Water-Soluble Carbon Nanotube Enhances Gossypol Production in Cotton Cell Suspension Culture
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
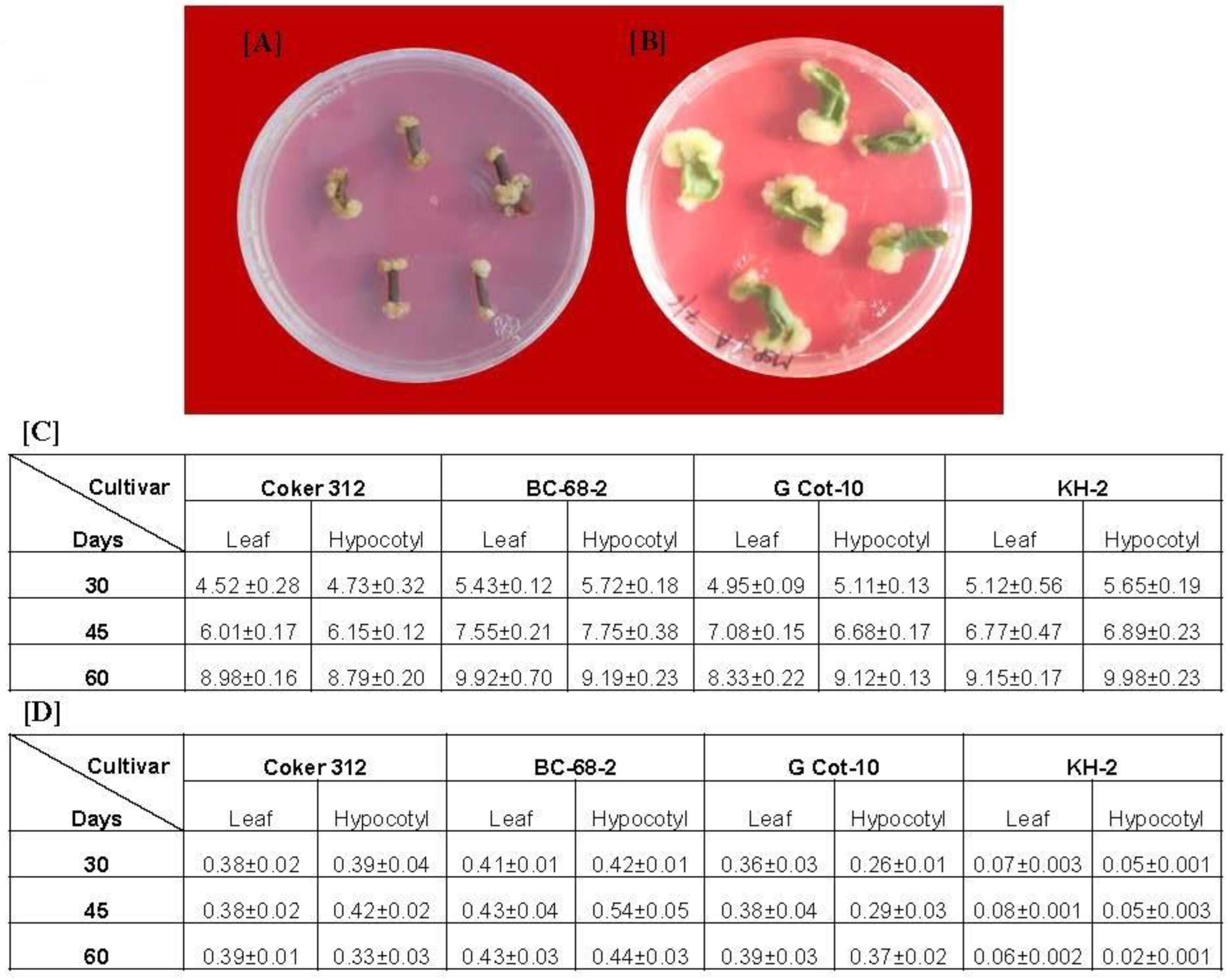
2.2. Induction and Maintenance of Cotton Callus
2.3. Cell Suspension Culture
2.4. Suspension Culture with CNT
2.5. Extraction and Quantification of Gossypol from Cotton Cell Suspension
2.6. Peroxidase Assay of Cotton Cell Suspension Culture
2.7. Confocal Imaging of Cotton Cell
2.8. SEM Imaging of Cotton Cell
2.9. Proliferation Assay
2.10. Statistical Analysis
3. Results
3.1. Callus Induction and Estimation of Gossypol Production
3.2. Effect of CNTs on Cotton Cell Suspension Culture and Gossypol Production
3.3. Peroxidase Assay of Cotton Cell
3.4. Microscopic Image Analysis of Cotton Cells
3.5. Antiproliferative Activity of Gossypol Produced from CNT-Induced Cotton Cell Suspension Culture
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hussein, R.A.; El-Anssary, A.A. Plants secondary metabolites: The key drivers of the pharmacological actions of medicinal plants. Herb. Med. 2019, 30, 1. [Google Scholar]
- Chandran, H.; Meena, M.; Barupal, T.; Sharma, K. Plant tissue culture as a perpetual source for production of industrially important bioactive compounds. Biotechnol. Rep. 2020, 26, e00450. [Google Scholar] [CrossRef] [PubMed]
- Dixit, S.; Shukla, A.; Singh, V.; Upadhyay, S.K. Bioprospecting of natural compounds for industrial and medical applications: Current scenario and bottleneck. Bioprospecting Plant Biodivers. Ind. Mol. 2021, 19, 53–71. [Google Scholar]
- Yue, W.; Ming, Q.L.; Lin, B.; Rahman, K.; Zheng, C.J.; Han, T.; Qin, L.P. Medicinal plant cell suspension cultures: Pharmaceutical applications and high-yielding strategies for the desired secondary metabolites. Crit. Rev. Biotechnol. 2016, 36, 215–232. [Google Scholar] [CrossRef]
- Yeshi, K.; Crayn, D.; Ritmejerytė, E.; Wangchuk, P. Plant secondary metabolites produced in response to abiotic stresses has potential application in pharmaceutical product development. Molecules 2022, 27, 313. [Google Scholar] [CrossRef]
- Hussain, M.S.; Fareed, S.; Ansari, S.; Rahman, M.A.; Ahmad, I.Z.; Saeed, M. Current approaches toward production of secondary plant metabolites. J. Pharm. Bioallied Sci. 2012, 4, 10. [Google Scholar] [CrossRef]
- Zhao, J.; Davis, L.C.; Verpoorte, R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol. Adv. 2005, 23, 283–333. [Google Scholar] [CrossRef]
- Verpoorte, R.; Alfermann, A.W. (Eds.) Metabolic Engineering of Plant Secondary Metabolism; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2000. [Google Scholar]
