Successful Treatment of Pancreatic Pseudocysto-Duodenum Fistula with Ultrasound Endoscopic Drainage: A Case Report
Abstract
1. Introduction
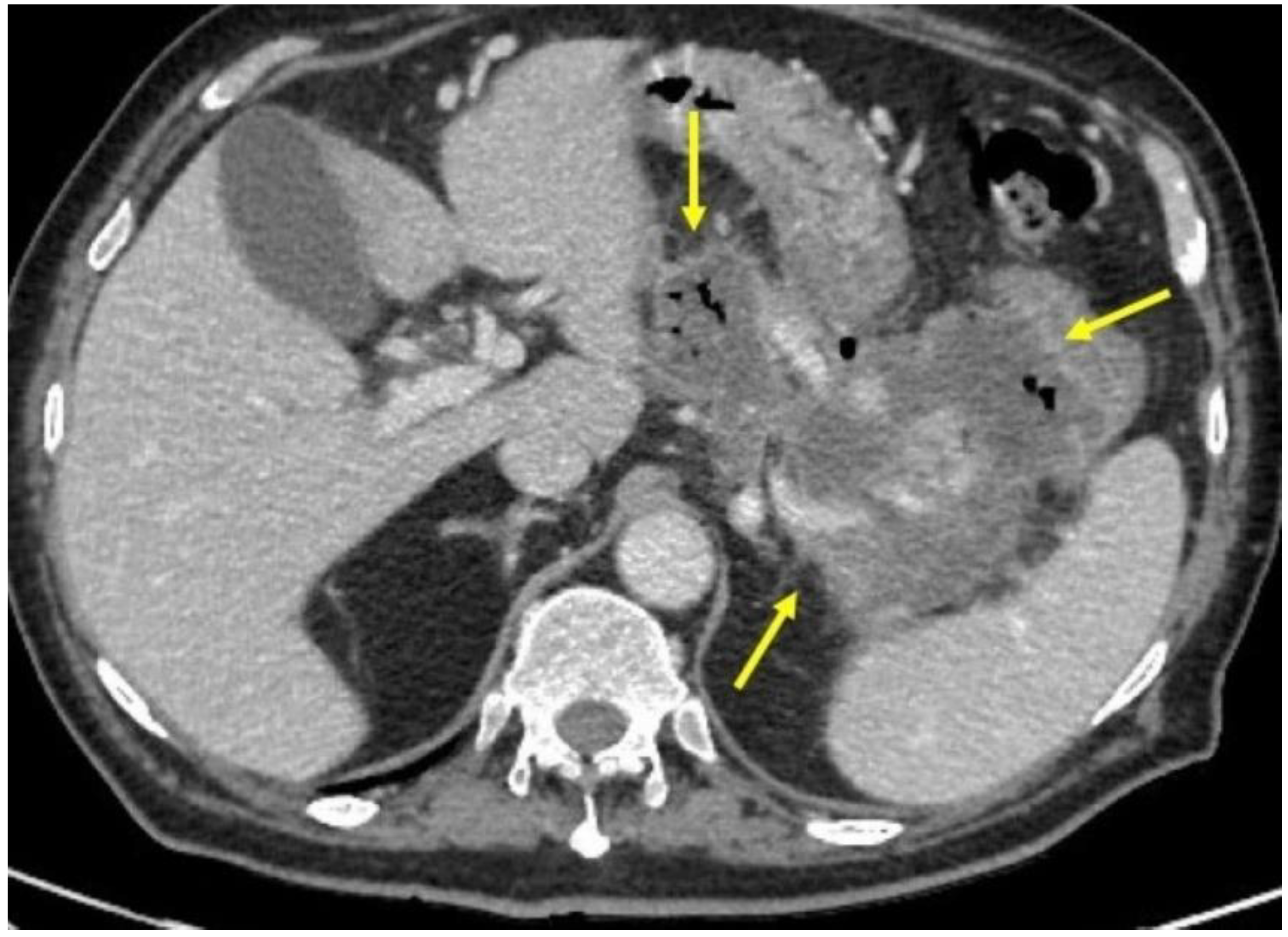
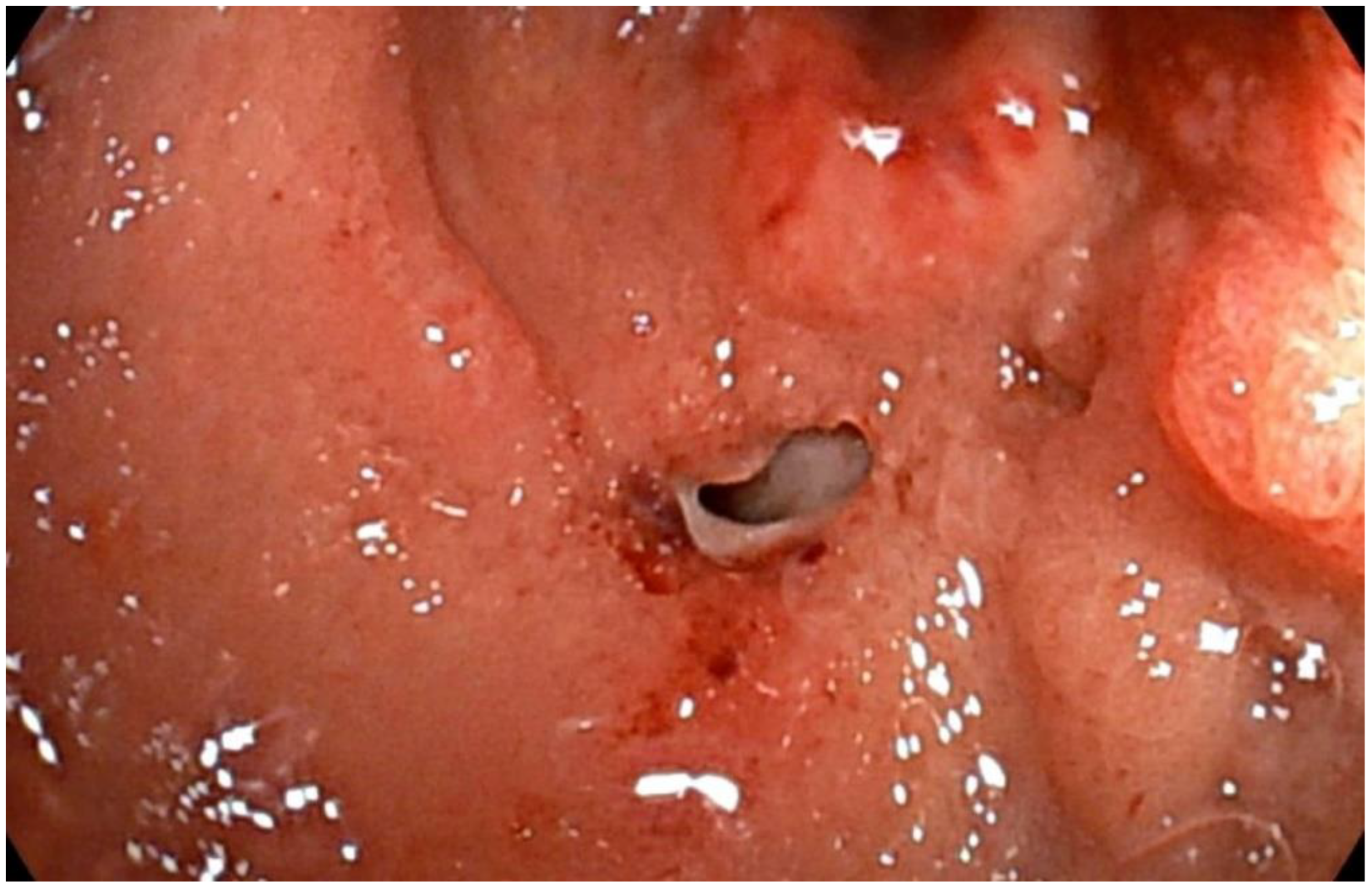
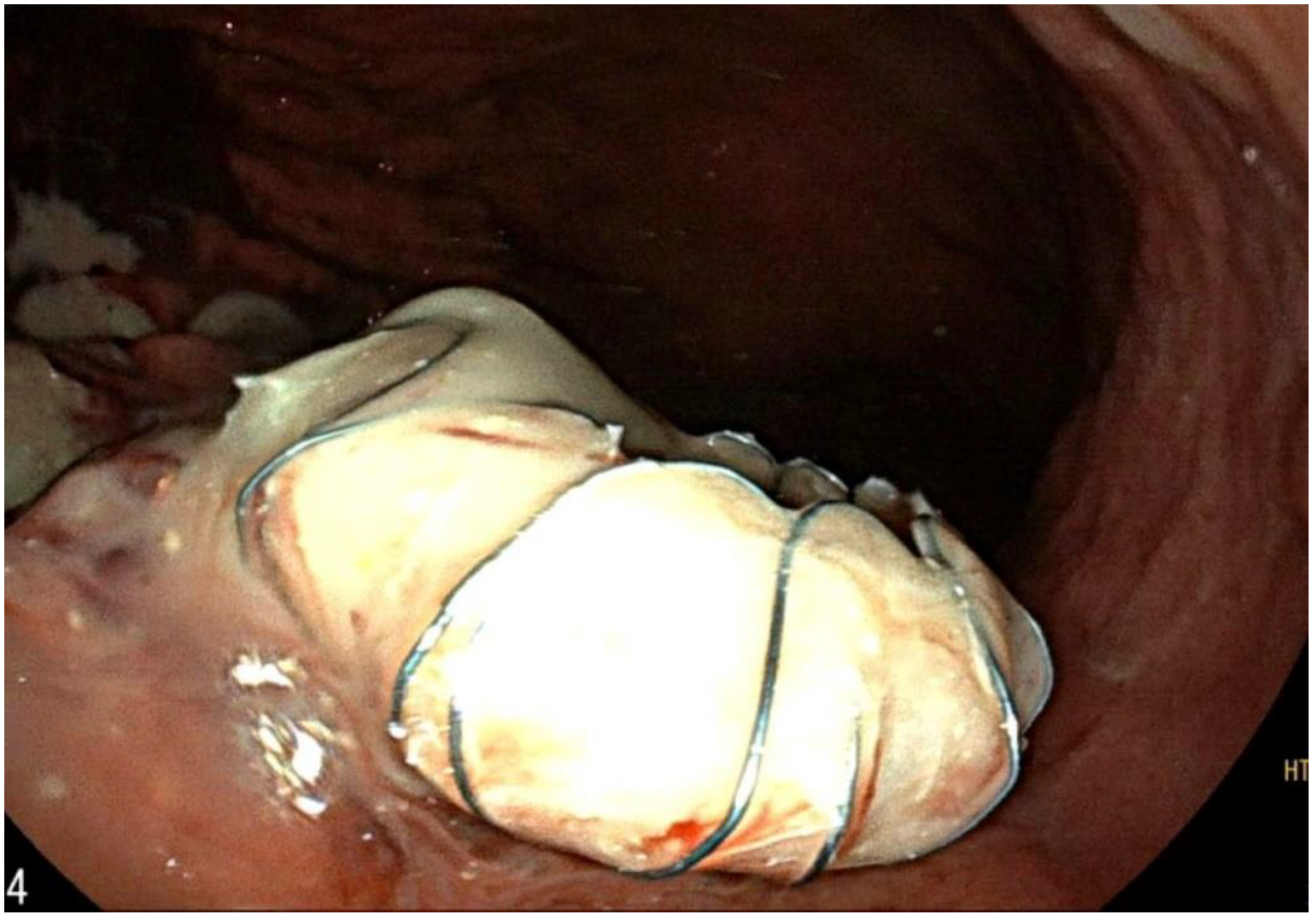
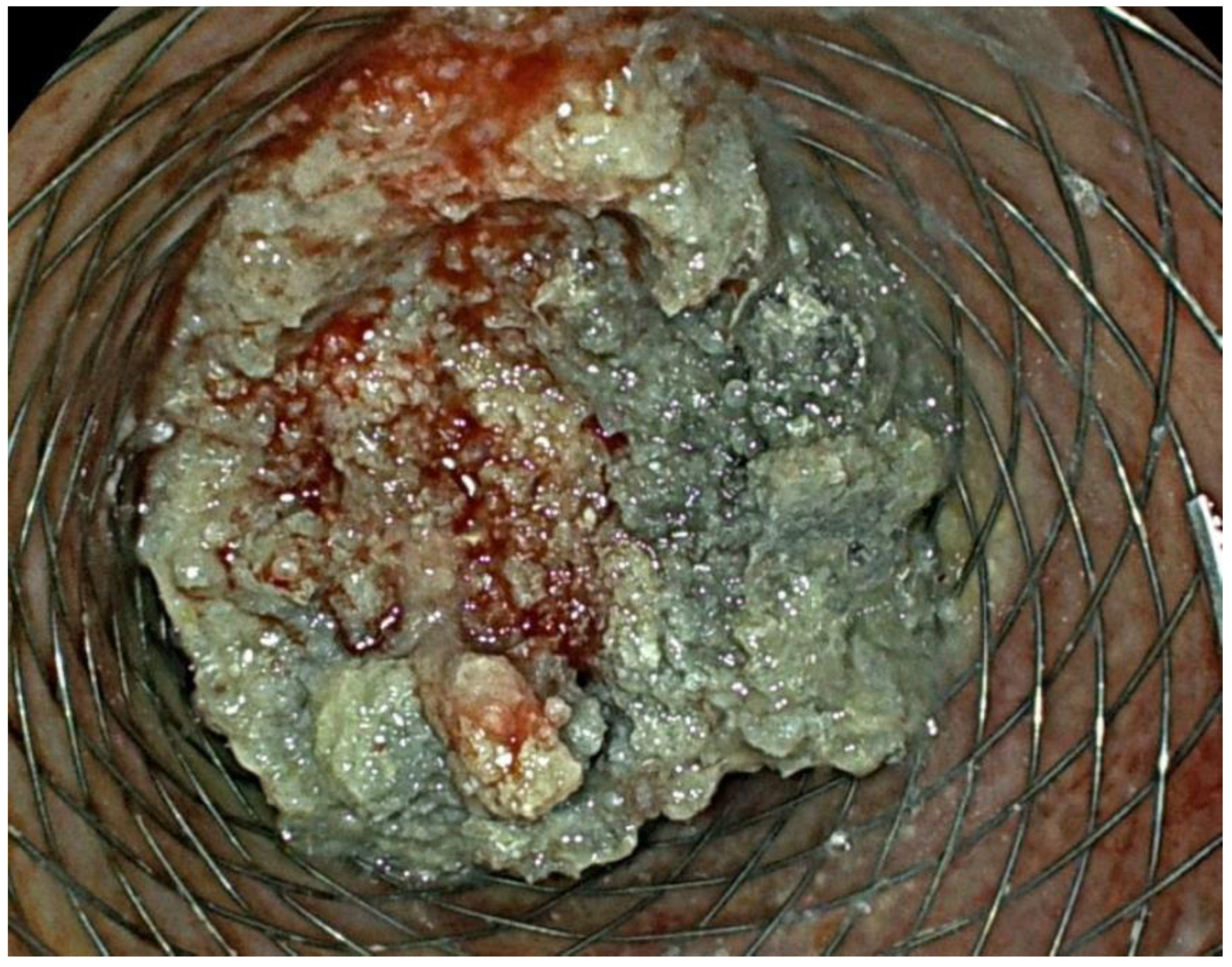
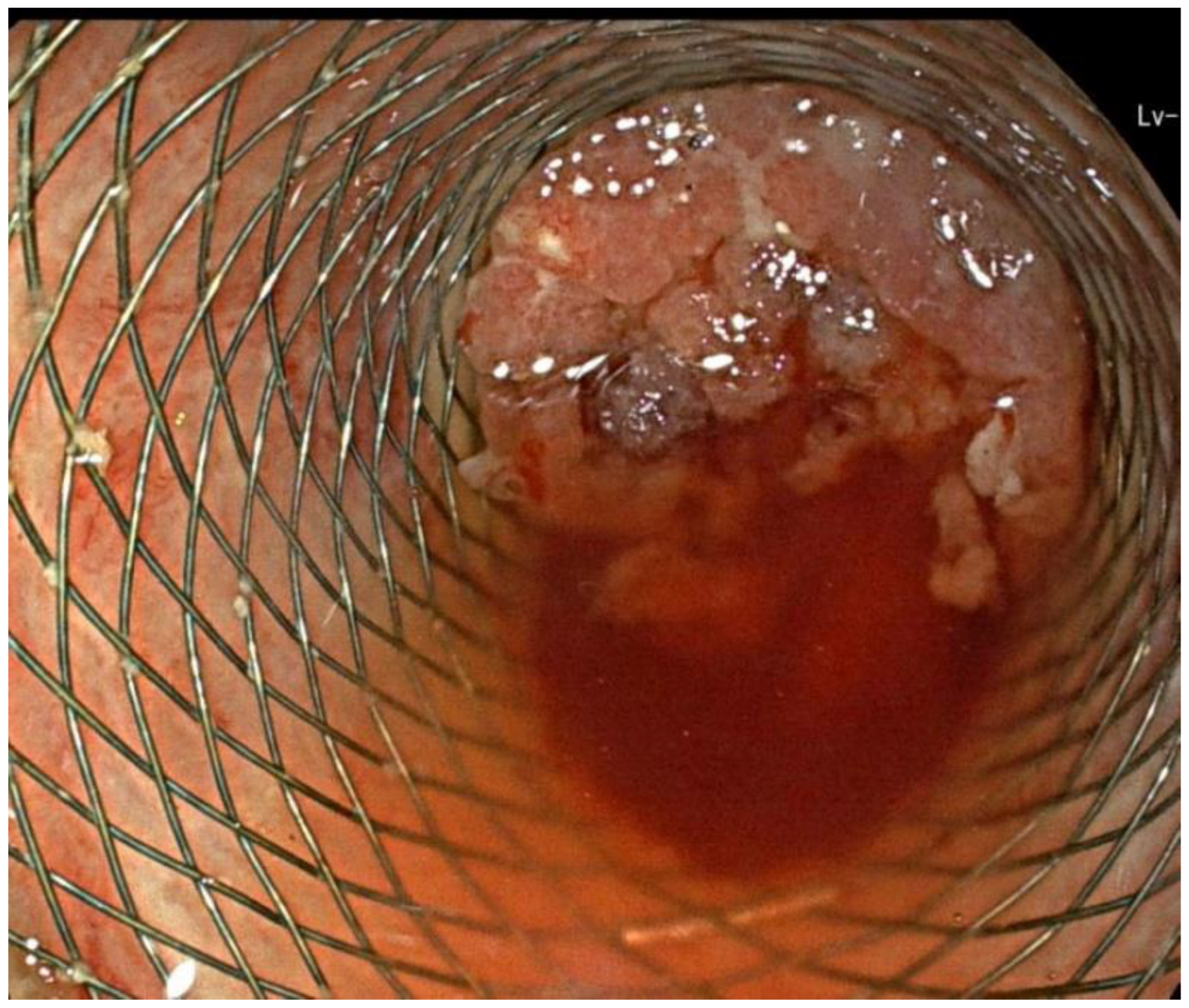
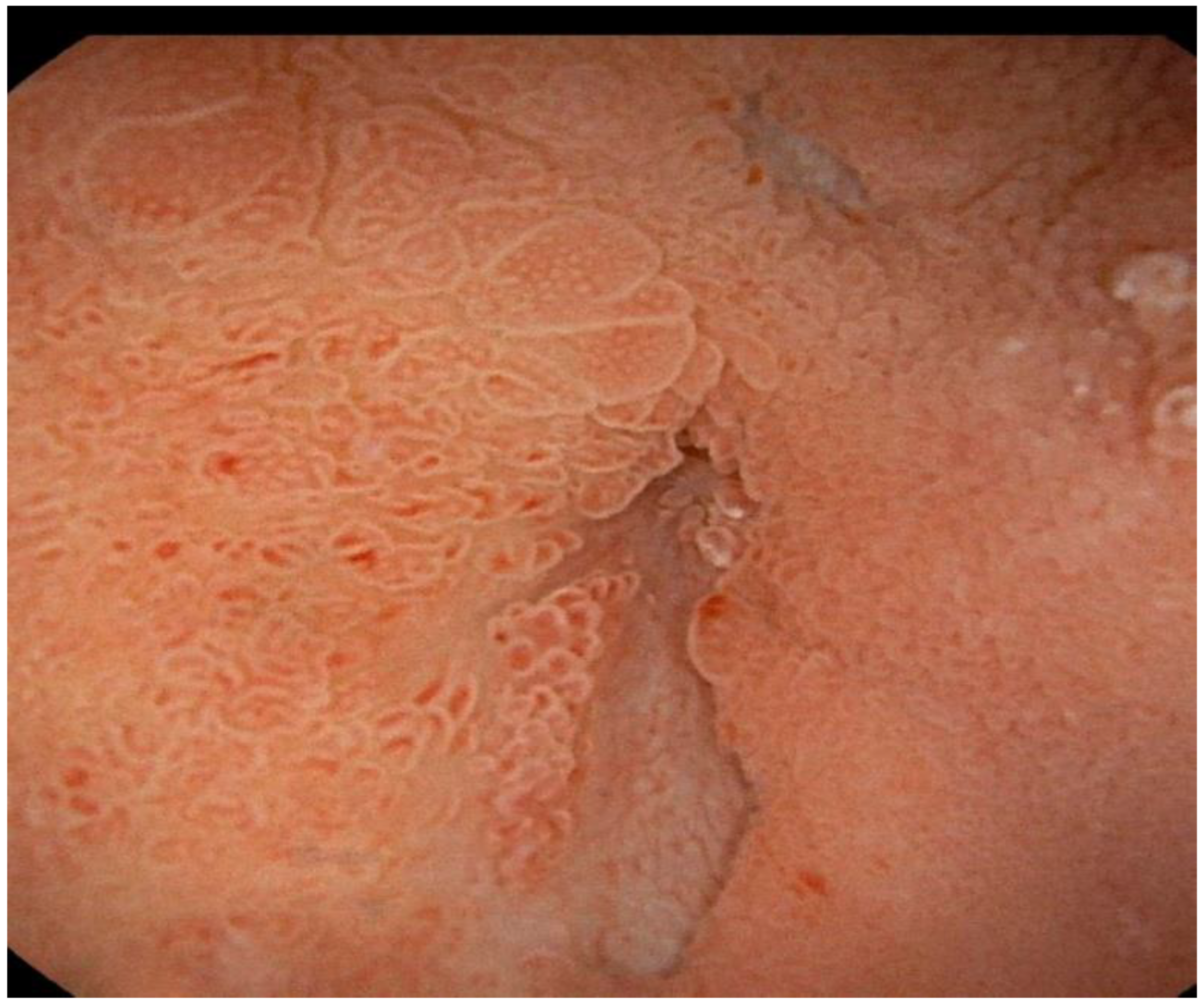
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Banks, P.A.; Bollen, T.L.; Dervenis, C.; Gooszen, H.G.; Johnson, C.D.; Sarr, M.G.; Tsiotos, G.G.; Vege, S.S.; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis—2012: