; He, X.; Zhou, T.; Zheng, J.; Chen, H. Novel Three Types of Neovascularization Elsewhere Determine the Differential Clinical Features of Proliferative Diabetic Retinopathy. Retina 2020, 41, 1265–1274. [Google Scholar] [CrossRef]
- Stefansson, E. Physiology of retinal oxygenation. Acta Ophthalmol. 2015, 93. [Google Scholar] [CrossRef]
- Stefánsson, E. Ocular Oxygenation and the Treatment of Diabetic Retinopathy. Surv. Ophthalmol. 2006, 51, 364–380. [Google Scholar] [CrossRef] [PubMed]
- Sharma, T.; Fong, A.; Lai, T.Y.; Lee, V.; Das, S.; Lam, D. Surgical treatment for diabetic vitreoretinal diseases: A review. Clin. Exp. Ophthalmol. 2016, 44, 340–354. [Google Scholar] [CrossRef]
- Elhamid, A.H.A.; Mohamed, A.A.E.A.; Khattab, A.M. Intravitreal Aflibercept injection with Panretinal photocoagulation versus early Vitrectomy for diabetic vitreous hemorrhage: Randomized clinical trial. BMC Ophthalmol. 2020, 20, 130–139. [Google Scholar] [CrossRef]
- Heydon, P.; Egan, C.; Bolter, L.; Chambers, R.; Anderson, J.; Aldington, S.; Stratton, I.M.; Scanlon, P.H.; Webster, L.; Mann, S.; et al. Prospective evaluation of an artificial intelligence-enabled algorithm for automated diabetic retinopathy screening of 30 000 patients. Br. J. Ophthalmol. 2021, 105, 723–728. [Google Scholar] [CrossRef]
- Babiuch, A.S.; Wykoff, C.C.; Srivastava, S.K.; Talcott, K.; Zhou, B.; Hach, J.; Hu, M.; Reese, J.L.; Ehlers, J.P. Retinal leakage index dynamics on ultra-widefield fluorescein angiography in eyes treated with intravitreal aflibercept for proliferative diabetic retinopathy in the recovery study. Retina 2020, 40, 2175–2183. [Google Scholar] [CrossRef] [PubMed]
- Abdelsalam, M.M. Effective blood vessels reconstruction methodology for early detection and classification of diabetic retinopathy using OCTA images by artificial neural network. Informatics Med. Unlocked 2020, 20, 100390. [Google Scholar] [CrossRef]
- Akil, H.; Karst, S.; Heisler, M.; Etminan, M.; Navajas, E.; Maberley, D. Application of optical coherence tomography angiography in diabetic retinopathy: A comprehensive review. Can. J. Ophthalmol. 2019, 54, 519–528. [Google Scholar] [CrossRef]
- Reshef, E.R.; Miller, J.B.; Vavvas, D.G. Hyperspectral Imaging of the Retina: A Review. Int. Ophthalmol. Clin. 2020, 60, 85–96. [Google Scholar] [CrossRef]
- Gillies, M.C. Laser Therapy Combined with Intravitreal Aflibercept vs Intravitreal Aflibercept Monotherapy (LADAMO). Available online: https://clinicaltrials.gov/ct2/show/NCT024325472019 (accessed on 1 June 2021).
