Cardiac Glycosides Increase Temozolomide Anticancer Activity in Therapy Resistant Glioblastoma Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. General Cell Culture
2.3. Drug Cytotoxicity Screening
2.4. MTT Assay
2.5. Immunohistochemical Monolayer Viability Analysis of T98G Resistant Glioma
2.6. Soft Agar Assay
3. Results
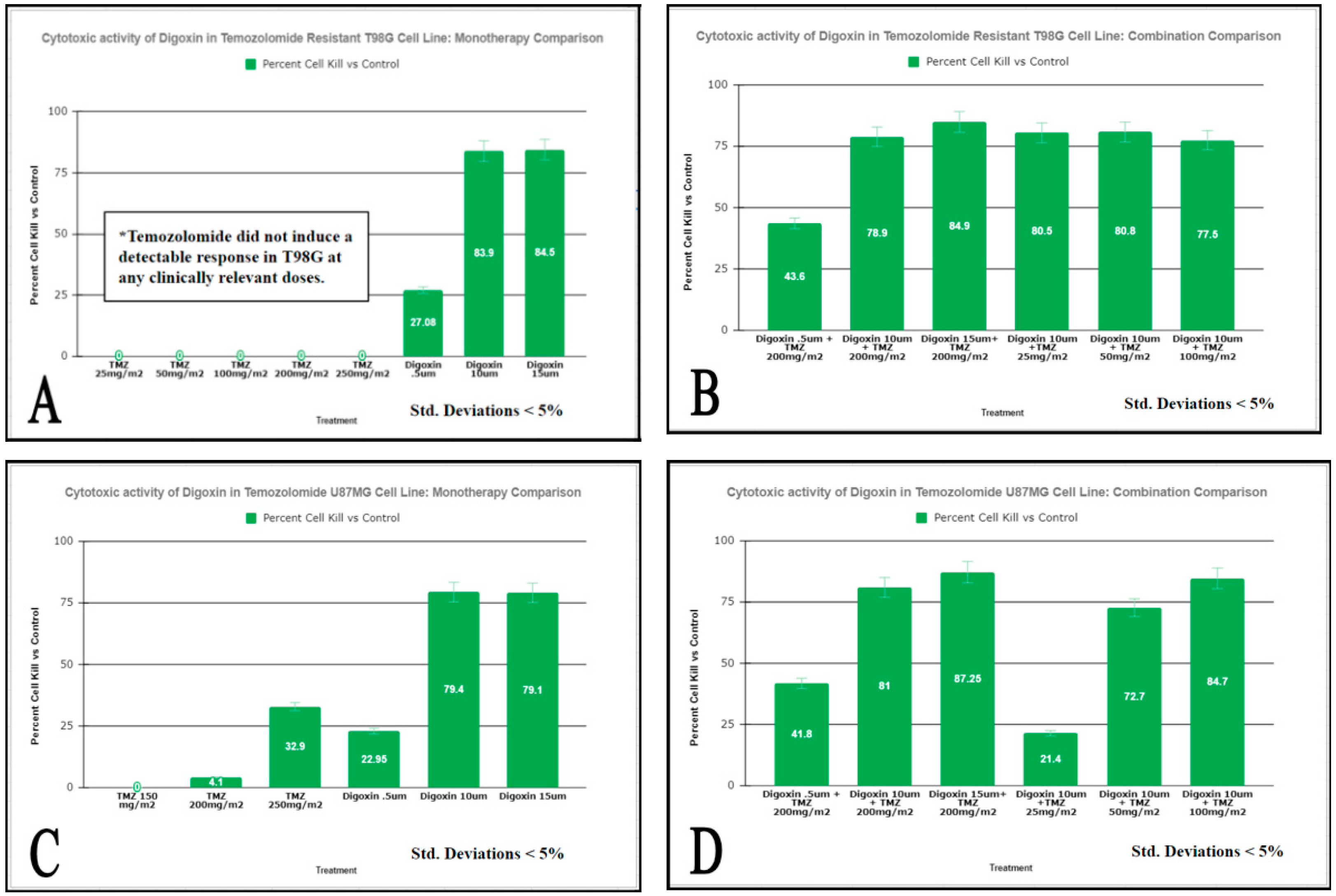
3.1. Digoxin Decreases the Viability of GBM Cells Resistant to TMZ
3.2. Digoxin Significantly Inhibited GBM Soft Agar Colony Formation
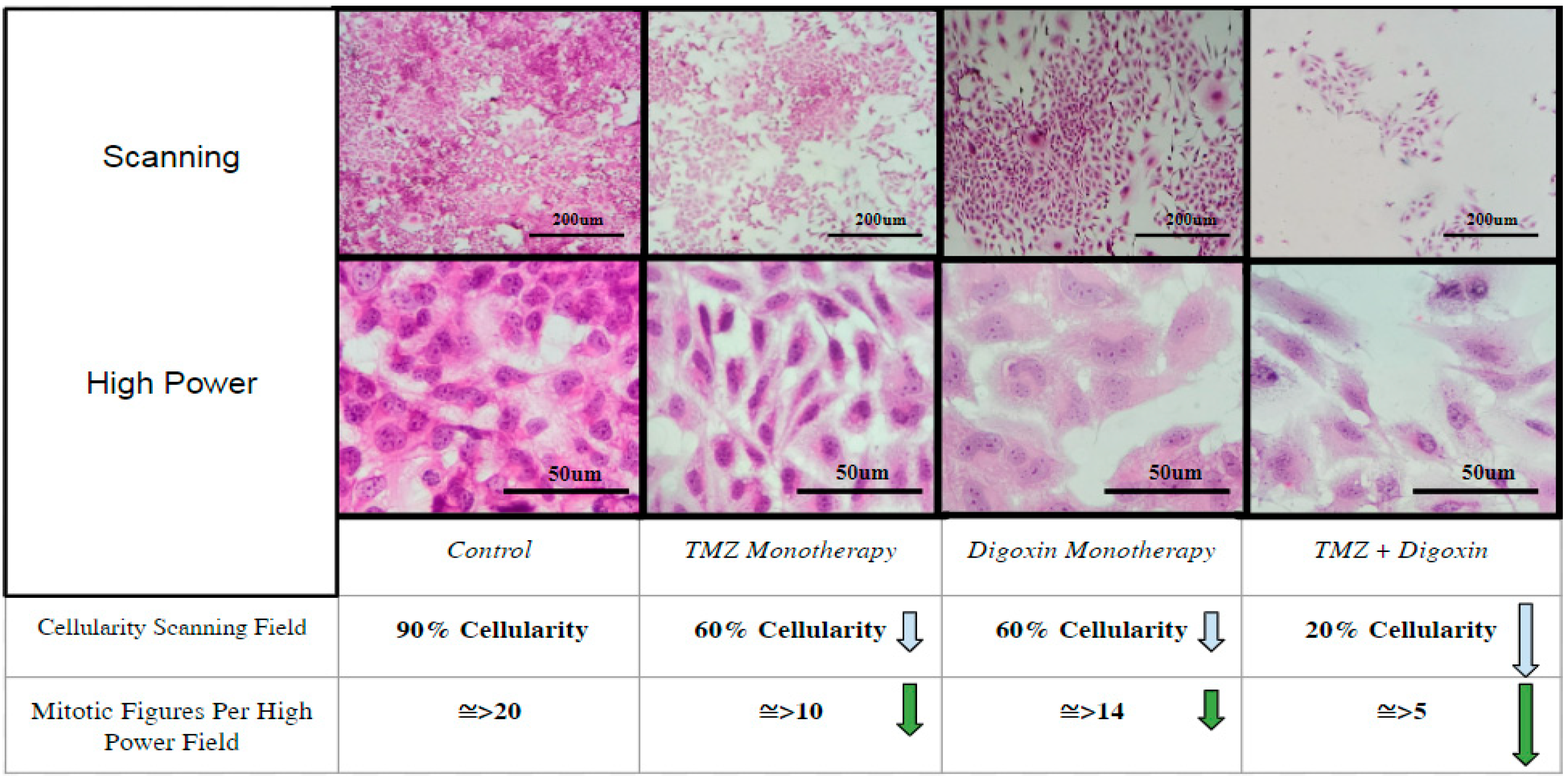
3.3. Detection of Digoxin Inhibition by Immunohistochemistry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Desjardins, A.; Rich, J.N.; Quinn, J.A.; Vredenburgh, J.; Gururangan, S.; Sathornsumetee, S.; Reardon, D.A.; Friedman, A.H.; Bigner, D.D.; Friedman, H.S. Chemotherapy and novel therapeutic approaches in malignant glioma. Front. Biosci. 2005, 10, 2645–2668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lefranc, F.; Brotchi, J.; Kiss, R. Possible future issues in the treatment of glioblastomas: Special emphasis on cell migration and the resistance of migrating glioblastoma cells to apoptosis. J. Clin. Oncol. 2005, 23, 2411–2422. [Google Scholar] [CrossRef] [PubMed]
- Giese, A.; Bjerkvig, R.; Berens, M.E.; Westphal, M. Cost of migration: Invasion of malignant gliomas and implications for treatment. J. Clin. Oncol. 2003, 21, 1624–1636. [Google Scholar] [CrossRef] [PubMed]