Revision of the Atlanta classification and definitions by international consensus. Gut 2013, 62, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Nabi, Z.; Basha, J.; Reddy, D.N. Endoscopic management of pancreatic fluid collections-revisited. World J. Gastroenterol. 2017, 23, 2660–2672. [Google Scholar] [CrossRef] [PubMed]
- Khanna, A.K.; Tiwary, S.K.; Kumar, P. Pancreatic pseudocyst: Therapeutic dilemma. Int. J. Inflam. 2012, 2012, 279476. [Google Scholar] [CrossRef] [PubMed]
- Seewald, S.; Ang, T.L.; Kida, M.; Teng, K.Y.; Soehendra, N.; EUS 2008 Working Group. EUS 2008 Working Group document: Evaluation of EUS-guided drainage of pancreatic-fluid collections (with video). Gastrointest. Endosc. 2009, 69, S13–S21. [Google Scholar] [CrossRef] [PubMed]
- Tyberg, A.; Karia, K.; Gabr, M.; Desai, A.; Doshi, R.; Gaidhane, M.; Sharaiha, R.Z.; Kahaleh, M. Management of pancreatic fluid collections: A comprehensive review of the literature. World J. Gastroenterol. 2016, 22, 2256–2270. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, C.; Luigiano, C.; Maimone, A.; Polifemo, A.M.; Tarantino, I.; Cennamo, V. Endoscopic ultrasound-guided drainage of pancreatic fluid collections. World J. Gastrointest. Endosc. 