- Lujan, B.J.; Calhoun, C.T.; Glassman, A.R.; Googe, J.M.; Jampol, L.M.; Melia, M.; Schlossman, D.K.; Sun, J.K. Optical Coherence Tomography Angiography Quality Across Three Multicenter Clinical Studies of Diabetic Retinopathy. Transl. Vis. Sci. Technol. 2021, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Maturi, R.K.; Glassman, A.R.; Josic, K.; Antoszyk, A.N.; Blodi, B.A.; Jampol, L.M.; Marcus, D.M.; Martin, D.F.; Melia, M.; Salehi-Had, H.; et al. Effect of Intravitreous Anti–Vascular Endothelial Growth Factor vs Sham Treatment for Prevention of Vision-Threatening Complications of Diabetic Retinopathy. JAMA Ophthalmol. 2021, 139, 701–712. [Google Scholar] [CrossRef]
- Lee, A.Y.; Yanagihara, R.T.; Lee, C.S.; Blazes, M.; Jung, H.C.; Chee, Y.E.; Gencarella, M.D.; Gee, H.; Maa, A.Y.; Cockerham, G.C.; et al. Multicenter, Head-to-Head, Real-World Validation Study of Seven Automated Artificial Intelligence Diabetic Retinopathy Screening Systems. Diabetes Care 2021, 44, 1168–1175. [Google Scholar] [CrossRef] [PubMed]

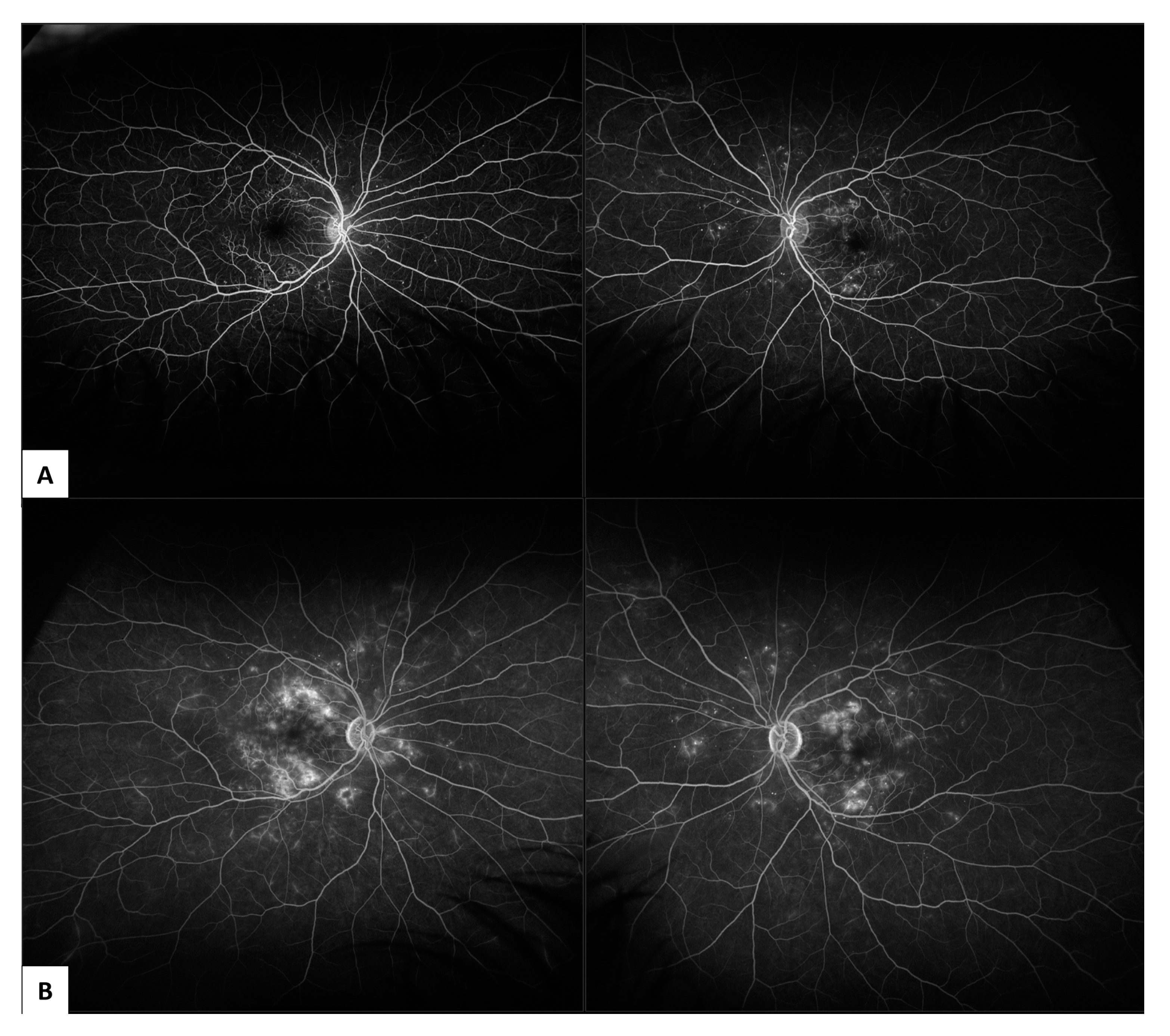
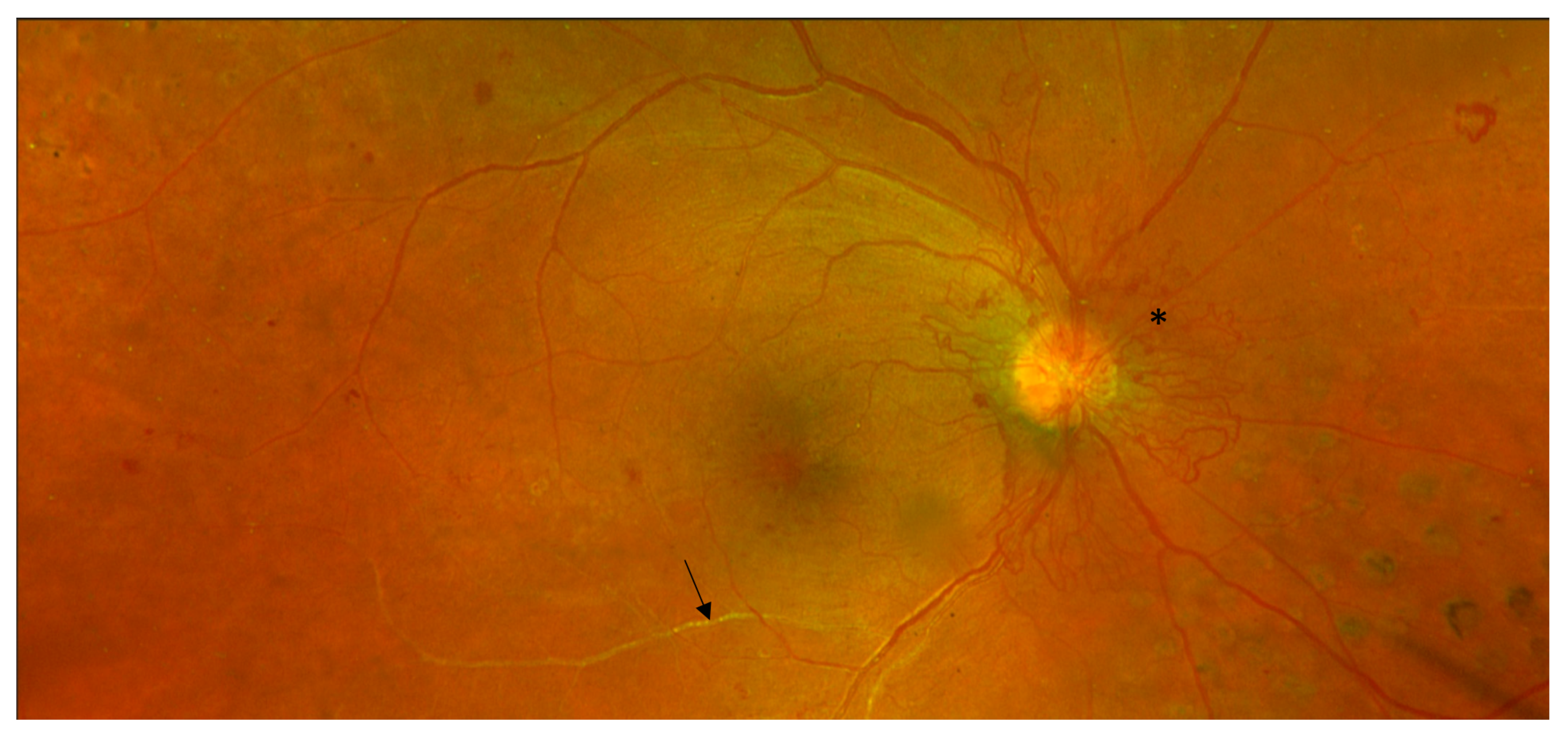

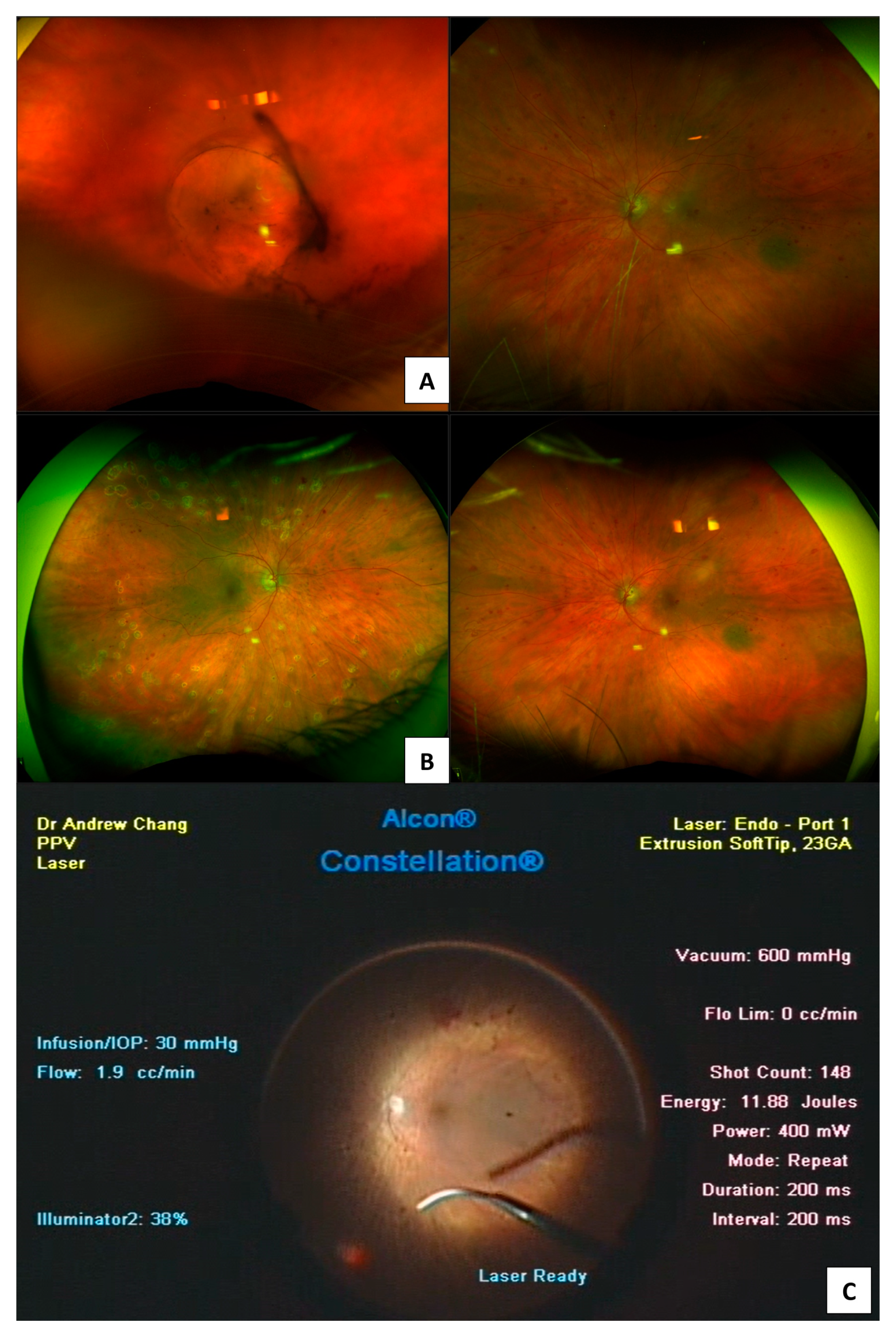
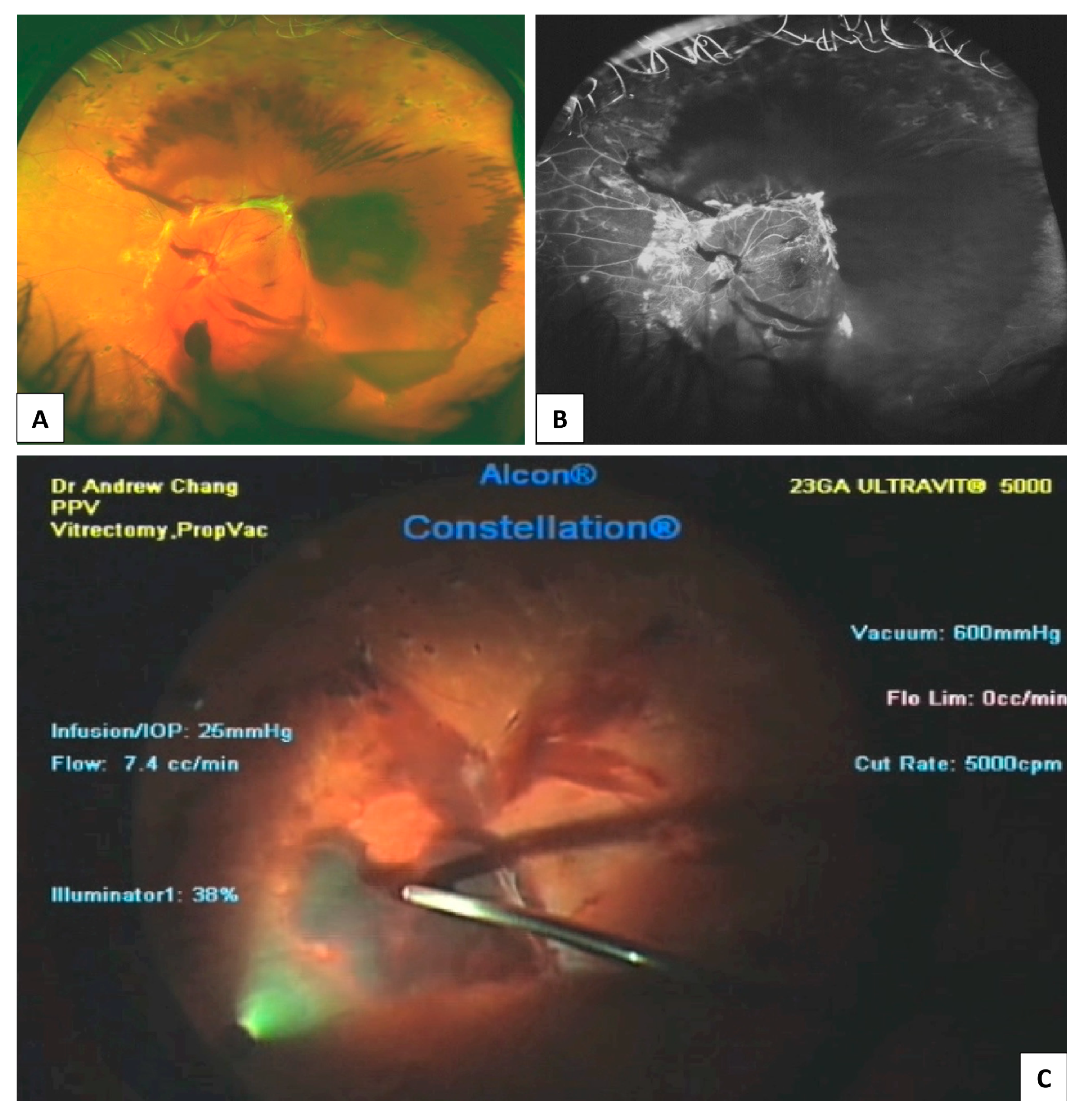
| DR Location or Feature | Imaging Findings |
|---|---|
| Retinal periphery | Capillary dropout (reduced vessel density) Dilated and tortuous capillaries |
| Choriocapillaris | Flow voids increase as DR level worsens Flow voids do not correlate with outer retinal changes Reduced mean subfoveal choroidal and choriocapillaris thickness on OCT |
| FAZ | Enlargement Loss of circularity Slower blood flow velocity in perifoveal capillaries |
| Capillary Integrity | Loss of vessel density in peripapillary plexuses and in parafovea Non-perfused parafoveal areas Reduced FD of vascular tree in far peripheral retina on UWF angiography Central non-perfused areas associated with macular thickening on OCT Deep capillary plexus vessel density decreases with increasing severity of PDR Vessel changes in the superficial retinal layers are present in later stages of PDR |
| Microaneurysms | Less easily detected on OCTA compared to FA Turnover may be used as an objective measure for therapeutic response Counts decrease following anti-VEGF treatment Usually located in the deep plexus |