- Hoelzinger, D.B.; Mariani, L.; Weis, J.; Woyke, T.; Berens, T.J.; McDonough, W.S.; Sloan, A.; Coons, S.W.; Berens, M.E. Gene expression profile of glioblastoma multiforme invasive phenotype points to new therapeutic targets. Neoplasia 2005, 7, 7–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mijatovic, T.; Ingrassia, L.; Facchini, V.; Kiss, R. Na+/K+-ATPase alpha subunits as new targets in anticancer therapy. Expert Opin. Ther. Targets 2008, 12, 1403–1417. [Google Scholar] [CrossRef] [PubMed]
- Lefranc, F.; Kiss, R. The sodium pump alpha1 subunit as a potential target to combat apoptosis-resistant glioblastomas. Neoplasia 2008, 10, 198–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haar, C.P.; Hebbar, P.; Wallace, G.C.T.; Das, A.; Vandergrift, W.A., 3rd; Smith, J.A.; Giglio, P.; Patel, S.J.; Ray, S.K.; Banik, N.L. Drug resistance in glioblastoma: A mini-review. Neurochem. Res. 2012, 37, 1192–1200. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.P.; Bhardwaj, G.; Gerlach, J.H.; Mackie, J.E.; Grant, C.E.; Almquist, K.C.; Stewart, A.J.; Kurz, E.U.; Duncan, A.M.; Deeley, R.G. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science 1992, 258, 1650–1654. [Google Scholar] [CrossRef] [PubMed]
- Prassas, I.; Diamandis, E.P. Novel therapeutic applications of cardiac glycosides. Nat. Rev. Drug Discov. 2008, 7, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Song, M.; Mohamad, O.; Yu, S.P. Inhibition of Na+/K+-ATPase induces hybrid cell death and enhanced sensitivity to chemotherapy in human glioblastoma cells. BMC Cancer 2014, 14, 716–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calderón-Montaño, J.M.; Burgos-Morón, E.; Orta, M.L.; Maldonado-Navas, D.; García-Domínguez, I.; López-Lázaro, M. Evaluating the cancer therapeutic potential of cardiac glycosides. BioMed Res. Int. 2014, 2014, 794930. