Osteopontin in Cancer: Mechanisms and Therapeutic Targets
Abstract
:1. Introduction
2. OPN Structure and Functions
3. OPN Expression in Tumors
4. Regulation of OPN Expression in Tumors
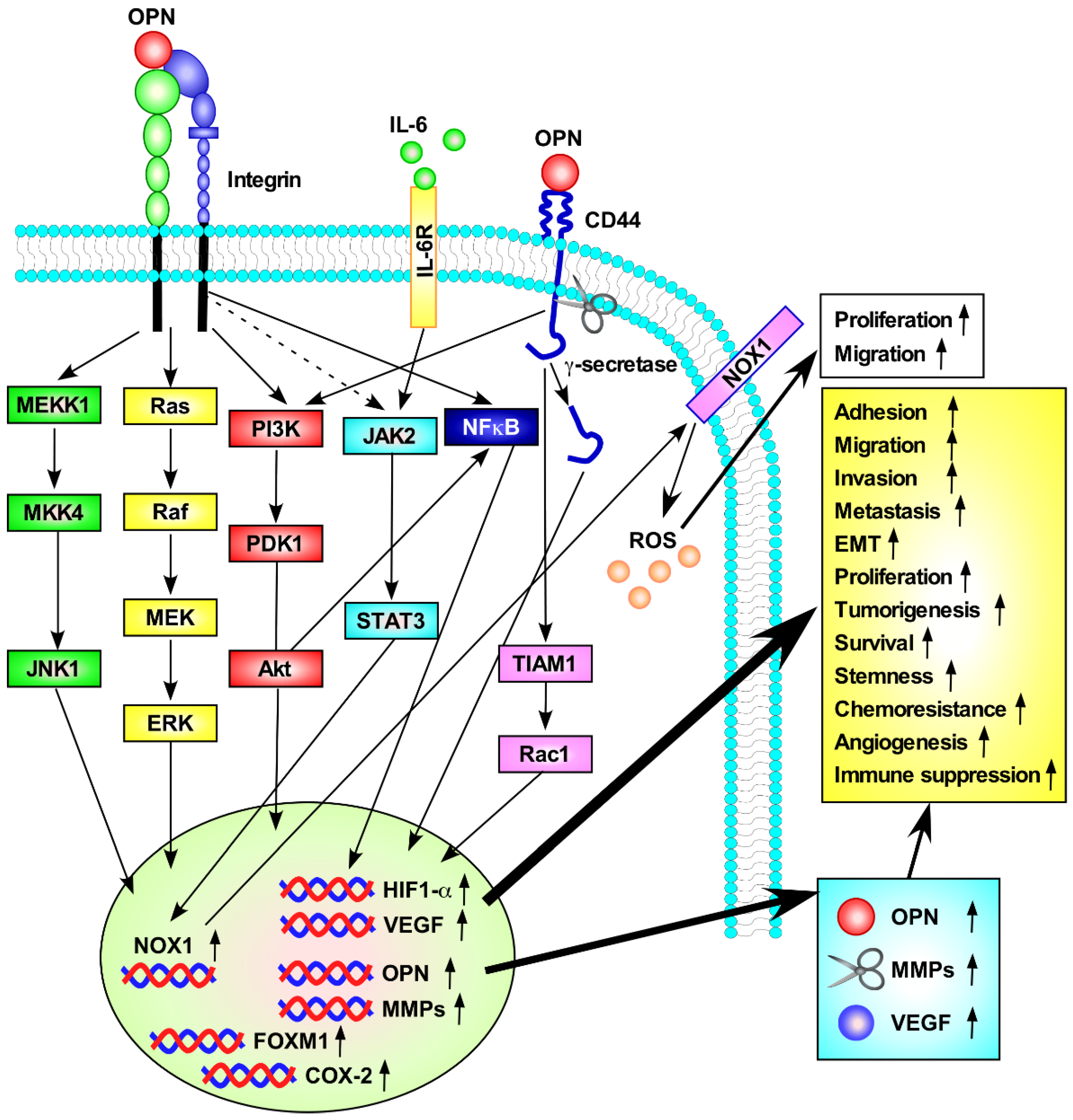
5. OPN in Tumor Progression
5.1. OPN Receptors and Their Relationship to the Progression of Tumors
5.1.1. Integrin Receptors
5.1.2. CD44 Receptors
5.2. Role of OPN in Key Events for Tumor Progression
5.2.1. EMT
5.2.2. CSC Property
5.2.3. Chemoresistance
5.2.4. Tumor Angiogenesis
5.2.5. Senescence
5.2.6. Bone Metastasis
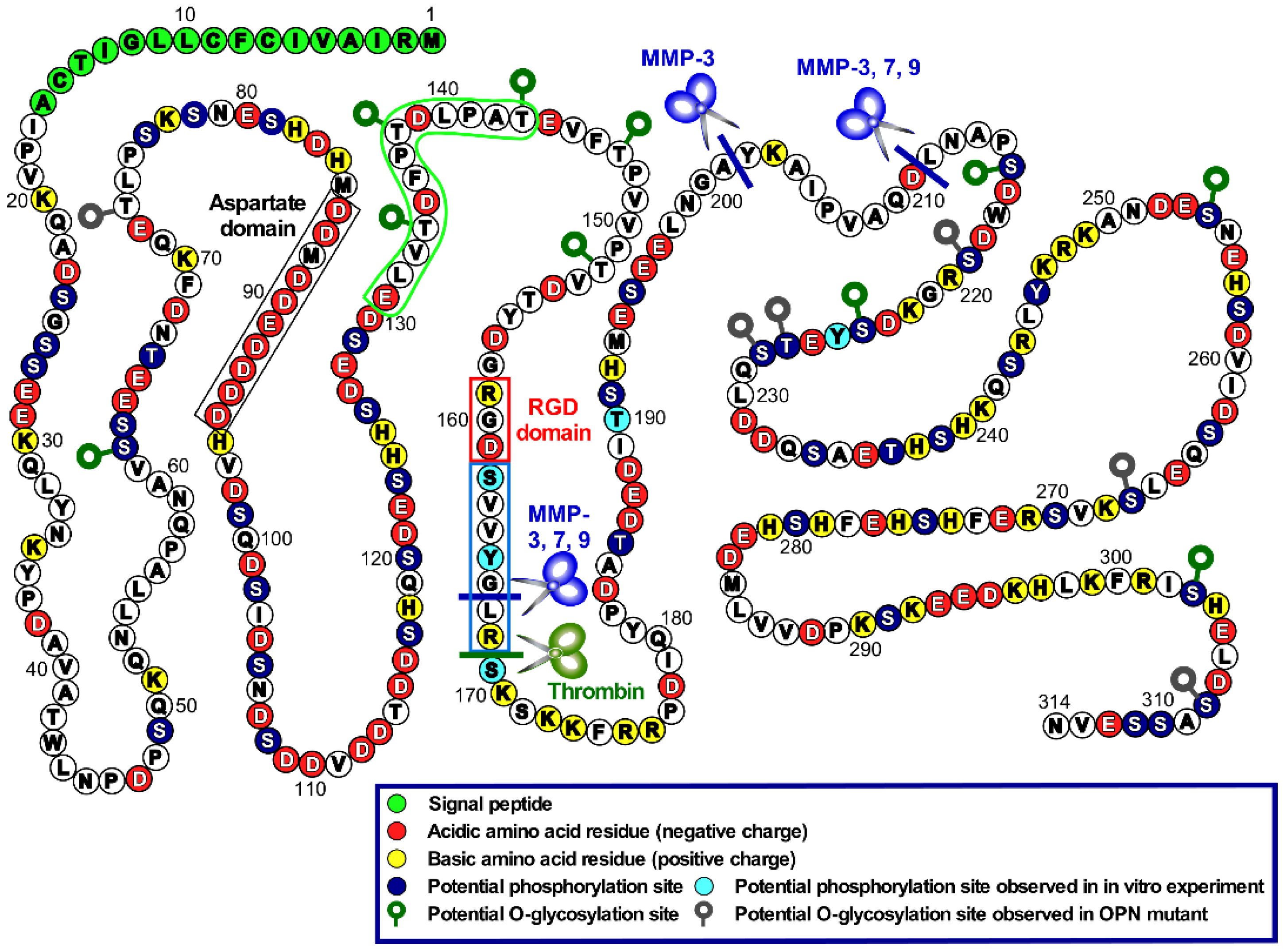
6. PTM of OPN in Tumors
6.1. Proteolytic Processing
6.2. Phosphorylation
6.3. Glycosylation
7. OPN and the Immune System in Cancer
7.1. Tumor-Associated Macrophages (TAMs)
7.2. Immune Checkpoint
8. OPN and Cancer-Associated Fibroblasts (CAFs)
9. Diagnostic and Therapeutic Applications of OPN in Cancer
9.1. Potential Applications as a Biomarker
9.2. Potential Applications as a Therapeutic Target
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.B.; Ha, S.-J.; Kim, H.R. Clinical Insights into Novel Immune Checkpoint Inhibitors. Front. Pharmacol. 2021, 12, 681320. [Google Scholar] [CrossRef] [PubMed]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Liu, S.; Zhang, S.; Min, L.; Zhu, S. Cellular and Extracellular Components in Tumor Microenvironment and Their Application in Early Diagnosis of Cancers. Anal. Cell. Pathol. 2020, 2020, 6283796. [Google Scholar] [CrossRef]
- Senger, D.R.; Wirth, D.F.; Hynes, R.O. Transformed mammalian cells secrete specific proteins and phosphoproteins. Cell 1979, 16, 885–893. [Google Scholar] [CrossRef]
- Moorman, H.R.; Poschel, D.; Klement, J.D.; Lu, C.; Redd, P.S.; Liu, K. Osteopontin: A Key Regulator of Tumor Progression and Immunomodulation. Cancers 2020, 12, 3379. [Google Scholar] [CrossRef]
- Pang, X.; Gong, K.; Zhang, X.; Wu, S.; Cui, Y.; Qian, B.-Z. Osteopontin as a multifaceted driver of bone metastasis and drug resistance. Pharmacol. Res. 2019, 144, 235–244. [Google Scholar] [CrossRef]
- Kariya, Y.; Kanno, M.; Matsumoto-Morita, K.; Konno, M.; Yamaguchi, Y.; Hashimoto, Y. Osteopontin O-glycosylation contributes to its phosphorylation and cell-adhesion properties. Biochem. J. 2014, 463, 93–102. [Google Scholar] [CrossRef]
- Kariya, Y.; Oyama, M.; Kariya, Y.; Hashimoto, Y. Phosphorylated Osteopontin Secreted from Cancer Cells Induces Cancer Cell Motility. Biomolecules 2021, 11, 1323. [Google Scholar] [CrossRef]
- Peraramelli, S.; Zhou, Q.; Zhou, Q.; Wanko, B.; Zhao, L.; Nishimura, T.; Leung, T.H.; Mizuno, S.; Ito, M.; Myles, T.; et al. Thrombin cleavage of osteopontin initiates osteopontin’s tumor-promoting activity. J. Thromb. Haemost. 2022, 20, 1256–1270. [Google Scholar] [CrossRef]
- Minai-Tehrani, A.; Chang, S.-H.; Park, S.B.; Cho, M.-H. The O-glycosylation mutant osteopontin alters lung cancer cell growth and migration in vitro and in vivo. Int. J. Mol. Med. 2013, 32, 1137–1149. [Google Scholar] [CrossRef] [PubMed]
- Kariya, Y.; Oyama, M.; Hashimoto, Y.; Gu, J.; Kariya, Y. β4-Integrin/PI3K Signaling Promotes Tumor Progression through the Galectin-3-N-Glycan Complex. Mol. Cancer Res. 2018, 16, 1024–1034. [Google Scholar] [CrossRef]
- Schytte, G.N.; Christensen, B.; Bregenov, I.; Kjøge, K.; Scavenius, C.; Petersen, S.V.; Enghild, J.J.; Sørensen, E.S. FAM20C phosphorylation of the RGDSVVYGLR motif in osteopontin inhibits interaction with the αvβ3 integrin. J. Cell. Biochem. 2020, 121, 4809–4818. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Zhang, B.; Wang, L.; Wu, L.; Kan, Q.; Fan, K. MMP-9-cleaved osteopontin isoform mediates tumor immune escape by inducing expansion of myeloid-derived suppressor cells. Biochem. Biophys. Res. Commun. 2017, 493, 1478–1484. [Google Scholar] [CrossRef]
- Virchow, R. Cellular Pathology as Based upon Physiological and Pathological Histology; J. B. Lippincott: Philadelphia, PA, USA, 1863. [Google Scholar]
- Gerarduzzi, C.; Hartmann, U.; Leask, A.; Drobetsky, E. The Matrix Revolution: Matricellular Proteins and Restructuring of the Cancer Microenvironment. Cancer Res. 2020, 80, 2705–2717. [Google Scholar] [CrossRef] [PubMed]
- Lamort, A.S.; Giopanou, I.; Psallidas, I.; Stathopoulos, G.T. Osteopontin as a Link between Inflammation and Cancer: The Thorax in the Spotlight. Cells 2019, 8, 815. [Google Scholar] [CrossRef] [PubMed]
- Rosmus, D.-D.; Lange, C.; Ludwig, F.; Ajami, B.; Wieghofer, P. The Role of Osteopontin in Microglia Biology: Current Concepts and Future Perspectives. Biomedicines 2022, 10, 840. [Google Scholar] [CrossRef]
- Singh, A.; Gill, G.; Kaur, H.; Amhmed, M.; Jakhu, H. Role of osteopontin in bone remodeling and orthodontic tooth movement: A review. Prog. Orthod. 2018, 19, 18. [Google Scholar] [CrossRef]
- Kariya, Y.; Kariya, Y.; Saito, T.; Nishiyama, S.; Honda, T.; Tanaka, K.; Yoshida, M.; Fujihara, K.; Hashimoto, Y. Increased cerebrospinal fluid osteopontin levels and its involvement in macrophage infiltration in neuromyelitis optica. BBA Clin. 2015, 3, 126–134. [Google Scholar] [CrossRef]
- Demmelmair, H.; Prell, C.; Timby, N.; Lönnerdal, B. Benefits of Lactoferrin, Osteopontin and Milk Fat Globule Membranes for Infants. Nutrients 2017, 9, 817. [Google Scholar] [CrossRef]
- Yang, L.; Chen, J.H.; Cai, D.; Wang, L.Y.; Zha, X.L. Osteopontin and integrin are involved in cholesterol gallstone formation. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2012, 18, BR16–BR23. [Google Scholar] [CrossRef]
- Kahles, F.; Findeisen, H.M.; Bruemmer, D. Osteopontin: A novel regulator at the cross roads of inflammation, obesity and diabetes. Mol. Metab. 2014, 3, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Sodek, J.; Ganss, B.; McKee, M.D. Osteopontin. Critical reviews in oral biology and medicine: An official publication of the American Association of Oral Biologists. Crit. Rev. Oral Biol. Med. 2000, 11, 279–303. [Google Scholar] [CrossRef] [PubMed]
- Kazanecki, C.C.; Uzwiak, D.J.; Denhardt, D.T. Control of osteopontin signaling and function by post-translational phosphorylation and protein folding. J. Cell. Biochem. 2007, 102, 912–924. [Google Scholar] [CrossRef] [PubMed]
- Yim, A.; Smith, C.; Brown, A.M. Osteopontin/secreted phosphoprotein-1 harnesses glial-, immune-, and neuronal cell ligand-receptor interactions to sense and regulate acute and chronic neuroinflammation. Immunol. Rev. 2022. early view. [Google Scholar] [CrossRef]
- Song, Z.; Chen, W.; Athavale, D.; Ge, X.; Desert, R.; Das, S.; Han, H.; Nieto, N. Osteopontin Takes Center Stage in Chronic Liver Disease. Hepatology 2021, 73, 1594–1608. [Google Scholar] [CrossRef]
- Anan, G.; Yoneyama, T.; Noro, D.; Tobisawa, Y.; Hatakeyama, S.; Sutoh Yoneyama, M.; Yamamoto, H.; Imai, A.; Iwamura, H.; Kohada, Y.; et al. The Impact of Glycosylation of Osteopontin on Urinary Stone Formation. Int. J. Mol. Sci. 2020, 21, 93. [Google Scholar] [CrossRef]
- Kurzbach, D.; Platzer, G.; Schwarz, T.C.; Henen, M.A.; Konrat, R.; Hinderberger, D. Cooperative Unfolding of Compact Conformations of the Intrinsically Disordered Protein Osteopontin. Biochemistry 2013, 52, 5167–5175. [Google Scholar] [CrossRef]
- Wright, P.E.; Dyson, H.J. Intrinsically disordered proteins in cellular signalling and regulation. Nat. Rev. Mol. Cell Biol. 2015, 16, 18–29. [Google Scholar] [CrossRef]
- Martinelli, A.; Lopes, F.; John, E.; Carlini, C.; Ligabue-Braun, R. Modulation of Disordered Proteins with a Focus on Neurodegenerative Diseases and Other Pathologies. Int. J. Mol. Sci. 2019, 20, 1322. [Google Scholar] [CrossRef]
