Prostate Cancer, Treatment and Response of the Hematological System in Mexican Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Data
2.2. Study Design
2.3. Population
- sensitive to treatment(s) (TSPC);
- admitted in a palliative state and with abandonment of treatment (AT), and
- resistance to treatment(s) or presented biochemical recurrence (TRPC).
2.4. Clinical Test
2.5. Prostate Cancer Surgical Procedures
2.6. Statistical Analysis
3. Results
3.1. Characteristics of the Population with Prostate Cancer
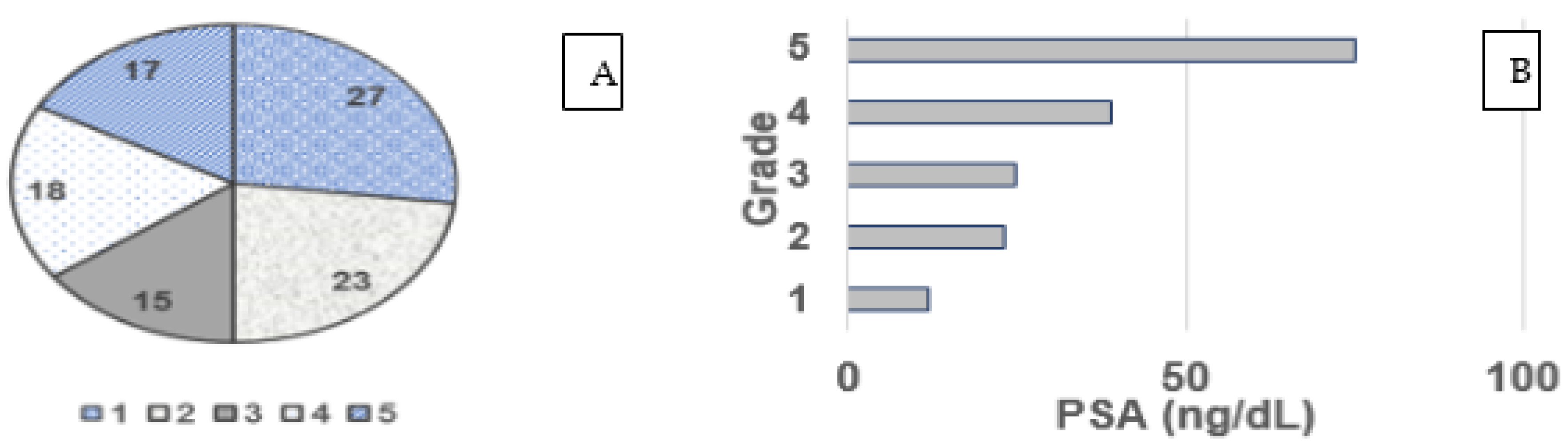
3.2. Gleason Grade and PSA
3.3. Pharmacological Response to Prostate Cancer