| IRMA | Do not breach the ILM Greater calibre than adjacent capillaries Some progress to NVE |
| DME | Chorioscleral interface may be not clearly identified in some areas OCTA flow areas in deep plexus correspond to cystic changes OCTA demontrates reperfusion following treatment with anti-VEGF Increased vessel density of microaneurysms in perifoveal retina |
| NVD | Vessels originate outside of physiologic cup Size and vascular pattern changes are visible in OCTA Arise from retinal arteries or veins, posterior ciliary arteries, or the choroid |
| NVE | Located adjacent to areas of non-perfusion on OCTA New classification systems based on NVE origin and branching pattern |
| Study (Year, Author) | Non-Invasive Imaging Modality | Main Findings |
|---|---|---|
| 2020, Um et al. [38] | OCTA 3 mm × 3 mm field AngioVue OCT-A system using an Avanti SD-OCT device (Optovue, Inc., Fremont, CA, USA) | The FAZ area in both the superior and deep capillary plexus increased with DR progression, whereas VD progressively decreased. Changes in the FAZ area and VD were greater in the deep capillary plexus compared to the superficial capillary plexus in PDR. |
| 2020, Vaz-Pereira et al. [39] | SD-OCT (Spectralis, Heidelberg Engineering, Heidelberg, Germany) | Near-infrared reflectance imaging with OCT was used to qualitatively grade both NVE and NVD neovascular complexes and IRMA growing into NVE over a mean follow-up 3.2 ± 1.7 years of DR progression in 20 eyes. Neovascular complexes were observed for progression or regression by the identification of hyporeflective vascular fronds. |
| 2020, Tan et al. [12] | Wide-field OCTA 12 mm × 12 mm field SS-OCT system (PlexElite 9000; Zeiss Meditec) | Wide-field (12 mm × 12 mm) fovea-centred OCTA examined retinal perfusion density, capillary perfusion density, large vessel density and capillary dropout density in 76 diabetic eyes. Microvascular perfusion may be useful for detecting predominant peripheral capillary dropout in eyes with DR. |