- Gao, J.; Xu, D. Correlation between posttranslational modification and intrinsic disorder in protein. Biocomputing 2012, 94–103. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, S.; Dunker, A.K. Intrinsically Disordered Proteins Link Alternative Splicing and Post-translational Modifications to Complex Cell Signaling and Regulation. J. Mol. Biol. 2018, 430, 2342–2359. [Google Scholar] [CrossRef] [PubMed]
- Gimba, E.; Brum, M.; Nestal De Moraes, G. Full-length osteopontin and its splice variants as modulators of chemoresistance and radioresistance (Review). Int. J. Oncol. 2019, 54, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, Y.U.; Sleeman, J.; Fujii, H.; Herrlich, P.; Hotta, H.; Tanaka, K.; Chikuma, S.; Yagita, H.; Okumura, K.; Murakami, M.; et al. CD44 variants but not CD44s cooperate with beta1-containing integrins to permit cells to bind to osteopontin independently of arginine-glycine-aspartic acid, thereby stimulating cell motility and chemotaxis. Cancer Res. 1999, 59, 219–226. [Google Scholar] [PubMed]
- Kanayama, M.; Xu, S.; Danzaki, K.; Gibson, J.R.; Inoue, M.; Gregory, S.G.; Shinohara, M.L. Skewing of the population balance of lymphoid and myeloid cells by secreted and intracellular osteopontin. Nat. Immunol. 2017, 18, 973–984. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, G.; Dong, B.; Huang, L.; Zhang, C.; Sheng, Y.; Wu, B.; Han, L.; Wu, C.; Qi, Y. A pan-cancer analysis of the oncogenic role of secreted phosphoprotein 1 (SPP1) in human cancers. Ann. Transl. Med. 2022, 10, 279. [Google Scholar] [CrossRef]
- Subraman, V.; Thiyagarajan, M.; Malathi, N.; Rajan, S.T. OPN—Revisited. J. Clin. Diagn. Res. 2015, 9, ZE10–ZE13. [Google Scholar] [CrossRef]
- Hao, C.; Cui, Y.; Owen, S.; Li, W.; Cheng, S.; Jiang, W.G. Human osteopontin: Potential clinical applications in cancer (Review). Int. J. Mol. Med. 2017, 39, 1327–1337. [Google Scholar] [CrossRef]
- Chen, P.; Zhao, D.; Li, J.; Liang, X.; Li, J.; Chang, A.; Henry, V.K.; Lan, Z.; Spring, D.J.; Rao, G.; et al. Symbiotic Macrophage-Glioma Cell Interactions Reveal Synthetic Lethality in PTEN-Null Glioma. Cancer Cell 2019, 35, 868–884. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Z.; Skrzypczynska, K.M.; Fang, Q.; Zhang, W.; O’Brien, S.A.; He, Y.; Wang, L.; Zhang, Q.; Kim, A.; et al. Single-Cell Analyses Inform Mechanisms of Myeloid-Targeted Therapies in Colon Cancer. Cell 2020, 181, 442–459. [Google Scholar] [CrossRef]
- Yonemitsu, K.; Miyasato, Y.; Shiota, T.; Shinchi, Y.; Fujiwara, Y.; Hosaka, S.; Yamamoto, Y.; Komohara, Y. Soluble Factors Involved in Cancer Cell-Macrophage Interaction Promote Breast Cancer Growth. Anticancer Res. 2021, 41, 4249–4258. [Google Scholar] [CrossRef] [PubMed]
- Muchlińska, A.; Nagel, A.; Popęda, M.; Szade, J.; Niemira, M.; Zieliński, J.; Skokowski, J.; Bednarz-Knoll, N.; Żaczek, A.J. Alpha-smooth muscle actin-positive cancer-associated fibroblasts secreting osteopontin promote growth of luminal breast cancer. Cell. Mol. Biol. Lett. 2022, 27, 45. [Google Scholar] [CrossRef]
- Shiomi, A.; Kusuhara, M.; Sugino, T.; Sugiura, T.; Ohshima, K.; Nagashima, T.; Urakami, K.; Serizawa, M.; Saya, H.; Yamaguchi, K. Comprehensive genomic analysis contrasting primary colorectal cancer and matched liver metastases. Oncol. Lett. 2021, 21, 466. [Google Scholar] [CrossRef] [PubMed]
- Briones-Orta, M.A.; Avendaño-Vázquez, S.E.; Aparicio-Bautista, D.I.; Coombes, J.D.; Weber, G.F.; Syn, W.-K. Osteopontin splice variants and polymorphisms in cancer progression and prognosis. Biochim. Biophys. Acta BBA Rev. Cancer 2017, 1868, 93–108.A. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.R.; Htwe, H.H.; Marsh, T.; Wang, A.L.; Montoya, M.L.; Subbaraj, L.; Tward, A.D.; Bardeesy, N.; Perera, R.M. Transcriptional control of subtype switching ensures adaptation and growth of pancreatic cancer. eLife 2019, 8, e45313. [Google Scholar] [CrossRef]
- Feng, Y.-H.; Su, Y.-C.; Lin, S.-F.; Lin, P.-R.; Wu, C.-L.; Tung, C.-L.; Li, C.-F.; Shieh, G.-S.; Shiau, A.-L. Oct4 upregulates osteopontin via Egr1 and is associated with poor outcome in human lung cancer. BMC Cancer 2019, 19, 791. [Google Scholar] [CrossRef]
- Krstic, M.; Hassan, H.M.; Kolendowski, B.; Hague, M.N.; Anborgh, P.H.; Postenka, C.O.; Torchia, J.; Chambers, A.F.; Tuck, A.B. Isoform-specific promotion of breast cancer tumorigenicity by TBX3 involves induction of angiogenesis. Lab. Investig. 2020, 100, 400–413. [Google Scholar] [CrossRef]
- Liu, K.; Hu, H.; Jiang, H.; Zhang, H.; Gong, S.; Wei, D.; Yu, Z. RUNX1 promotes MAPK signaling to increase tumor progression and metastasis via OPN in head and neck cancer. Carcinogenesis 2021, 42, 414–422. [Google Scholar] [CrossRef]
- Deiana, M.; Dalle Carbonare, L.; Serena, M.; Cheri, S.; Mutascio, S.; Gandini, A.; Innamorati, G.; Lorenzi, P.; Cumerlato, M.; Bertacco, J.; et al. A Potential Role of RUNX2- RUNT Domain in Modulating the Expression of Genes Involved in Bone Metastases: An In Vitro Study with Melanoma Cells. Cells 2020, 9, 751. [Google Scholar] [CrossRef]
- Whittle, M.C.; Izeradjene, K.; Rani, P.G.; Feng, L.; Carlson, M.A.; DelGiorno, K.E.; Wood, L.D.; Goggins, M.; Hruban, R.H.; Chang, A.E.; et al. RUNX3 Controls a Metastatic Switch in Pancreatic Ductal Adenocarcinoma. Cell 2015, 161, 1345–1360. [Google Scholar] [CrossRef]
- Amilca-Seba, K.; Tan, T.Z.; Thiery, J.-P.; Louadj, L.; Thouroude, S.; Bouygues, A.; Sabbah, M.; Larsen, A.K.; Denis, J.A. Osteopontin (OPN/SPP1), a Mediator of Tumor Progression, Is Regulated by the Mesenchymal Transcription Factor Slug/SNAI2 in Colorectal Cancer (CRC). Cells 2022, 11, 1808. [Google Scholar] [CrossRef] [PubMed]
- Thomann, S.; Weiler, S.M.E.; Marquard, S.; Rose, F.; Ball, C.R.; Tóth, M.; Wei, T.; Sticht, C.; Fritzsche, S.; Roessler, S.; et al. YAP Orchestrates Heterotypic Endothelial Cell Communication via HGF/c-MET Signaling in Liver Tumorigenesis. Cancer Res. 2020, 80, 5502–5514. [Google Scholar] [CrossRef] [PubMed]
- Klement, J.D.; Paschall, A.V.; Redd, P.S.; Ibrahim, M.L.; Lu, C.; Yang, D.; Celis, E.; Abrams, S.I.; Ozato, K.; Liu, K. An osteopontin/CD44 immune checkpoint controls CD8+ T cell activation and tumor immune evasion. J. Clin. Investig. 2018, 128, 5549–5560. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wang, C.; Hu, B.; Gao, X.; Zou, T.; Luo, Q.; Chen, M.; Fu, Y.; Sheng, Y.; Zhang, K.; et al. Exosomal S100A4 derived from highly metastatic hepatocellular carcinoma cells promotes metastasis by activating STAT3. Signal Transduct. Target. Ther. 2021, 6, 187. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guo, S.; Zhao, K.; Conrad, C.; Driescher, C.; Rothbart, V.; Schlomann, U.; Guerreiro, H.; Bopp, M.H.; König, A.; et al. ADAM8 affects glioblastoma progression by regulating osteopontin-mediated angiogenesis. Biol. Chem. 2021, 402, 195–206. [Google Scholar] [CrossRef]
- Gan, N.; Zou, S.; Hang, W.; Yang, D.; Zhang, X.; Yin, Y. Osteopontin is Critical for Hyperactive mTOR-Induced Tumorigenesis in Oral Squamous Cell Carcinoma. J. Cancer 2017, 8, 1362–1370. [Google Scholar] [CrossRef]
- Schulz, A.; Gorodetska, I.; Behrendt, R.; Fuessel, S.; Erdmann, K.; Foerster, S.; Datta, K.; Mayr, T.; Dubrovska, A.; Muders, M.H. Linking NRP2 With EMT and Chemoradioresistance in Bladder Cancer. Front. Oncol. 2020, 9, 1461. [Google Scholar] [CrossRef]
- Yang, Y.-F.; Chang, Y.-C.; Jan, Y.-H.; Yang, C.-J.; Huang, M.-S.; Hsiao, M. Squalene synthase promotes the invasion of lung cancer cells via the osteopontin/ERK pathway. Oncogenesis 2020, 9, 78. [Google Scholar] [CrossRef]
- Curtis, K.J.; Schiavi, J.; Mc Garrigle, M.J.; Kumar, V.; McNamara, L.M.; Niebur, G.L. Mechanical stimuli and matrix properties modulate cancer spheroid growth in three-dimensional gelatin culture. J. R. Soc. Interface 2020, 17, 20200568. [Google Scholar] [CrossRef]