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Sliwinska, P.; Scapozza, L.; Ruiz Altaba, A. Drug repurposing in oncology: Compounds, pathways, phenotypes and computational approaches for colorectal cancer. Biochimica et Biophysica Acta. Rev. Cancer 2019, 1871, 434–454. [Google Scholar] [CrossRef]
- Wu, B.; Zhu, J.-S.; Zhang, Y.; Shen, W.-M.; Zhang, Q. Predictive value of MTT assay as an in vitro chemosensitivity testing for gastric cancer: One institution’s experience. World J. Gastroenterol. 2008, 14, 3064–3068. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valluri, A.; Lawrence, L.; Denning, K.L.; Cuda, J.; Zhu, G.-Z. Cardiac Glycosides Increase Temozolomide Anticancer Activity in Therapy Resistant Glioblastoma Cells. Int. J. Transl. Med. 2022, 2, 148-155. https://doi.org/10.3390/ijtm2020012
Valluri A, Lawrence L, Denning KL, Cuda J, Zhu G-Z. Cardiac Glycosides Increase Temozolomide Anticancer Activity in Therapy Resistant Glioblastoma Cells. International Journal of Translational Medicine. 2022; 2(2):148-155. https://doi.org/10.3390/ijtm2020012
Chicago/Turabian StyleValluri, Anisha, Logan Lawrence, Krista L. Denning, Jonathan Cuda, and Guo-Zhang Zhu. 2022. "Cardiac Glycosides Increase Temozolomide Anticancer Activity in Therapy Resistant Glioblastoma Cells" International Journal of Translational Medicine 2, no. 2: 148-155. https://doi.org/10.3390/ijtm2020012
APA StyleValluri, A., Lawrence, L., Denning, K. L., Cuda, J., & Zhu, G.-Z. (2022). Cardiac Glycosides Increase Temozolomide Anticancer Activity in Therapy Resistant Glioblastoma Cells. International Journal of Translational Medicine, 2(2), 148-155. https://doi.org/10.3390/ijtm2020012