| 2020, Schwartz et al. [40] | OCT 12 mm × 9 mm field & OCTA 6 mm × 6 mm field Topcon OCT (DRI OCT-1, Triton, Topcon, 151 Tokyo, Japan) | Structural OCT had a higher detection of new-onset NVD and NVE compared to B-scan OCTA, en face OCTA and colour photography. Change in NVD or NVE (either regression or progression) was best detected by B-scan OCTA. |
| 2020, Levine et al. [41] | OCTA 3 mm × 3 mm field SD-RTVue XR Avanti with AngioVue (Optovue, Inc., Fremont, CA, USA), the SD- Cirrus HD-OCT 5000 (Carl Zeiss Meditec, Dublin, CA, USA), and the swept-source PLEX Elite 9000 (Carl Zeiss Meditec, Dublin, CA, USA) | Full retinal layer vessel density is more precise for follow-up than OCTA images of the superficial or deep capillary plexus alone when comparing progression of PDR in the same and among different OCTA devices. |
| 2020, Kase et al. [42] | OCT 9 mm × 9 mm field (Cirrus HD OCT; Carl Zeiss Meditec, Dublin, CA, USA) | Choroidal morphology analysis in treatment-naïve eye with DR showed significantly lower ratio of luminal area to total choroidal area in diabetic eyes compared to normal eyes. No change in central choroidal thickness was noted between eyes with and without DR. |
| 2020, Hirano et al. [43] | OCTA 12 mm × 12 mm field PLEX Elite 9000 (Carl Zeiss Meditec, Dublin, CA, USA) | The efficacy of OCTA for detecting NVD and NVE in PDR was comparable to the sensitivity of that detected by FA. Additionally, OCTA may be better than FA for detecting IRMA. |
| 2020, Ashraf et al. [44] | OCTA 3 mm × 3 mm field RTVue XR Avanti SD- OCT device with AngioVue software (Optovue, Fremont, CA, USA) | OCTA metrics showed reducing vessel density in the superficial, intermediate and deep layers of the capillary plexus with increasing severity of DR. In eyes with advanced DR vascular changes were present primarily in the superficial capillary plexus. |
| 2019, Wang & Tao [45] | OCTA (Spectralis, Heidelberg Engineering, Heidelberg, Germany) | The ratio of the luminal to choroidal area was decreased in eyes with DR compared to normal controls. The choroidal vascularity index decreased with increasing severity of DR. Changes in the luminal area to choroidal area ratio may predict DR development before other clinical signs are evident. |