- Kolb, A.; Kleeff, J.; Guweidhi, A.; Esposito, I.; Giese, N.A.; Adwan, H.; Giese, T.; Büchler, M.W.; Berger, M.R.; Friess, H. Osteopontin influences the invasiveness of pancreatic cancer cells and is increased in neoplastic and inflammatory conditions. Cancer Biol. Ther. 2005, 4, 740–746. [Google Scholar] [CrossRef]
- Chang, J.; Bhasin, S.S.; Bielenberg, D.R.; Sukhatme, V.P.; Bhasin, M.; Huang, S.; Kieran, M.W.; Panigrahy, D. Chemotherapy-generated cell debris stimulates colon carcinoma tumor growth via osteopontin. FASEB J. 2019, 33, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-Y.; Chen, C.-L.; Hu, Y.-C.; Chi, Y.; Huang, Y.-H.; Su, C.-W.; Jeng, W.-J.; Liang, Y.-J.; Wu, J.-C. High Expression of MicroRNA-196a is Associated with Progression of Hepatocellular Carcinoma in Younger Patients. Cancers 2019, 11, 1549. [Google Scholar] [CrossRef] [PubMed]
- Marisetty, A.; Wei, J.; Kong, L.-Y.; Ott, M.; Fang, D.; Sabbagh, A.; Heimberger, A.B. MiR-181 Family Modulates Osteopontin in Glioblastoma Multiforme. Cancers 2020, 12, 3813. [Google Scholar] [CrossRef] [PubMed]
- Taipaleenmäki, H.; Farina, N.H.; van Wijnen, A.J.; Stein, J.L.; Hesse, E.; Stein, G.S.; Lian, J.B. Antagonizing miR-218-5p attenuates Wnt signaling and reduces metastatic bone disease of triple negative breast cancer cells. Oncotarget 2016, 7, 79032–79046. [Google Scholar] [CrossRef] [PubMed]
- Colden, M.; Dar, A.A.; Saini, S.; Dahiya, P.V.; Shahryari, V.; Yamamura, S.; Tanaka, Y.; Stein, G.; Dahiya, R.; Majid, S. MicroRNA-466 inhibits tumor growth and bone metastasis in prostate cancer by direct regulation of osteogenic transcription factor RUNX2. Cell Death Dis. 2017, 8, e2572. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Li, C.; Zheng, Q.; Fang, S.; Xu, J.; Wang, H.; Guo, H. Metformin suppresses lung adenocarcinoma by downregulating long non-coding RNA (lncRNA) AFAP1-AS1 and secreted phosphoprotein 1 (SPP1) while upregulating miR-3163. Bioengineered 2022, 13, 11987–12002. [Google Scholar] [CrossRef] [PubMed]
- Amilca-Seba, K.; Sabbah, M.; Larsen, A.K.; Denis, J.A. Osteopontin as a Regulator of Colorectal Cancer Progression and Its Clinical Applications. Cancers 2021, 13, 3793. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Liu, Z.; Klement, J.D.; Yang, D.; Merting, A.D.; Poschel, D.; Albers, T.; Waller, J.L.; Shi, H.; Liu, K. WDR5-H3K4me3 epigenetic axis regulates OPN expression to compensate PD-L1 function to promote pancreatic cancer immune escape. J. Immunother. Cancer 2021, 9, e002624. [Google Scholar] [CrossRef]
- Giopanou, I.; Kanellakis, N.I.; Giannou, A.D.; Lilis, I.; Marazioti, A.; Spella, M.; Papaleonidopoulos, V.; Simoes, D.C.M.; Zazara, D.E.; Agalioti, T.; et al. Osteopontin drives KRAS-mutant lung adenocarcinoma. Carcinogenesis 2020, 41, 1134–1144. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Li, L.; Miao, C.; Hasnat, M.; Sun, L.; Jiang, Z.; Zhang, L. Osteopontin promotes hepatocellular carcinoma progression through inducing JAK2/STAT3/NOX1-mediated ROS production. Cell Death Dis. 2022, 13, 341. [Google Scholar] [CrossRef]
- Cao, J.; Li, J.; Sun, L.; Qin, T.; Xiao, Y.; Chen, K.; Qian, W.; Duan, W.; Lei, J.; Ma, J.; et al. Hypoxia-driven paracrine osteopontin/integrin αvβ3 signaling promotes pancreatic cancer cell epithelial–mesenchymal transition and cancer stem cell-like properties by modulating forkhead box protein M1. Mol. Oncol. 2019, 13, 228–245. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Ruhland, M.K.; Pazolli, E.; Lind, A.C.; Stewart, S.A. Osteopontin Stimulates Preneoplastic Cellular Proliferation Through Activation of the MAPK Pathway. Mol. Cancer Res. 2011, 9, 1018–1029. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; LeSavage, B.L.; Hubka, K.M.; Ma, C.; Natarajan, S.; Eggold, J.T.; Xiao, Y.; Fuh, K.C.; Krishnan, V.; Enejder, A.; et al. Cancer-associated mesothelial cells promote ovarian cancer chemoresistance through paracrine osteopontin signaling. J. Clin. Investig. 2021, 131, e146186. [Google Scholar] [CrossRef]
- Cao, L.; Fan, X.; Jing, W.; Liang, Y.; Chen, R.; Liu, Y.; Zhu, M.; Jia, R.; Wang, H.; Zhang, X.; et al. Osteopontin promotes a cancer stem cell-like phenotype in hepatocellular carcinoma cells via an integrin-NF-κB-HIF-1α pathway. Oncotarget 2015, 6, 6627–6640. [Google Scholar] [CrossRef] [PubMed]
- Rao, G.; Wang, H.; Li, B.; Huang, L.; Xue, D.; Wang, X.; Jin, H.; Wang, J.; Zhu, Y.; Lu, Y.; et al. Reciprocal Interactions between Tumor-Associated Macrophages and CD44-Positive Cancer Cells via Osteopontin/CD44 Promote Tumorigenicity in Colorectal Cancer. Clin. Cancer Res. 2013, 19, 785–797. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.-H.; Quan, Y.-J.; Chen, J.-H.; Wang, T.-F.; Xu, M.; Ye, M.; Yuan, H.; Zhang, C.-J.; Liu, X.-J.; Min, Z.-J. Osteopontin Promotes Cell Migration and Invasion, and Inhibits Apoptosis and Autophagy in Colorectal Cancer by activating the p38 MAPK Signaling Pathway. Cell. Physiol. Biochem. 2017, 41, 1851–1864. [Google Scholar] [CrossRef]
- Ahmed, M.; Sottnik, J.L.; Dancik, G.M.; Sahu, D.; Hansel, D.E.; Theodorescu, D.; Schwartz, M.A. An Osteopontin/CD44 Axis in RhoGDI2-Mediated Metastasis Suppression. Cancer Cell 2016, 30, 432–443. [Google Scholar] [CrossRef]
- Gupta, A.; Zhou, C.; Chellaiah, M. Osteopontin and MMP9: Associations with VEGF Expression/Secretion and Angiogenesis in PC3 Prostate Cancer Cells. Cancers 2013, 5, 617–638. [Google Scholar] [CrossRef]
- Pitarresi, J.R.; Norgard, R.J.; Chiarella, A.M.; Suzuki, K.; Bakir, B.; Sahu, V.; Li, J.; Zhao, J.; Marchand, B.; Wengyn, M.D.; et al. PTHrP Drives Pancreatic Cancer Growth and Metastasis and Reveals a New Therapeutic Vulnerability. Cancer Discov. 2021, 11, 1774–1791. [Google Scholar] [CrossRef]
- Liu, H.; Wei, S.; Zhang, L.; Yuan, C.; Duan, Y.; Wang, Q. Secreted Phosphoprotein 1 Promotes the Development of Small Cell Lung Cancer Cells by Inhibiting Autophagy and Apoptosis. Pathol. Oncol. Res. 2019, 25, 1487–1495. [Google Scholar] [CrossRef]
- Napoli, S.; Scuderi, C.; Gattuso, G.; Di Bella, V.; Candido, S.; Basile, M.S.; Libra, M.; Falzone, L. Functional Roles of Matrix Metalloproteinases and Their Inhibitors in Melanoma. Cells 2020, 9, 1151. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.X.; Xia, Y.H.; Xue, T.C.; Zhang, H.; Ye, S.L. Down-regulation of osteopontin inhibits metastasis of hepatocellular carcinoma cells via a mechanism involving MMP-2 and uPA. Oncol. Rep. 2011, 25, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Kechagia, J.Z.; Ivaska, J.; Roca-Cusachs, P. Integrins as biomechanical sensors of the microenvironment. Nat. Rev. Mol. Cell Biol. 2019, 20, 457–473. [Google Scholar] [CrossRef] [PubMed]
- Kariya, Y.; Kariya, Y.; Gu, J. Roles of Integrin α6β4 Glycosylation in Cancer. Cancers 2017, 9, 79. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.; Giancotti, F.G. Integrin Signaling in Cancer: Mechanotransduction, Stemness, Epithelial Plasticity, and Therapeutic Resistance. Cancer Cell 2019, 35, 347–367. [Google Scholar] [CrossRef]
- Ludwig, B.S.; Kessler, H.; Kossatz, S.; Reuning, U. RGD-Binding Integrins Revisited: How Recently Discovered Functions and Novel Synthetic Ligands (Re-)Shape an Ever-Evolving Field. Cancers 2021, 13, 1711. [Google Scholar] [CrossRef]
- Desgrosellier, J.S.; Cheresh, D.A. Integrins in cancer: Biological implications and therapeutic opportunities. Nat. Rev. Cancer 2010, 10, 9–22. [Google Scholar] [CrossRef]