- Khan, T.; Khan, M.A.; Karam, K.; Ullah, N.; Mashwani, Z.U.; Nadhman, A. Plant in vitro culture technologies; a promise into factories of secondary metabolites against COVID-19. Front. Plant Sci. 2021, 12, 356. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Ahmar, S.; Mahmood, T.; Fiaz, S.; Mora-Poblete, F.; Shafique, M.S.; Chattha, M.S.; Jung, K.H. Advantage of nanotechnology-based genome editing system and its application in crop improvement. Front. Plant Sci. 2021, 12, 663849. [Google Scholar] [CrossRef]
- Wang, P.; Lombi, E.; Zhao, F.J.; Kopittke, P.M. Nanotechnology: A new opportunity in plant sciences. Trends Plant Sci. 2016, 21, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Ruttkay-Nedecky, B.; Krystofova, O.; Nejdl, L.; Adam, V. Nanoparticles based on essential metals and their phytotoxicity. J. Nanobiotechnol. 2017, 15, 1–9. [Google Scholar] [CrossRef]
- Ghorbanpour, M.; Hadian, J. Multi-walled carbon nanotubes stimulate callus induction, secondary metabolites biosynthesis and antioxidant capacity in medicinal plant Satureja khuzestanica grown in vitro. Carbon 2015, 94, 749–759. [Google Scholar] [CrossRef]
- Terrones, M. Science and technology of the twenty-first century: Synthesis, properties, and applications of carbon nanotubes. Annu. Rev. Mater. Res. 2003, 33, 419–501. [Google Scholar] [CrossRef]
- Verma, P.C.; Trivedi, I.; Singh, H.; Shukla, A.K.; Kumar, M.; Upadhyay, S.K.; Pandey, P.; Hans, A.L.; Singh, P.K. Efficient production of gossypol from hairy root cultures of cotton (Gossypium hirsutum L.). Curr. Pharm. Biotechnol. 2009, 10, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Dixit, S.; Verma, P.C.; Singh, P.K. Differential peroxidase activities in three different crops upon insect feeding. Plant Signal. Behav. 2013, 8, e25615. [Google Scholar] [CrossRef]
- Tripathi, S.; Sonkar, S.K.; Sarkar, S. Growth stimulation of gram (Cicer arietinum) plant by water soluble carbon nanotubes. Nanoscale 2011, 3, 1176–1181. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Huang, X.F.; Mu, S.J.; An, Q.X.; Xia, A.J.; Chen, R.; Wu, D.C. Inhibition of proliferation of prostate cancer cell line, PC-3, in vitro and in vivo using (−)-gossypol. Asian J. Androl. 2010, 12, 390. [Google Scholar] [CrossRef]
- Shukla, A.; Rajawat, J.; Dixit, S.; Mishra, S. Assays to evaluate tumor angiogenesis. In Protocol Handbook for Cancer Biology; Academic Press: Cambridge, MA, USA, 2021; pp. 43–67. [Google Scholar]
- Kumar, M.; Singh, H.; Shukla, A.K.; Verma, P.C.; Singh, P.K. Induction and establishment of somatic embryogenesis in elite Indian cotton cultivar (Gossypium hirsutum L. cv Khandwa-2). Plant Signal. Behav. 2013, 8, e26762. [Google Scholar] [CrossRef][Green Version]
- Wilkins, T.A.; Mishra, R.; Trolinder, N.L. Agrobacterium mediated transformation and regeneration of cotton. J. Food Agric. Environ. 2004, 2, 179–187. [Google Scholar]