| 2019, Motulsky et al., [46] | Wide-field OCTA 12 mm × 12 mm field PLEX Elite 9000 (Carl Zeiss Meditec, Dublin, CA, USA) | Decreased retinal perfusion, increased retinal thickness and neovascularization can be identified from 12 mm × 12 mm OCTA imaging in PDR. |
| 2019, La Mantia et al. [47] | OCTA 4.5 mm × 4.5 mm & 3 mm × 3 mm field Topcon DRI OCT Triton device (Topcon Corporation, Tokyo, Japan) | There is good agreement between FFA and 4.5 mm × 4.5 mm and 3 mm × 3 mm OCTA FAZ area measurements in grading diabetic macular ischaemia in patients with DR. |
| 2019, Hsiao et al. [48] | OCTA 3 mm × 3 mm field (AngioVue; Optovue Inc., Fremont, CA, USA) | Nineteen eyes with PDR showed reductions in vascular density with increasing progression of DR. Decreased deep retinal vascular density were proportional to severity of DR. |
| 2019, Hirano et al. [49] | OCTA 3 mm × 3 mm, 6 mm × 6 mm & 12 mm × 12 mm field (PLEX Elite 9000; Carl Zeiss Meditec, Dublin, CA, USA) | Twenty-three eyes with PDR showed worse perfusion density, vessel length density and fractal dimension with worsening DR in all scan sizes compared to normal eyes. Of all OCTA parameters, perfusion density, vessel length density, and fractal dimension best predict DR. |
| 2019, Cui et al. [50] | Wide-field OCTA 15 mm × 9 mm field PLEX Elite 9000 (Carl Zeiss Meditec, Dublin, CA, USA) | Motion artifacts and segmentation errors in montage images may affect reliability of results in widefield OCTA analysis. Higher levels of motion artefact limit the ability to visualise fine capillary vessels and DR features. |
| 2019, Alonso-Plasencia et al. [51] | OCTA 4.5 mm × 4.5 mm field Nidek RS-3000 Advance 2 AngioScan (Nidek, Japan) Microperimetry MP-3 (Nidek, Gamagori, Japan) | OCTA: Reduced vessel density in DR patients compared to normal controls. Microperimetry: Reduced mean retinal sensitivity in DR (27.68 ± 2.71 dB) compared to normal controls (31.68 ± 1.46) (p < 0.05). Correlation (r = 0.501, p = 0.01) between microperimetry and OCTA in the area temporal to the fovea was found in patients with DR. |
| 2018, Savastano et al. [52] | OCTA 3 mm × 3 mm & 6 mm × 6 mm fields (AngioVue; Optovue Inc., Fremont, CA, USA) | OCTA shows blood flow in neovascularisations without artefacts due to dye leakage in PDR. Direct morphological signs of pathologic microvessels were able to be visualised to diagnose NVD in PDR. |