- Vogetseder, A.; Thies, S.; Ingold, B.; Roth, P.; Weller, M.; Schraml, P.; Goodman, S.L.; Moch, H. αv-Integrin isoform expression in primary human tumors and brain metastases. Int. J. Cancer 2013, 133, 2362–2371. [Google Scholar] [CrossRef]
- Avraamides, C.J.; Garmy-Susini, B.; Varner, J.A. Integrins in angiogenesis and lymphangiogenesis. Nat. Rev. Cancer 2008, 8, 604–617. [Google Scholar] [CrossRef]
- Böger, C.; Warneke, V.S.; Behrens, H.-M.; Kalthoff, H.; Goodman, S.L.; Becker, T.; Röcken, C. Integrins αvβ3 and αvβ5 as prognostic, diagnostic, and therapeutic targets in gastric cancer. Gastric Cancer 2015, 18, 784–795. [Google Scholar] [CrossRef]
- Baiula, M.; Spampinato, S.; Gentilucci, L.; Tolomelli, A. Novel Ligands Targeting α4β1 Integrin: Therapeutic Applications and Perspectives. Front. Chem. 2019, 7, 489. [Google Scholar] [CrossRef] [PubMed]
- LaFoya, B.; Munroe, J.A.; Miyamoto, A.; Detweiler, M.A.; Crow, J.J.; Gazdik, T.; Albig, A.R. Beyond the Matrix: The Many Non-ECM Ligands for Integrins. Int. J. Mol. Sci. 2018, 19, 449. [Google Scholar] [CrossRef] [PubMed]
- Taooka, Y.; Chen, J.; Yednock, T.; Sheppard, D. The integrin alpha9beta1 mediates adhesion to activated endothelial cells and transendothelial neutrophil migration through interaction with vascular cell adhesion molecule-1. J. Cell Biol. 1999, 145, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Kale, S.; Raja, R.; Thorat, D.; Soundararajan, G.; Patil, T.V.; Kundu, G.C. Osteopontin signaling upregulates cyclooxygenase-2 expression in tumor-associated macrophages leading to enhanced angiogenesis and melanoma growth via α9β1 integrin. Oncogene 2014, 33, 2295–2306. [Google Scholar] [CrossRef] [PubMed]
- Krishn, S.R.; Salem, I.; Quaglia, F.; Naranjo, N.M.; Agarwal, E.; Liu, Q.; Sarker, S.; Kopenhaver, J.; McCue, P.A.; Weinreb, P.H.; et al. The αvβ6 integrin in cancer cell-derived small extracellular vesicles enhances angiogenesis. J. Extracell. Vesicles 2020, 9, 1763594. [Google Scholar] [CrossRef]
- Oyama, M.; Kariya, Y.; Kariya, Y.; Matsumoto, K.; Kanno, M.; Yamaguchi, Y.; Hashimoto, Y. Biological role of site-specific O-glycosylation in cell adhesion activity and phosphorylation of osteopontin. Biochem. J. 2018, 475, 1583–1595. [Google Scholar] [CrossRef]
- Fan, C.S.; Chen, W.S.; Chen, L.L.; Chen, C.C.; Hsu, Y.T.; Chua, K.V.; Wang, H.D.; Huang, T.S. Osteopontin-integrin engagement induces HIF-1α-TCF12-mediated endothelial-mesenchymal transition to exacerbate colorectal cancer. Oncotarget 2018, 9, 4998–5015. [Google Scholar] [CrossRef]
- Hsieh, I.S.; Huang, W.H.; Liou, H.C.; Chuang, W.J.; Yang, R.S.; Fu, W.M. Upregulation of drug transporter expression by osteopontin in prostate cancer cells. Mol. Pharmacol. 2013, 83, 968–977. [Google Scholar] [CrossRef]
- Lu, C.; Fang, S.; Weng, Q.; Lv, X.; Meng, M.; Zhu, J.; Zheng, L.; Hu, Y.; Gao, Y.; Wu, X.; et al. Integrated analysis reveals critical glycolytic regulators in hepatocellular carcinoma. Cell Commun. Signal. 2020, 18, 97. [Google Scholar] [CrossRef]
- Che, P.; Yu, L.; Friedman, G.K.; Wang, M.; Ke, X.; Wang, H.; Zhang, W.; Nabors, B.; Ding, Q.; Han, X. Integrin αvβ3 Engagement Regulates Glucose Metabolism and Migration through Focal Adhesion Kinase (FAK) and Protein Arginine Methyltransferase 5 (PRMT5) in Glioblastoma Cells. Cancers 2021, 13, 1111. [Google Scholar] [CrossRef]
- Luengo, A.; Gui, D.Y.; Vander Heiden, M.G. Targeting Metabolism for Cancer Therapy. Cell Chem. Biol. 2017, 24, 1161–1180. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Pyun, W.Y.; Park, H.W. Cancer Metabolism: Phenotype, Signaling and Therapeutic Targets. Cells 2020, 9, 2308. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Wang, B.; Chihanga, T.; Kennedy, M.A.; Weber, G.F. Energy Metabolism during Anchorage-Independence. Induction by Osteopontin-c. PLoS ONE 2014, 9, e105675. [Google Scholar] [CrossRef] [PubMed]
- Senbanjo, L.T.; Chellaiah, M.A. CD44: A Multifunctional Cell Surface Adhesion Receptor Is a Regulator of Progression and Metastasis of Cancer Cells. Front. Cell Dev. Biol. 2017, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Hassn Mesrati, M.; Syafruddin, S.E.; Mohtar, M.A.; Syahir, A. CD44: A Multifunctional Mediator of Cancer Progression. Biomolecules 2021, 11, 1850. [Google Scholar] [CrossRef]
- Fnu, G.; Agrawal, P.; Kundu, G.C.; Weber, G.F. Structural Constraint of Osteopontin Facilitates Efficient Binding to CD44. Biomolecules 2021, 11, 813. [Google Scholar] [CrossRef]
- Weber, G.F.; Ashkar, S.; Glimcher, M.J.; Cantor, H. Receptor-ligand interaction between CD44 and osteopontin (Eta-1). Science 1996, 271, 509–512. [Google Scholar] [CrossRef]
- Yan, Y.; Zuo, X.; Wei, D. Concise Review: Emerging Role of CD44 in Cancer Stem Cells: A Promising Biomarker and Therapeutic Target. Stem Cells Transl. Med. 2015, 4, 1033–1043. [Google Scholar] [CrossRef]
- Nallasamy, P.; Nimmakayala, R.K.; Karmakar, S.; Leon, F.; Seshacharyulu, P.; Lakshmanan, I.; Rachagani, S.; Mallya, K.; Zhang, C.; Ly, Q.P.; et al. Pancreatic Tumor Microenvironment Factor Promotes Cancer Stemness via SPP1–CD44 Axis. Gastroenterology 2021, 161, 1998–2013. [Google Scholar] [CrossRef]
- Pietras, A.; Katz, A.M.; Ekström, E.J.; Wee, B.; Halliday, J.J.; Pitter, K.L.; Werbeck, J.L.; Amankulor, N.M.; Huse, J.T.; Holland, E.C. Osteopontin-CD44 Signaling in the Glioma Perivascular Niche Enhances Cancer Stem Cell Phenotypes and Promotes Aggressive Tumor Growth. Cell Stem Cell 2014, 14, 357–369. [Google Scholar] [CrossRef]
- Viana, B.P.P.B.; Gomes, A.V.P.; Gimba, E.R.P.; Ferreira, L.B. Osteopontin Expression in Thyroid Cancer: Deciphering EMT-Related Molecular Mechanisms. Biomedicines 2021, 9, 1372. [Google Scholar] [CrossRef] [PubMed]
- Kothari, A.; Arffa, M.; Chang, V.; Blackwell, R.; Syn, W.-K.; Zhang, J.; Mi, Z.; Kuo, P. Osteopontin—A Master Regulator of Epithelial-Mesenchymal Transition. J. Clin. Med. 2016, 5, 39. [Google Scholar] [CrossRef] [PubMed]
- Aiello, N.M.; Kang, Y. Context-dependent EMT programs in cancer metastasis. J. Exp. Med. 2019, 216, 1016–1026. [Google Scholar] [CrossRef] [PubMed]
- Bakir, B.; Chiarella, A.M.; Pitarresi, J.R.; Rustgi, A.K. EMT, MET, Plasticity, and Tumor Metastasis. Trends Cell Biol. 2020, 30, 764–776. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Hou, J.; Wang, L.; Fu, H.; Zhang, Y.; Song, Y.; Wang, X. Regulatory roles of osteopontin in human lung cancer cell epithelial-to-mesenchymal transitions and responses. Clin. Transl. Med. 2021, 11, e486. [Google Scholar] [CrossRef]
- Hao, C.; Cui, Y.; Chang, S.; Huang, J.; Birkin, E.; Hu, M.; Zhi, X.; Li, W.; Zhang, L.; Cheng, S.; et al. OPN promotes the aggressiveness of non-small-cell lung cancer cells through the activation of the RON tyrosine kinase. Sci. Rep. 2019, 9, 18101. [Google Scholar] [CrossRef]
- Jia, R.; Liang, Y.; Chen, R.; Liu, G.; Wang, H.; Tang, M.; Zhou, X.; Wang, H.; Yang, Y.; Wei, H.; et al. Osteopontin facilitates tumor metastasis by regulating epithelial–mesenchymal plasticity. Cell Death Dis. 2016, 7, e2564. [Google Scholar] [CrossRef]
- Saitoh, M. Involvement of partial EMT in cancer progression. J. Biochem. 2018, 164, 257–264. [Google Scholar] [CrossRef]
- Kariya, Y.; Oyama, M.; Suzuki, T.; Kariya, Y. αvβ3 Integrin induces partial EMT independent of TGF-β signaling. Commun. Biol. 2021, 4, 490. [Google Scholar] [CrossRef]
- Ding, K.U.N.; Fan, L.U.; Chen, S.; Wang, Y.; Yu, H.; Sun, Y.; Yu, J.; Wang, L.I.; Liu, X.; Liu, Y. Overexpression of osteopontin promotes resistance to cisplatin treatment in HCC. Oncol. Rep. 2015, 34, 3297–3303. [Google Scholar] [CrossRef]