- Khodakovskaya, M.V.; De Silva, K.; Biris, A.S.; Dervishi, E.; Villagarcia, H. Carbon nanotubes induce growth enhancement of tobacco cells. ACS Nano 2012, 6, 2128–2135. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.R.; Ravishankar, G.A. Plant cell cultures: Chemical factories of secondary metabolites. Biotechnol. Adv. 2002, 20, 101–153. [Google Scholar]
- Nigra, H.M.; Alvarez, M.A.; Giulietti, A.M. Effect of carbon and nitrogen sources on growth and solasodine production in batch suspension cultures of Solanum eleagnifolium Cav. Plant Cell Tissue Organ Cult. 1990, 21, 55–60. [Google Scholar] [CrossRef]
- Bao Do, C.; Cormier, F. Effects of low nitrate and high sugar concentrations on anthocyanin content and composition of grape (Vitis vinifera L.) cell suspension. Plant Cell Rep. 1991, 9, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Gershenzon, J. Changes in the levels of plant secondary metabolites under water and nutrient stress. In Phytochemical Adaptations to Stress; Springer: Boston, MA, USA, 1984; pp. 273–320. [Google Scholar]
- Khan, A.W.; Farooq, M.; Haseeb, M.; Choi, S. Role of plant-derived active constituents in cancer treatment and their mechanisms of action. Cells 2022, 11, 1326. [Google Scholar] [CrossRef]
- Fazili, M.A.; Bashir, I.; Ahmad, M.; Yaqoob, U.; Geelani, S.N. In vitro strategies for the enhancement of secondary metabolite production in plants: A review. Bull. Natl. Res. Cent. 2022, 46, 35. [Google Scholar] [CrossRef]




| Supplements | Dry Weight (g) | Fresh Weight (mg) | Gossypol (mg/g) |
|---|---|---|---|
| Control | 1.47 ± 0.30 | 5.46 ± 0.45 | 0.46 ± 0.03 |
| Charcoal | 1.39 ± 0.20 | 5.00 ± 0.45 | 0.44 ± 0.03 |
| CNT 10 µg/g | 2.26 ± 0.45 | 7.98 ± 0.30 | 0.50 ± 0.02 |
| CNT 20 µg/g | 3.14 ± 0.60 | 10.42 ± 0.60 | 0.54 ± 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dixit, S.; Shukla, A.; Upadhyay, S.K.; Verma, P.C. Water-Soluble Carbon Nanotube Enhances Gossypol Production in Cotton Cell Suspension Culture. Int. J. Transl. Med. 2022, 2, 607-617. https://doi.org/10.3390/ijtm2040046
Dixit S, Shukla A, Upadhyay SK, Verma PC. Water-Soluble Carbon Nanotube Enhances Gossypol Production in Cotton Cell Suspension Culture. International Journal of Translational Medicine. 2022; 2(4):607-617. https://doi.org/10.3390/ijtm2040046
Chicago/Turabian StyleDixit, Sameer, Akanchha Shukla, Santosh Kumar Upadhyay, and Praveen Chandra Verma. 2022. "Water-Soluble Carbon Nanotube Enhances Gossypol Production in Cotton Cell Suspension Culture" International Journal of Translational Medicine 2, no. 4: 607-617. https://doi.org/10.3390/ijtm2040046
APA StyleDixit, S., Shukla, A., Upadhyay, S. K., & Verma, P. C. (2022). Water-Soluble Carbon Nanotube Enhances Gossypol Production in Cotton Cell Suspension Culture. International Journal of Translational Medicine, 2(4), 607-617. https://doi.org/10.3390/ijtm2040046