| 2018, Pan et al. [8] | OCTA 3 mm × 3 mm & 4.5 mm × 4.5 mm fields RTVue XR Avanti (RT-VUE XR, Optivue, Fremont, CA, USA) | OCTA was able to detect microstructure of new vessels to identify their origins and locations and to distinguish preretinal new vessels from IRMAs. Thirty-five eyes with PDR showed three distinct origins of NVE and NVDs; originating from the venous side, originating from capillary networks, or originating from IRMAs. |
| Study | Non-Invasive Imaging Modality | Main Findings |
|---|---|---|
| 2021, Kim et al. [53] | SD-OCT 12 mm × 12 mm field Cirrus HD-OCT (Carl Zeiss Meditec, Inc., Dublin, CA, USA) OCTA 3 mm × 3 mm & 12 mm × 12 mm fields (PLEX Elite 9000, Carl Zeiss Meditec, Dublin, CA, USA) | Retinal microvascular changes in 27 treatment-naïve eyes with PDR subjected to argon laser PRP; SD-OCT: Mean sub-foveal choroidal thickness progressively decreased at 1, 3, 6, and 12 months following PRP, although the change was not statistically significant (p = 0.108). Mean CFT progressively increased over 12 months following PRP (p = 0.103). Mean macular ganglion cell inner plexiform layer thickness progressively decreased over 12 months following PRP (p = 0.351). OCTA: FAZ area in the superficial capillary plexus and deep capillary plexus decreased after 12 months from PRP treatment. Perfusion density and vessel length density of the superficial capillary plexus increased from 3 months after PRP treatment. The total non-perfusion area progressively decreased over 12 months (p = 0.082). |
| 2020, Vergmann et al. [54] | OCTA 4.5 mm × 4.5 mm field DRI OCT Triton, swept source OCT, (Topcon Corporation, Tokyo, Japan) | Retinal microvascular changes in 21 treatment-naïve eyes with PDR subjected to PRP; the area of NVE measured by OCTA was able to distinguish between progression and non-progression of PDR from 6 months after PRP treatment. |
| 2020, Russell et al. [55] | Wide-field OCTA 12 mm × 12 mm field WF SS-OCTA (PLEX Elite 9000, Carl Zeiss Meditec, Inc., Dublin, CA, USA) | Areas of retinal non-perfusion identified on wide-field OCTA co-localised with areas identified by invasive UWF FA. No significant changes in retinal non-perfusion areas were seen on OCTA 3 months following PRP in patients with PDR. |