- Ng, L.; Wan, T.; Chow, A.; Iyer, D.; Man, J.; Chen, G.; Yau, T.C.-C.; Lo, O.; Foo, C.-C.; Poon, J.T.-C.; et al. Osteopontin Overexpression Induced Tumor Progression and Chemoresistance to Oxaliplatin through Induction of Stem-Like Properties in Human Colorectal Cancer. Stem Cells Int. 2015, 2015, 247892. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; Li, P.; Yan, W.E.I.; Shi, L.E.I.; Zhang, J.; Wang, Y.; Liu, H.; You, Y. Downregulation of osteopontin enhances the sensitivity of glioma U251 cells to temozolomide and cisplatin by targeting the NF-κB/Bcl-2 pathway. Mol. Med. Rep. 2015, 11, 1951–1955. [Google Scholar] [CrossRef] [PubMed]
- Insua-Rodríguez, J.; Pein, M.; Hongu, T.; Meier, J.; Descot, A.; Lowy, C.M.; De Braekeleer, E.; Sinn, H.P.; Spaich, S.; Sütterlin, M.; et al. Stress signaling in breast cancer cells induces matrix components that promote chemoresistant metastasis. EMBO Mol. Med. 2018, 10, e9003. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.-C.; Wang, H.-C.; Hou, Y.-C.; Tung, H.-L.; Chiu, T.-J.; Shan, Y.-S. Blockade of autophagy reduces pancreatic cancer stem cell activity and potentiates the tumoricidal effect of gemcitabine. Mol. Cancer 2015, 14, 179. [Google Scholar] [CrossRef]
- Liu, G.; Fan, X.; Tang, M.; Chen, R.; Wang, H.; Jia, R.; Zhou, X.; Jing, W.; Wang, H.; Yang, Y.; et al. Osteopontin induces autophagy to promote chemo-resistance in human hepatocellular carcinoma cells. Cancer Lett. 2016, 383, 171–182. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, Y.; Lei, Z.; Liu, T.; Cai, T.; Wang, A.; Du, W.; Zeng, Y.; Zhu, J.; Liu, Z.; et al. Abnormally activated OPN/integrin αVβ3/FAK signalling is responsible for EGFR-TKI resistance in EGFR mutant non-small-cell lung cancer. J. Hematol. Oncol. 2020, 13, 169. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, R.; Wang, M.; Luo, J.; Liu, C. Inhibition of osteopontin overcomes acquired resistance to afatinib in EGFR-mutant non-small-cell lung cancer. Transl. Cancer Res. 2020, 9, 754–762. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, F.; Yang, X.; Xue, M.; Li, X.; Gao, Y.; Liu, L. Secreted Phosphoprotein 1 (SPP1) Contributes to Second-Generation EGFR Tyrosine Kinase Inhibitor Resistance in Non-Small Cell Lung Cancer. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2019, 27, 871–877. [Google Scholar] [CrossRef]
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The Different Mechanisms of Cancer Drug Resistance: A Brief Review. Adv. Pharm. Bull. 2017, 7, 339–348. [Google Scholar] [CrossRef]
- Graessmann, M.; Berg, B.; Fuchs, B.; Klein, A.; Graessmann, A. Chemotherapy resistance of mouse WAP-SVT/t breast cancer cells is mediated by osteopontin, inhibiting apoptosis downstream of caspase-3. Oncogene 2007, 26, 2840–2850. [Google Scholar] [CrossRef]
- Horala, A.; Swiatly, A.; Matysiak, J.; Banach, P.; Nowak-Markwitz, E.; Kokot, Z. Diagnostic Value of Serum Angiogenesis Markers in Ovarian Cancer Using Multiplex Immunoassay. Int. J. Mol. Sci. 2017, 18, 123. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, G.; Jain, S.; Kundu, G.C. Osteopontin Promotes Vascular Endothelial Growth Factor–Dependent Breast Tumor Growth and Angiogenesis via Autocrine and Paracrine Mechanisms. Cancer Res. 2008, 68, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Peng, L.; Fan, K.; Wang, H.; Wei, R.; Ji, G.; Cai, J.; Lu, B.; Li, B.; Zhang, D.; et al. Osteopontin induces angiogenesis through activation of PI3K/AKT and ERK1/2 in endothelial cells. Oncogene 2009, 28, 3412–3422. [Google Scholar] [CrossRef] [PubMed]
- Raja, R.; Kale, S.; Thorat, D.; Soundararajan, G.; Lohite, K.; Mane, A.; Karnik, S.; Kundu, G.C. Hypoxia-driven osteopontin contributes to breast tumor growth through modulation of HIF1α-mediated VEGF-dependent angiogenesis. Oncogene 2014, 33, 2053–2064. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Chakraborty, G.; Kundu, G.C. The Crucial Role of Cyclooxygenase-2 in Osteopontin-Induced Protein Kinase C α/c-Src/IκB Kinase α/β–Dependent Prostate Tumor Progression and Angiogenesis. Cancer Res. 2006, 66, 6638–6648. [Google Scholar] [CrossRef]
- Łukaszewicz-Zając, M.; Pączek, S.; Mroczko, B. A Disintegrin and Metalloproteinase (ADAM) Family—Novel Biomarkers of Selected Gastrointestinal (GI) Malignancies? Cancers 2022, 14, 2307. [Google Scholar] [CrossRef]
- Kobori, T.; Hamasaki, S.; Kitaura, A.; Yamazaki, Y.; Nishinaka, T.; Niwa, A.; Nakao, S.; Wake, H.; Mori, S.; Yoshino, T.; et al. Interleukin-18 Amplifies Macrophage Polarization and Morphological Alteration, Leading to Excessive Angiogenesis. Front. Immunol. 2018, 9, 334. [Google Scholar] [CrossRef]
- Nakagami, H. Cellular senescence and senescence-associated T cells as a potential therapeutic target. Geriatr. Gerontol. Int. 2020, 20, 97–100. [Google Scholar] [CrossRef]
- Ohtani, N. The roles and mechanisms of senescence-associated secretory phenotype (SASP): Can it be controlled by senolysis? Inflamm. Regen. 2022, 42, 11. [Google Scholar] [CrossRef]
- Flanagan, K.C.; Alspach, E.; Pazolli, E.; Parajuli, S.; Ren, Q.; Arthur, L.L.; Tapia, R.; Stewart, S.A. c-Myb and C/EBPβ regulate OPN and other senescence-associated secretory phenotype factors. Oncotarget 2018, 9, 21–36. [Google Scholar] [CrossRef]
- Liu, J.; Xu, K.; Chase, M.; Ji, Y.; Logan, J.K.; Buchsbaum, R.J. Tiam1-regulated osteopontin in senescent fibroblasts contributes to the migration and invasion of associated epithelial cells. J. Cell Sci. 2012, 125, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Tian, X.; Oh, S.Y.; Movassaghi, M.; Naber, S.P.; Kuperwasser, C.; Buchsbaum, R.J. The fibroblast Tiam1-osteopontin pathway modulates breast cancer invasion and metastasis. Breast Cancer Res. 2016, 18, 14. [Google Scholar] [CrossRef] [PubMed]
- Kyjacova, L.; Saup, R.; Rönsch, K.; Wallbaum, S.; Dukowic-Schulze, S.; Foss, A.; Scherer, S.D.; Rothley, M.; Neeb, A.; Grau, N.; et al. IER2-induced senescence drives melanoma invasion through osteopontin. Oncogene 2021, 40, 6494–6512. [Google Scholar] [CrossRef]
- Zuo, H.; Yang, D.; Wan, Y. Fam20C Regulates Bone Resorption and Breast Cancer Bone Metastasis through Osteopontin and BMP4. Cancer Res. 2021, 81, 5242–5254. [Google Scholar] [CrossRef]
- Kovacheva, M.; Zepp, M.; Schraad, M.; Berger, S.; Berger, M.R. Conditional Knockdown of Osteopontin Inhibits Breast Cancer Skeletal Metastasis. Int. J. Mol. Sci. 2019, 20, 4918. [Google Scholar] [CrossRef]
- Zhao, Y.; Bachelier, R.; Treilleux, I.; Pujuguet, P.; Peyruchaud, O.; Baron, R.; Clément-Lacroix, P.; Clézardin, P. Tumor αvβ3 Integrin Is a Therapeutic Target for Breast Cancer Bone Metastases. Cancer Res. 2007, 67, 5821–5830. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Kiagia, M.; Karapanagiotou, E.M.; Charpidou, A.; Dilana, K.D.; Nasothimiou, E.; Harrington, K.J.; Polyzos, A.; Syrigos, K.N. The clinical significance of serum markers of bone turnover in NSCLC patients: Surveillance, management and prognostic implications. Anticancer Res. 2009, 29, 1651–1657. [Google Scholar]
- Chang, W.-M.; Lin, Y.-F.; Su, C.-Y.; Peng, H.-Y.; Chang, Y.-C.; Hsiao, J.-R.; Chen, C.-L.; Chang, J.-Y.; Shieh, Y.-S.; Hsiao, M.; et al. Parathyroid Hormone-Like Hormone is a Poor Prognosis Marker of Head and Neck Cancer and Promotes Cell Growth via RUNX2 Regulation. Sci. Rep. 2017, 7, 41131. [Google Scholar] [CrossRef]
- Edwards, C.M.; Johnson, R.W. From Good to Bad: The Opposing Effects of PTHrP on Tumor Growth, Dormancy, and Metastasis Throughout Cancer Progression. Front. Oncol. 2021, 11, 644303. [Google Scholar] [CrossRef]
- Pinho, S.S.; Reis, C.A. Glycosylation in cancer: Mechanisms and clinical implications. Nat. Rev. Cancer 2015, 15, 540–555. [Google Scholar] [CrossRef]