2012, 4, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.E.; Benrajab, K.; Mardini, H.; Su, L.; Gabr, M.; Frandah, W.M. Anchoring lumen-apposing metal stent with coaxial plastic stent for endoscopic ultrasound-guided drainage of pancreatic fluid collections: Any benefit? Ann. Gastroenterol. 2019, 32, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Hammad, T.; Khan, M.A.; Alastal, Y.; Lee, W.; Nawras, A.; Ismail, M.K.; Kahaleh, M. Efficacy and safety of lumen-apposing metal stents in management of pancreatic fluid collections: Are they better than plastic stents? A systematic review and meta-analysis. Dig. Dis. Sci. 2018, 63, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Priyanka, P.; Hsueh, W.; Nasr, J. Use of lumen-apposing stents for the treatment of postsurgical fluid collections: A case series and a review of literature. Case Rep. Gastrointest. Med. 2019, 2019, 7656950. [Google Scholar] [CrossRef] [PubMed]
- Shatney, C.H.; Sosin, H. Spontaneous perforation of a pancreatic pseudocyst into the colon and duodenum. Am. J. Surg. 1973, 126, 433–438. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lattanzi, B.; Febbraro, I.; Pironti, E.; Bianchi, M. Successful Treatment of Pancreatic Pseudocysto-Duodenum Fistula with Ultrasound Endoscopic Drainage: A Case Report. Int. J. Transl. Med. 2022, 2, 537-542. https://doi.org/10.3390/ijtm2040040
Lattanzi B, Febbraro I, Pironti E, Bianchi M. Successful Treatment of Pancreatic Pseudocysto-Duodenum Fistula with Ultrasound Endoscopic Drainage: A Case Report. International Journal of Translational Medicine. 2022; 2(4):537-542. https://doi.org/10.3390/ijtm2040040
Chicago/Turabian StyleLattanzi, Barbara, Ingrid Febbraro, Eliseo Pironti, and Marco Bianchi. 2022. "Successful Treatment of Pancreatic Pseudocysto-Duodenum Fistula with Ultrasound Endoscopic Drainage: A Case Report" International Journal of Translational Medicine 2, no. 4: 537-542. https://doi.org/10.3390/ijtm2040040
APA StyleLattanzi, B., Febbraro, I., Pironti, E., & Bianchi, M. (2022). Successful Treatment of Pancreatic Pseudocysto-Duodenum Fistula with Ultrasound Endoscopic Drainage: A Case Report. International Journal of Translational Medicine, 2(4), 537-542. https://doi.org/10.3390/ijtm2040040