| 2020, Lupidi et al. [56] | OCTA 3 mm × 3 mm field (Spectralis HRA OCT, Heidelberg Engineering, Heidelberg, Germany) | Retinal neovascular area, vascular perfusion density, and fractal dimension were assessed by OCTA pre- and post-PRP in 15 eyes with PDR. A reduction in the retinal neovascular area detected by non-invasive imaging is predictive of PRP treatment efficacy and may reveal those eyes that may not require additional treatment. |
| 2020, Zacharias et al. [57] | OCT SD-OCT (Heidelberg Engineering, Heidelberg, Germany | PRP was not found to cause significant changes in peripapillary RNFL thickness in diabetic PDR patients 12 months following treatment. |
| 2019, Mirshahi et al. [58] | OCTA 12 mm × 12 mm field DRI OCT swept source Triton Plus, (Topcon Corporation, Tokyo, Japan) | The FAZ area was unchanged, whilst foveal vascular density and retinal thickness increased in 11 eyes with PDR following PRP. |
| 2019, Lorusso et al. [59] | OCTA (AngioVue XR Avanti; Optovue, Fremont, CA, USA) | Vessel density in the superficial and deep capillary plexus and the size of the foveal avascular zone was unchanged 6 months after PRP treatment for PDR. |
| Study | Non-Invasive Imaging Modality | Main Findings |
|---|---|---|
| 2020, Choi et al. [60] | SD-OCT SD-OCT device (Spectralis OCT, Heidelberg Engineering, Franklin, MA, USA) | Intravitreal bevacizumab injection before PRP leads to decreased CMT in patients diagnosed with PDR (p = 0.002). |
| 2020, Chatziralli et al. [61] | OCT Spectralis HRA + OCT, Heidelberg Engineering, Germany | Twenty-four patients with PDR treated with concurrent PRP plus at least three intravitreal injections of ranibizumab showed greater regression of neovascularization with a smaller number of injections over twenty-four months compared to twenty-three patients receiving anti-VEGF injections alone. |
| 2020, Bressler et al. [62] | OCT (multi-centre RCTs) | Anti-VEGF therapy to manage DMO or PDR was not found to increase the risk of TRD. In eyes with PDR without macula-threatening TRD, anti-VEGF therapy did not increase the risk of developing TRD in a pooled analysis from the five DRCR Retina Network RCTs. |