- Mereiter, S.; Balmaña, M.; Campos, D.; Gomes, J.; Reis, C.A. Glycosylation in the Era of Cancer-Targeted Therapy: Where Are We Heading? Cancer Cell 2019, 36, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Darling, A.L.; Uversky, V.N. Intrinsic Disorder and Posttranslational Modifications: The Darker Side of the Biological Dark Matter. Front. Genet. 2018, 9, 158. [Google Scholar] [CrossRef] [PubMed]
- Dean, R.A.; Overall, C.M. Proteomics Discovery of Metalloproteinase Substrates in the Cellular Context by iTRAQ™ Labeling Reveals a Diverse MMP-2 Substrate Degradome. Mol. Cell. Proteom. 2007, 6, 611–623. [Google Scholar] [CrossRef]
- Agnihotri, R.; Crawford, H.C.; Haro, H.; Matrisian, L.M.; Havrda, M.C.; Liaw, L. Osteopontin, a Novel Substrate for Matrix Metalloproteinase-3 (Stromelysin-1) and Matrix Metalloproteinase-7 (Matrilysin). J. Biol. Chem. 2001, 276, 28261–28267. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Shao, Z.; Sharif, S.; Du, X.-Y.; Myles, T.; Merchant, M.; Harsh, G.; Glantz, M.; Recht, L.; Morser, J.; et al. Thrombin-cleaved Fragments of Osteopontin Are Overexpressed in Malignant Glial Tumors and Provide a Molecular Niche with Survival Advantage. J. Biol. Chem. 2013, 288, 3097–3111. [Google Scholar] [CrossRef]
- Takafuji, V.; Forgues, M.; Unsworth, E.; Goldsmith, P.; Wang, X.W. An osteopontin fragment is essential for tumor cell invasion in hepatocellular carcinoma. Oncogene 2007, 26, 6361–6371. [Google Scholar] [CrossRef] [PubMed]
- Mi, Z.; Oliver, T.; Guo, H.; Gao, C.; Kuo, P.C. Thrombin-Cleaved COOH-Terminal Osteopontin Peptide Binds with Cyclophilin C to CD147 in Murine Breast Cancer Cells. Cancer Res. 2007, 67, 4088–4097. [Google Scholar] [CrossRef]
- Yokosaki, Y.; Matsuura, N.; Sasaki, T.; Murakami, I.; Schneider, H.; Higashiyama, S.; Saitoh, Y.; Yamakido, M.; Taooka, Y.; Sheppard, D. The Integrin α9β1 Binds to a Novel Recognition Sequence (SVVYGLR) in the Thrombin-cleaved Amino-terminal Fragment of Osteopontin. J. Biol. Chem. 1999, 274, 36328–36334. [Google Scholar] [CrossRef]
- Senger, D.R.; Perruzzi, C.A.; Papadopoulos-Sergiou, A.; Van de Water, L. Adhesive properties of osteopontin: Regulation by a naturally occurring thrombin-cleavage in close proximity to the GRGDS cell-binding domain. Mol. Biol. Cell 1994, 5, 565–574. [Google Scholar] [CrossRef]
- Yokosaki, Y.; Tanaka, K.; Higashikawa, F.; Yamashita, K.; Eboshida, A. Distinct structural requirements for binding of the integrins alphavbeta6, alphavbeta3, alphavbeta5, alpha5beta1 and alpha9beta1 to osteopontin. Matrix Biol. J. Int. Soc. Matrix Biol. 2005, 24, 418–427. [Google Scholar] [CrossRef]
- Malaponte, G.; Hafsi, S.; Polesel, J.; Castellano, G.; Spessotto, P.; Guarneri, C.; Canevari, S.; Signorelli, S.S.; McCubrey, J.A.; Libra, M. Tumor microenvironment in diffuse large B-cell lymphoma: Matrixmetalloproteinases activation is mediated by osteopontin overexpression. Biochim. Biophys. Acta 2016, 1863, 483–489. [Google Scholar] [CrossRef]
- Lee, M.J.; Yaffe, M.B. Protein Regulation in Signal Transduction. Cold Spring Harb. Perspect. Biol. 2016, 8, a005918. [Google Scholar] [CrossRef] [PubMed]
- Mateos, B.; Holzinger, J.; Conrad-Billroth, C.; Platzer, G.; Żerko, S.; Sealey-Cardona, M.; Anrather, D.; Koźmiński, W.; Konrat, R. Hyperphosphorylation of Human Osteopontin and Its Impact on Structural Dynamics and Molecular Recognition. Biochemistry 2021, 60, 1347–1355. [Google Scholar] [CrossRef] [PubMed]
- Yalak, G.; Vogel, V. Extracellular phosphorylation and phosphorylated proteins: Not just curiosities but physiologically important. Sci. Signal. 2012, 5, re7. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Hanashima, S.; Yagi, H.; Takahashi, Y.; Sasakawa, H.; Kurimoto, E.; Iguchi, T.; Kon, S.; Uede, T.; Kato, K. NMR characterization of intramolecular interaction of osteopontin, an intrinsically disordered protein with cryptic integrin-binding motifs. Biochem. Biophys. Res. Commun. 2010, 393, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Weber, G.F.; Zawaideh, S.; Hikita, S.; Kumar, V.A.; Cantor, H.; Ashkar, S. Phosphorylation-dependent interaction of osteopontin with its receptors regulates macrophage migration and activation. J. Leukoc. Biol. 2002, 72, 752–761. [Google Scholar]
- Zhang, H.; Cai, Y.-H.; Ding, Y.; Zhang, G.; Liu, Y.; Sun, J.; Yang, Y.; Zhan, Z.; Iliuk, A.; Gu, Z.; et al. Proteomics, Phosphoproteomics and Mirna Analysis of Circulating Extracellular Vesicles through Automated and High-Throughput Isolation. Cells 2022, 11, 2070. [Google Scholar] [CrossRef]
- Tagliabracci, V.S.; Wiley, S.E.; Guo, X.; Kinch, L.N.; Durrant, E.; Wen, J.; Xiao, J.; Cui, J.; Nguyen, K.B.; Engel, J.L.; et al. A Single Kinase Generates the Majority of the Secreted Phosphoproteome. Cell 2015, 161, 1619–1632. [Google Scholar] [CrossRef]
- Liu, X.; Zhan, Y.; Xu, W.; Liu, X.; Geng, Y.; Liu, L.; Da, J.; Wang, J.; Zhang, X.; Jin, H.; et al. Prognostic and immunological role of Fam20C in pan-cancer. Biosci. Rep. 2021, 41, BSR20201920. [Google Scholar] [CrossRef]
- Stowell, S.R.; Ju, T.; Cummings, R.D. Protein Glycosylation in Cancer. Annu. Rev. Pathol. Mech. Dis. 2015, 10, 473–510. [Google Scholar] [CrossRef]
- Kariya, Y.; Oyama, M.; Ohtsuka, M.; Kikuchi, N.; Hashimoto, Y.; Yamamoto, T. Quantitative analysis of β1,6GlcNAc-branched N-glycans on β4 integrin in cutaneous squamous cell carcinoma. Fukushima J. Med. Sci. 2020, 66, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Kariya, Y.; Kariya, Y.; Gu, J. Laminin-332 and Integrins: Signaling Platform for Cell Adhesion and Migration and its Regulation by N-glycosylation. In Laminins: Structure, Biological Activity and Role in Disease; Nova Biomedical: New York, NY, USA, 2013; pp. 29–51. [Google Scholar]
- Masuda, K.; Takahashi, N.; Tsukamoto, Y.; Honma, H.; Kohri, K. N-Glycan structures of an osteopontin from human bone. Biochem. Biophys. Res. Commun. 2000, 268, 814–817. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, V.; Chackalaparampil, I.; Kundu, G.C.; Mukherjee, A.B.; Mukherjee, B.B. Altered sialylation of osteopontin prevents its receptor-mediated binding on the surface of oncogenically transformed tsB77 cells. Biochemistry 1997, 36, 5729–5738. [Google Scholar] [CrossRef] [PubMed]
- Patarca, R.; Freeman, G.J.; Singh, R.P.; Wei, F.Y.; Durfee, T.; Blattner, F.; Regnier, D.C.; Kozak, C.A.; Mock, B.A.; Morse, H.C., 3rd; et al. Structural and functional studies of the early T lymphocyte activation 1 (Eta-1) gene. Definition of a novel T cell-dependent response associated with genetic resistance to bacterial infection. J. Exp. Med. 1989, 170, 145–161. [Google Scholar] [CrossRef] [PubMed]
- Klement, J.D.; Poschel, D.B.; Lu, C.; Merting, A.D.; Yang, D.; Redd, P.S.; Liu, K. Osteopontin Blockade Immunotherapy Increases Cytotoxic T Lymphocyte Lytic Activity and Suppresses Colon Tumor Progression. Cancers 2021, 13, 1006. [Google Scholar] [CrossRef]
- Wei, J.; Marisetty, A.; Schrand, B.; Gabrusiewicz, K.; Hashimoto, Y.; Ott, M.; Grami, Z.; Kong, L.-Y.; Ling, X.; Caruso, H.; et al. Osteopontin mediates glioblastoma-associated macrophage infiltration and is a potential therapeutic target. J. Clin. Investig. 2019, 129, 137–149. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, J.; Xu, D.; Gao, X.-M.; Zhang, Z.; Hsu, J.L.; Li, C.-W.; Lim, S.-O.; Sheng, Y.-Y.; Zhang, Y.; et al. Disruption of tumour-associated macrophage trafficking by the osteopontin-induced colony-stimulating factor-1 signalling sensitises hepatocellular carcinoma to anti-PD-L1 blockade. Gut 2019, 68, 1653–1666. [Google Scholar] [CrossRef]