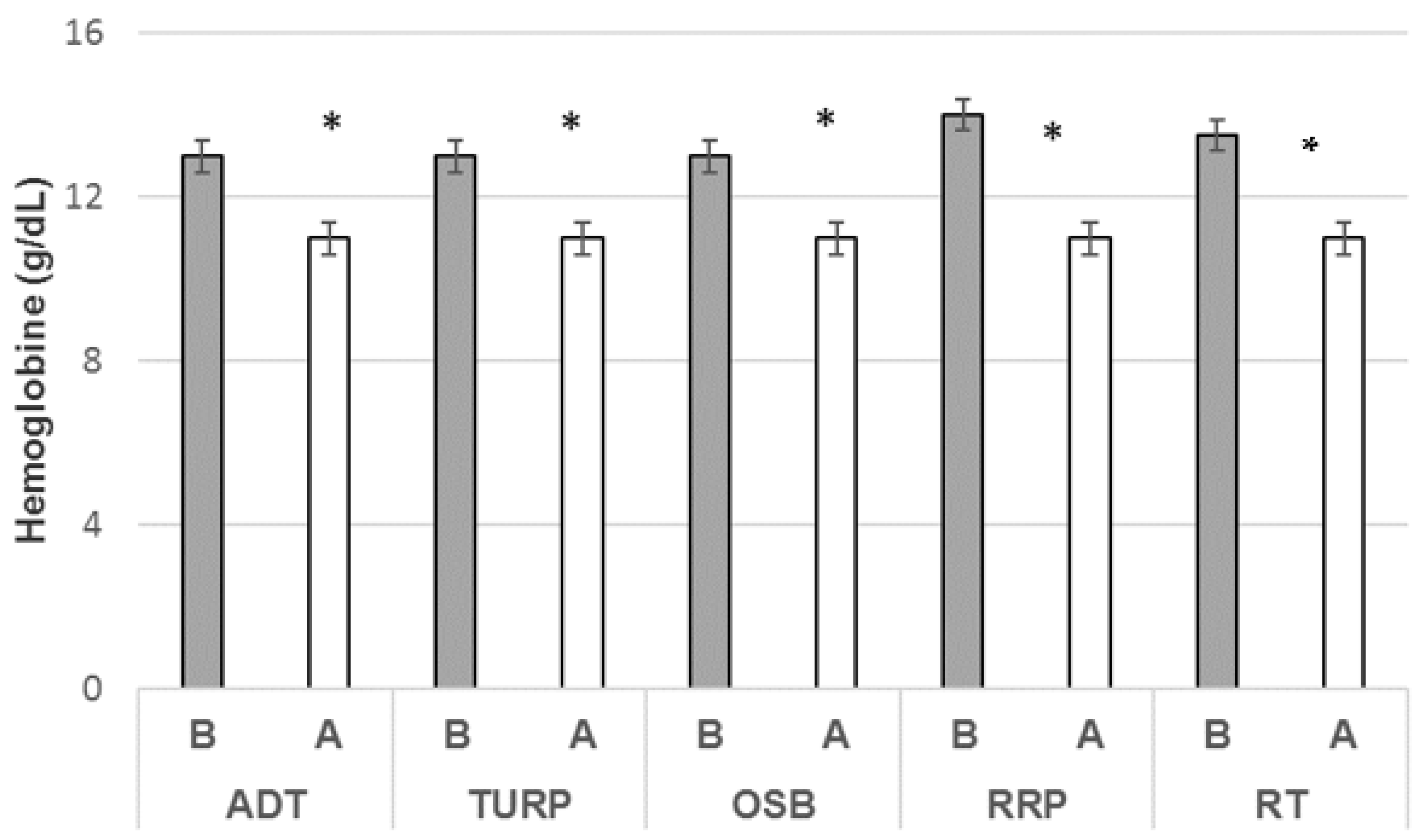
3.4. Hemoglobin Ratio and Treatment
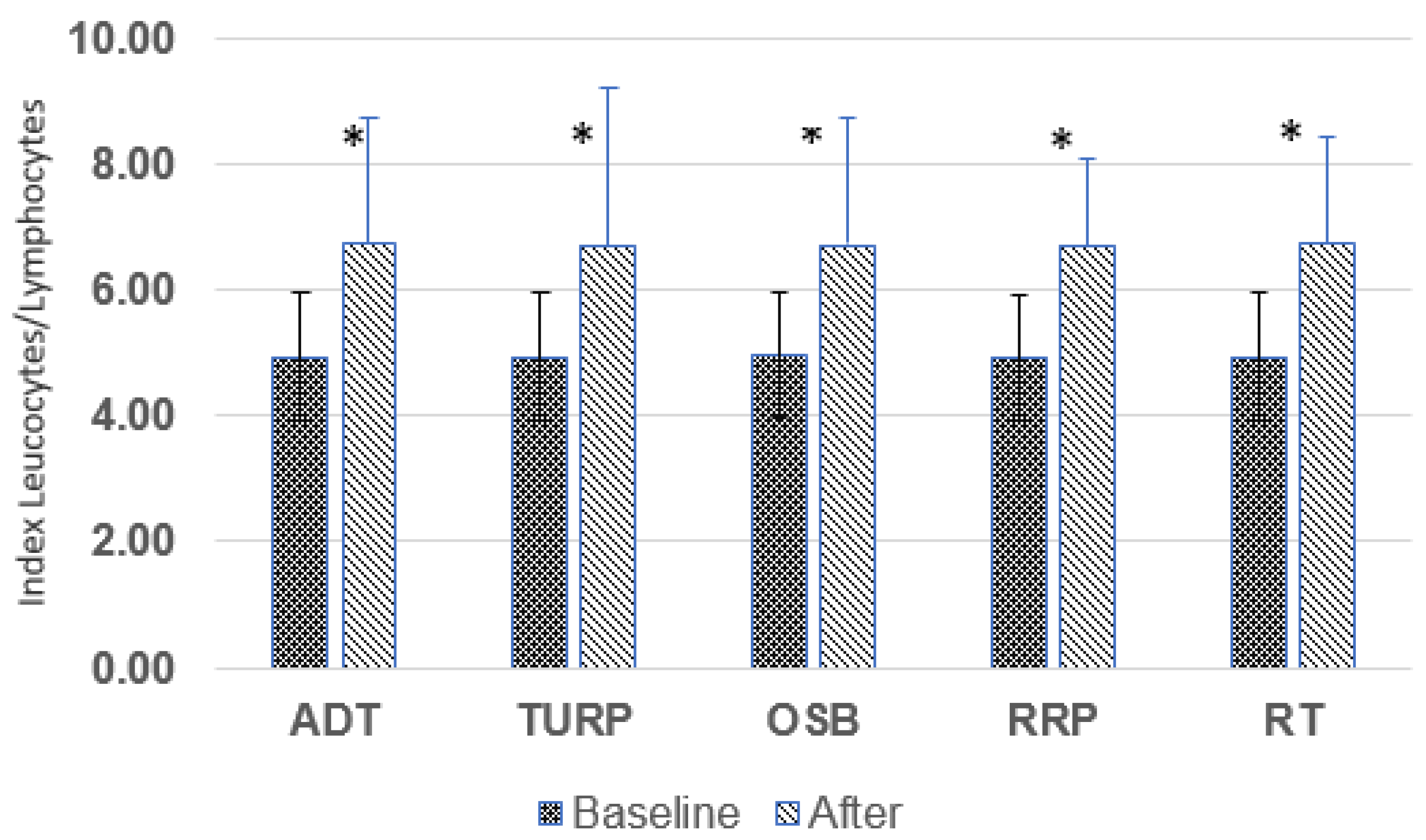
3.5. Leukocyte/Lymphocyte Ratio (L/L)
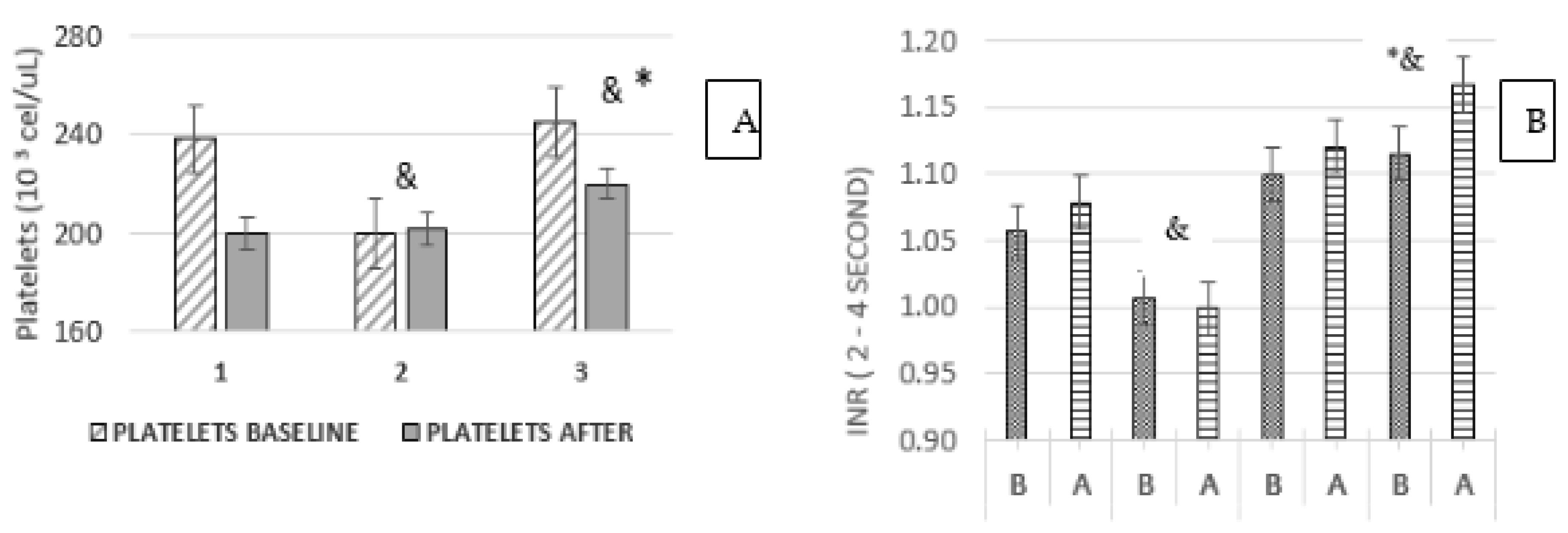
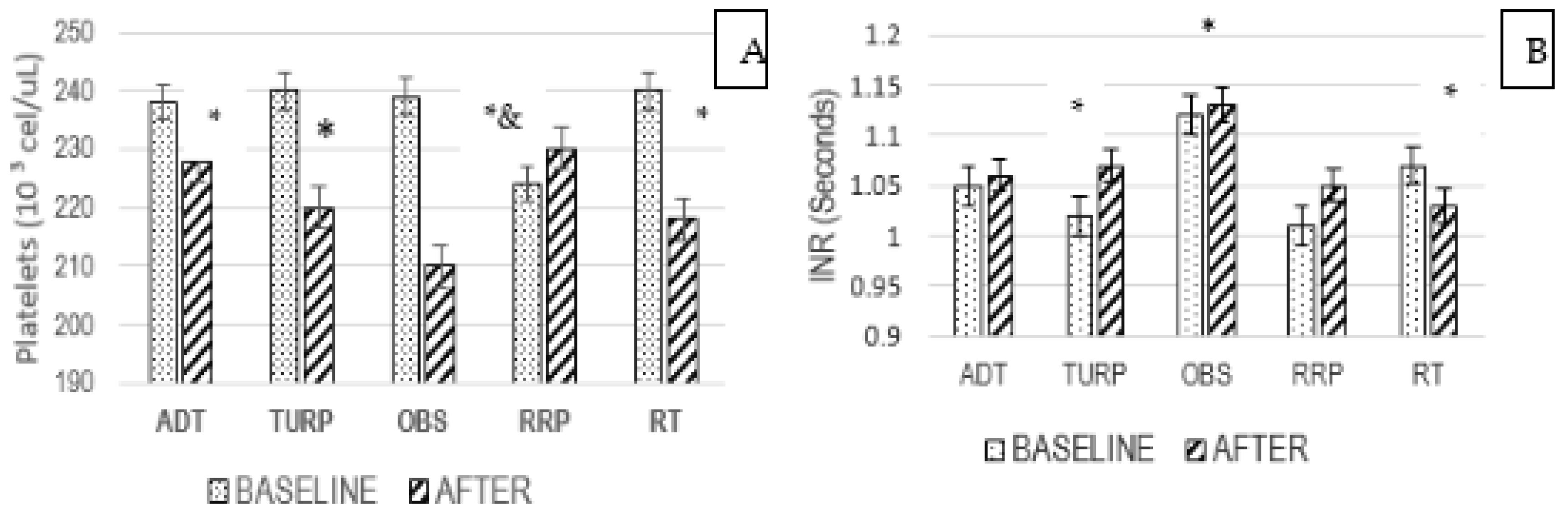
3.6. Platelets and INR Ratio
3.7. Platelets and Treatment
3.8. Age and Hematological Response
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Agency for Research on Cancer. Global Cancer Observatory; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Ferlay, J.; Colombet, M.; Mery, L.