| 2019, Arevalo et al. [63] | OCT (multi-centre RCT) | Preoperative intravitreal bevacizumab reduced the risk of intraoperative bleeding and improved surgical field visualization in 102 eyes with TRD secondary to PDR undergoing PPV compared to PPV alone. |
| Study | Non-Invasive Imaging Modality | Main Findings |
|---|---|---|
| 2020, Rush et al. [9] | OCT (Zeiss Cirrus HD-OCT; Carl Zeiss Meditec, Inc., Dublin, CA, USA) | Improved vision, fewer postoperative diabetic macular oedema treatments and lower incidence of epiretinal membrane when ILM peeling was performed in patients (n = 207) with PDR undergoing PPV for VH. CMT tended to be lower in patients who had ILM peeled compared to those who did not. |
| 2020, Abd Elhamid et al. [68] | Fundus examination with indirect ophthalmoscopy | Early vitrectomy for diabetic VH leads to faster gains in visual acuity with less incidence of recurrent VH compared to intravitreal anti-VEGF alone. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bowditch, E.; Chang, A.; Mehta, H. Developments in Non-Invasive Imaging to Guide Diagnosis and Treatment of Proliferative Diabetic Retinopathy: A Systematic Review. Int. J. Transl. Med. 2021, 1, 332-352. https://doi.org/10.3390/ijtm1030020
Bowditch E, Chang A, Mehta H. Developments in Non-Invasive Imaging to Guide Diagnosis and Treatment of Proliferative Diabetic Retinopathy: A Systematic Review. International Journal of Translational Medicine. 2021; 1(3):332-352. https://doi.org/10.3390/ijtm1030020
Chicago/Turabian StyleBowditch, Ellie, Andrew Chang, and Hemal Mehta. 2021. "Developments in Non-Invasive Imaging to Guide Diagnosis and Treatment of Proliferative Diabetic Retinopathy: A Systematic Review" International Journal of Translational Medicine 1, no. 3: 332-352. https://doi.org/10.3390/ijtm1030020
APA StyleBowditch, E., Chang, A., & Mehta, H. (2021). Developments in Non-Invasive Imaging to Guide Diagnosis and Treatment of Proliferative Diabetic Retinopathy: A Systematic Review. International Journal of Translational Medicine, 1(3), 332-352. https://doi.org/10.3390/ijtm1030020