- Ellert-Miklaszewska, A.; Wisniewski, P.; Kijewska, M.; Gajdanowicz, P.; Pszczolkowska, D.; Przanowski, P.; Dabrowski, M.; Maleszewska, M.; Kaminska, B. Tumour-processed osteopontin and lactadherin drive the protumorigenic reprogramming of microglia and glioma progression. Oncogene 2016, 35, 6366–6377. [Google Scholar] [CrossRef]
- Sangaletti, S.; Tripodo, C.; Sandri, S.; Torselli, I.; Vitali, C.; Ratti, C.; Botti, L.; Burocchi, A.; Porcasi, R.; Tomirotti, A.; et al. Osteopontin Shapes Immunosuppression in the Metastatic Niche. Cancer Res. 2014, 74, 4706–4719. [Google Scholar] [CrossRef]
- Kim, E.-K.; Jeon, I.; Seo, H.; Park, Y.-J.; Song, B.; Lee, K.-A.; Jang, Y.; Chung, Y.; Kang, C.-Y. Tumor-Derived Osteopontin Suppresses Antitumor Immunity by Promoting Extramedullary Myelopoiesis. Cancer Res. 2014, 74, 6705–6716. [Google Scholar] [CrossRef]
- Lin, Y.; Xu, J.; Lan, H. Tumor-associated macrophages in tumor metastasis: Biological roles and clinical therapeutic applications. J. Hematol. Oncol. 2019, 12, 76. [Google Scholar] [CrossRef] [PubMed]
- DeNardo, D.G.; Ruffell, B. Macrophages as regulators of tumour immunity and immunotherapy. Nat. Rev. Immunol. 2019, 19, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, Q.; Chen, G.; Luo, D. Multi-Omics Analysis Showed the Clinical Value of Gene Signatures of C1QC+ and SPP1+ TAMs in Cervical Cancer. Front. Immunol. 2021, 12, 694801. [Google Scholar] [CrossRef]
- Qi, J.; Sun, H.; Zhang, Y.; Wang, Z.; Xun, Z.; Li, Z.; Ding, X.; Bao, R.; Hong, L.; Jia, W.; et al. Single-cell and spatial analysis reveal interaction of FAP+ fibroblasts and SPP1+ macrophages in colorectal cancer. Nat. Commun. 2022, 13, 1742. [Google Scholar] [CrossRef] [PubMed]
- Castello, L.M.; Raineri, D.; Salmi, L.; Clemente, N.; Vaschetto, R.; Quaglia, M.; Garzaro, M.; Gentilli, S.; Navalesi, P.; Cantaluppi, V.; et al. Osteopontin at the Crossroads of Inflammation and Tumor Progression. Mediat. Inflamm. 2017, 2017, 4049098. [Google Scholar] [CrossRef]
- Wykes, M.N.; Lewin, S.R. Immune checkpoint blockade in infectious diseases. Nat. Rev. Immunol. 2018, 18, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, H.; Zhao, Y.; Yue, D.; Chen, C.; Li, C.; Zhang, Z.; Wang, C. Tumor-associated macrophages (TAMs)-derived osteopontin (OPN) upregulates PD-L1 expression and predicts poor prognosis in non-small cell lung cancer (NSCLC). Thorac. Cancer 2021, 12, 2698–2709. [Google Scholar] [CrossRef] [PubMed]
- Raineri, D.; Dianzani, C.; Cappellano, G.; Maione, F.; Baldanzi, G.; Iacobucci, I.; Clemente, N.; Baldone, G.; Boggio, E.; Gigliotti, C.L.; et al. Osteopontin binds ICOSL promoting tumor metastasis. Commun. Biol. 2020, 3, 615. [Google Scholar] [CrossRef]
- Raineri, D.; Cappellano, G.; Vilardo, B.; Maione, F.; Clemente, N.; Canciani, E.; Boggio, E.; Gigliotti, C.L.; Monge, C.; Dianzani, C.; et al. Inducible T-Cell Costimulator Ligand Plays a Dual Role in Melanoma Metastasis upon Binding to Osteopontin or Inducible T-Cell Costimulator. Biomedicines 2021, 10, 51. [Google Scholar] [CrossRef]
- Louault, K.; Li, R.-R.; DeClerck, Y.A. Cancer-Associated Fibroblasts: Understanding Their Heterogeneity. Cancers 2020, 12, 3108. [Google Scholar] [CrossRef]
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T.; et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Mi, Z.; Bhattacharya, S.D.; Kim, V.M.; Guo, H.; Talbot, L.J.; Kuo, P.C. Osteopontin promotes CCL5-mesenchymal stromal cell-mediated breast cancer metastasis. Carcinogenesis 2011, 32, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Butti, R.; Nimma, R.; Kundu, G.; Bulbule, A.; Kumar, T.V.S.; Gunasekaran, V.P.; Tomar, D.; Kumar, D.; Mane, A.; Gill, S.S.; et al. Tumor-derived osteopontin drives the resident fibroblast to myofibroblast differentiation through Twist1 to promote breast cancer progression. Oncogene 2021, 40, 2002–2017. [Google Scholar] [CrossRef]
- Sharon, Y.; Raz, Y.; Cohen, N.; Ben-Shmuel, A.; Schwartz, H.; Geiger, T.; Erez, N. Tumor-Derived Osteopontin Reprograms Normal Mammary Fibroblasts to Promote Inflammation and Tumor Growth in Breast Cancer. Cancer Res. 2015, 75, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.E.; Kothari, A.N.; Wai, P.Y.; Li, N.Y.; Driver, J.; Zapf, M.A.C.; Franzen, C.A.; Gupta, G.N.; Osipo, C.; Zlobin, A.; et al. Osteopontin mediates an MZF1–TGF-β1-dependent transformation of mesenchymal stem cells into cancer-associated fibroblasts in breast cancer. Oncogene 2015, 34, 4821–4833. [Google Scholar] [CrossRef] [PubMed]
- Tokuda, K.; Morine, Y.; Miyazaki, K.; Yamada, S.; Saito, Y.; Nishi, M.; Tokunaga, T.; Ikemoto, T.; Imura, S.; Shimada, M. The interaction between cancer associated fibroblasts and tumor associated macrophages via the osteopontin pathway in the tumor microenvironment of hepatocellular carcinoma. Oncotarget 2021, 12, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Gu, J.; Zhang, J.; Liu, S.; Wang, Q.; Tian, T.; Chen, Z.; Zhang, J. MyD88 in myofibroblasts enhances colitis-associated tumorigenesis via promoting macrophage M2 polarization. Cell Rep. 2021, 34, 108724. [Google Scholar] [CrossRef]
- Pestell, T.G.; Jiao, X.; Kumar, M.; Peck, A.R.; Prisco, M.; Deng, S.; Li, Z.; Ertel, A.; Casimiro, M.C.; Ju, X.; et al. Stromal cyclin D1 promotes heterotypic immune signaling and breast cancer growth. Oncotarget 2017, 8, 81754–81775. [Google Scholar] [CrossRef]
- Lenos, K.J.; Miedema, D.M.; Lodestijn, S.C.; Nijman, L.E.; van den Bosch, T.; Romero Ros, X.; Lourenço, F.C.; Lecca, M.C.; van der Heijden, M.; van Neerven, S.M.; et al. Stem cell functionality is microenvironmentally defined during tumour expansion and therapy response in colon cancer. Nat. Cell Biol. 2018, 20, 1193–1202. [Google Scholar] [CrossRef]
- Qin, X.; Yan, M.; Wang, X.; Xu, Q.; Wang, X.; Zhu, X.; Shi, J.; Li, Z.; Zhang, J.; Chen, W. Cancer-associated Fibroblast-derived IL-6 Promotes Head and Neck Cancer Progression via the Osteopontin-NF-kappa B Signaling Pathway. Theranostics 2018, 8, 921–940. [Google Scholar] [CrossRef]
- Jing, C.-Y.; Fu, Y.-P.; Zhou, C.; Zhang, M.-X.; Yi, Y.; Huang, J.-L.; Gan, W.; Zhang, J.; Zheng, S.-S.; Zhang, B.-H.; et al. Hepatic stellate cells promote intrahepatic cholangiocarcinoma progression via NR4A2/osteopontin/Wnt signaling axis. Oncogene 2021, 40, 2910–2922. [Google Scholar] [CrossRef] [PubMed]
- Nazarizadeh, A.; Alizadeh-Fanalou, S.; Hosseini, A.; Mirzaei, A.; Salimi, V.; Keshipour, H.; Safizadeh, B.; Jamshidi, K.; Bahrabadi, M.; Tavakoli-Yaraki, M. Evaluation of local and circulating osteopontin in malignant and benign primary bone tumors. J. Bone Oncol. 2021, 29, 100377. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Liu, Y.; Mei, F.; Li, X.; Zhang, M.; Yao, B.; Wu, R.; You, J.; Pei, F. SPP1 overexpression is associated with poor outcomes in ALK fusion lung cancer patients without receiving targeted therapy. Sci. Rep. 2021, 11, 14031. [Google Scholar] [CrossRef] [PubMed]
- Moldogazieva, N.; Mokhosoev, I.; Zavadskiy, S.; Terentiev, A. Proteomic Profiling and Artificial Intelligence for Hepatocellular Carcinoma Translational Medicine. Biomedicines 2021, 9, 159. [Google Scholar] [CrossRef]
- Anborgh, P.H.; Caria, L.B.; Chambers, A.F.; Tuck, A.B.; Stitt, L.W.; Brackstone, M. Role of plasma osteopontin as a biomarker in locally advanced breast cancer. Am. J. Transl. Res. 2015, 7, 723–732. [Google Scholar] [PubMed]
- Kohata, T.; Ito, S.; Masuda, T.; Furuta, T.; Nakada, M.; Ohtsuki, S. Laminin Subunit Alpha-4 and Osteopontin Are Glioblastoma-Selective Secreted Proteins That Are Increased in the Cerebrospinal Fluid of Glioblastoma Patients. J. Proteome Res. 2020, 19, 3542–3553. [Google Scholar] [CrossRef] [PubMed]
- Shang, S.; Plymoth, A.; Ge, S.; Feng, Z.; Rosen, H.R.; Sangrajrang, S.; Hainaut, P.; Marrero, J.A.; Beretta, L. Identification of osteopontin as a novel marker for early hepatocellular carcinoma. Hepatology 2012, 55, 483–490. [Google Scholar] [CrossRef]
- Sun, T.; Tang, Y.; Sun, D.; Bu, Q.; Li, P. Osteopontin versus alpha-fetoprotein as a diagnostic marker for hepatocellular carcinoma: A meta-analysis. Oncotargets Ther. 2018, 11, 8925–8935. [Google Scholar] [CrossRef]
- Sun, J.; Chen, X.; Wang, Y. Comparison of the diagnostic value of CEA combined with OPN or DKK1 in non-small cell lung cancer. Oncol. Lett. 2020, 20, 3046–3052. [Google Scholar] [CrossRef]
- Walker, C.; Nguyen, T.-M.; Jessel, S.; Alvero, A.B.; Silasi, D.-A.; Rutherford, T.; Draghici, S.; Mor, G. Automated Assay of a Four-Protein Biomarker Panel for Improved Detection of Ovarian Cancer. Cancers 2021, 13, 325. [Google Scholar] [CrossRef]
- Hasenburg, A.; Eichkorn, D.; Vosshagen, F.; Obermayr, E.; Geroldinger, A.; Zeillinger, R.; Bossart, M. Biomarker-based early detection of epithelial ovarian cancer based on a five-protein signature in patient’s plasma—A prospective trial. BMC Cancer 2021, 21, 1037. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, H.; Wu, W.; Li, Y.; Li, J. Osteopontin genetic variants are associated with overall survival in advanced non-small-cell lung cancer patients and bone metastasis. J. Exp. Clin. Cancer Res. 2013, 32, 45. [Google Scholar] [CrossRef] [PubMed]
- Miao, T.w.; Xiao, W.; Du, L.y.; Mao, B.; Huang, W.; Chen, X.m.; Li, C.; Wang, Y.; Fu, J.j. High expression of SPP1 in patients with chronic obstructive pulmonary disease (COPD) is correlated with increased risk of lung cancer. FEBS Open Bio 2021, 11, 1237–1249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bi, K.; Tu, X.; Zhang, Q.; Cao, Q.; Liang, Y.; Zeng, P.; Wang, L.; Liu, T.; Fang, W.; et al. Interleukin-33 as an early predictor of cetuximab treatment efficacy in patients with colorectal cancer. Cancer Med. 2021, 10, 8338–8351. [Google Scholar] [CrossRef]
- Armstrong, A.J.; Nixon, A.B.; Carmack, A.; Yang, Q.; Eisen, T.; Stadler, W.M.; Jones, R.J.; Garcia, J.A.; Vaishampayan, U.N.; Picus, J.; et al. Angiokines Associated with Targeted Therapy Outcomes in Patients with Non–Clear Cell Renal Cell Carcinoma. Clin. Cancer Res. 2021, 27, 3317–3328. [Google Scholar] [CrossRef]
- Carbone, F.; Grossi, F.; Bonaventura, A.; Vecchié, A.; Minetti, S.; Bardi, N.; Elia, E.; Ansaldo, A.M.; Ferrara, D.; Rijavec, E.; et al. Baseline serum levels of osteopontin predict clinical response to treatment with nivolumab in patients with non-small cell lung cancer. Clin. Exp. Metastasis 2019, 36, 449–456. [Google Scholar] [CrossRef]
- Sperlich, A.; Balmert, A.; Doll, D.; Bauer, S.; Franke, F.; Keller, G.; Wilhelm, D.; Mur, A.; Respondek, M.; Friess, H.; et al. Genetic and immunological biomarkers predict metastatic disease recurrence in stage III colon cancer. BMC Cancer 2018, 18, 998. [Google Scholar] [CrossRef]
- Shojaei, F.; Scott, N.; Kang, X.; Lappin, P.B.; Fitzgerald, A.A.; Karlicek, S.; Simmons, B.H.; Wu, A.; Lee, J.H.; Bergqvist, S.; et al. Osteopontin induces growth of metastatic tumors in a preclinical model of non-small lung cancer. J. Exp. Clin. Cancer Res. 2012, 31, 26. [Google Scholar] [CrossRef]
- Dai, J.; Li, B.; Shi, J.; Peng, L.; Zhang, D.; Qian, W.; Hou, S.; Zhao, L.; Gao, J.; Cao, Z.; et al. A humanized anti-osteopontin antibody inhibits breast cancer growth and metastasis in vivo. Cancer Immunol. Immunother. 2010, 59, 355–366. [Google Scholar] [CrossRef]
- Boumans, M.J.; Houbiers, J.G.; Verschueren, P.; Ishikura, H.; Westhovens, R.; Brouwer, E.; Rojkovich, B.; Kelly, S.; den Adel, M.; Isaacs, J.; et al. Safety, tolerability, pharmacokinetics, pharmacodynamics and efficacy of the monoclonal antibody ASK8007 blocking osteopontin in patients with rheumatoid arthritis: A randomised, placebo controlled, proof-of-concept study. Ann. Rheum. Dis. 2012, 71, 180–185. [Google Scholar] [CrossRef]
- Farrokhi, V.; Chabot, J.R.; Neubert, H.; Yang, Z. Assessing the Feasibility of Neutralizing Osteopontin with Various Therapeutic Antibody Modalities. Sci. Rep. 2018, 8, 7781. [Google Scholar] [CrossRef] [PubMed]
- Bergonzini, C.; Kroese, K.; Zweemer, A.J.M.; Danen, E.H.J. Targeting Integrins for Cancer Therapy—Disappointments and Opportunities. Front. Cell Dev. Biol. 2022, 10, 863850. [Google Scholar] [CrossRef]
- Slack, R.J.; Macdonald, S.J.F.; Roper, J.A.; Jenkins, R.G.; Hatley, R.J.D. Emerging therapeutic opportunities for integrin inhibitors. Nat. Rev. Drug Discov. 2022, 21, 60–78. [Google Scholar] [CrossRef] [PubMed]
- Ben-David-Naim, M.; Dagan, A.; Grad, E.; Aizik, G.; Nordling-David, M.; Morss Clyne, A.; Granot, Z.; Golomb, G. Targeted siRNA Nanoparticles for Mammary Carcinoma Therapy. Cancers 2019, 11, 442. [Google Scholar] [CrossRef] [PubMed]
- Noguchi-Yachide, T. BET Bromodomain as a Target of Epigenetic Therapy. Chem. Pharm. Bull. 2016, 64, 540–547. [Google Scholar] [CrossRef]
- Deng, G.; Zeng, F.; Su, J.; Zhao, S.; Hu, R.; Zhu, W.; Hu, S.; Chen, X.; Yin, M. BET inhibitor suppresses melanoma progression via the noncanonical NF-κB/SPP1 pathway. Theranostics 2020, 10, 11428–11443. [Google Scholar] [CrossRef]
- Yamanaka, T.; Harimoto, N.; Yokobori, T.; Muranushi, R.; Hoshino, K.; Hagiwara, K.; Gantumur, D.; Handa, T.; Ishii, N.; Tsukagoshi, M.; et al. Conophylline Inhibits Hepatocellular Carcinoma by Inhibiting Activated Cancer-associated Fibroblasts Through Suppression of G Protein–coupled Receptor 68. Mol. Cancer Ther. 2021, 20, 1019–1028. [Google Scholar] [CrossRef]
- Benedicto, A.; Hernandez-Unzueta, I.; Sanz, E.; Márquez, J. Ocoxin Increases the Antitumor Effect of BRAF Inhibition and Reduces Cancer Associated Fibroblast-Mediated Chemoresistance and Protumoral Activity in Metastatic Melanoma. Nutrients 2021, 13, 686. [Google Scholar] [CrossRef]
- Chiou, J.; Chang, Y.-C.; Tsai, H.-F.; Lin, Y.-F.; Huang, M.-S.; Yang, C.-J.; Hsiao, M. Follistatin-like Protein 1 Inhibits Lung Cancer Metastasis by Preventing Proteolytic Activation of Osteopontin. Cancer Res. 2019, 79, 6113–6125. [Google Scholar] [CrossRef]
- Marin-Acevedo, J.A.; Kimbrough, E.O.; Lou, Y. Next generation of immune checkpoint inhibitors and beyond. J. Hematol. Oncol. 2021, 14, 45. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Jia, W.; Deng, H.; Li, G.; Deng, W.; Chen, J.; Kim, B.Y.S.; Jiang, W.; Liu, Q.; et al. Low-Dose Anti-Angiogenic Therapy Sensitizes Breast Cancer to PD-1 Blockade. Clin. Cancer Res. 2020, 26, 1712–1724. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Mao, C.; Chen, H.; Liu, L.; Wang, Y.; Hussain, A.; Li, S.; Zhang, X.; Tuguntaev, R.G.; Liang, X.J.; et al. Osteopontin targeted theranostic nanoprobes for laser-induced synergistic regression of vulnerable atherosclerotic plaques. Acta Pharm. Sin. B 2022, 12, 2014–2028. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kariya, Y.; Kariya, Y. Osteopontin in Cancer: Mechanisms and Therapeutic Targets. Int. J. Transl. Med. 2022, 2, 419-447. https://doi.org/10.3390/ijtm2030033
Kariya Y, Kariya Y. Osteopontin in Cancer: Mechanisms and Therapeutic Targets. International Journal of Translational Medicine. 2022; 2(3):419-447. https://doi.org/10.3390/ijtm2030033
Chicago/Turabian StyleKariya, Yoshinobu, and Yukiko Kariya. 2022. "Osteopontin in Cancer: Mechanisms and Therapeutic Targets" International Journal of Translational Medicine 2, no. 3: 419-447. https://doi.org/10.3390/ijtm2030033
APA StyleKariya, Y., & Kariya, Y. (2022). Osteopontin in Cancer: Mechanisms and Therapeutic Targets. International Journal of Translational Medicine, 2(3), 419-447. https://doi.org/10.3390/ijtm2030033