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2020, 24, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Abida, W.; Patnaik, A.; Cambell, D.; Shapiro, J.; Bryce, A.H.; McDermott, R.; Sautois, B.; Vogelzang, N.J.; Bambury, R.M.; Voog, E.; et al. Rucaparib in men with metastatic castration resistant prostate cancer a BRCA1 or BRCA2 gene alteration. J. Clin. Oncol. 2020, 38, 3763–3772. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.; Martens, J.W.M.; Hoeck, A.V.; Cuppen, E. Pan-cancer landscape of homologous recombination deficiency. Nat. Commun. 2020, 11, 5584. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.J. Biomarkers for prostate cancer: Prostate-specific antigen and beyond. Clin. Chem. Lab. Med. 2020, 58, 326–339. [Google Scholar] [CrossRef]
- Van Poppel, H.; Roobol, M.J.; Chapple, C.R.; Catto, J.; N’Dow, J.; Sønksen, J.; Stenzl, A.; Wirth, M. Prostate-specific Antigen Testing as Part of Risk-Adapted Early Detection Strategy for Prostate Cancer: European Association of Urology Position and Recommendations for 2021. Eur. Urol. 2021, 80, 703–711. [Google Scholar] [CrossRef]
- American Joint Committee on Cancer AJCC. Cancer Staging Manual Prostate, 8th ed.; Springer: New York, NY, USA, 2017; pp. 715–725. [Google Scholar]
- Pérez-Hernández, A.I.; Catalan, V.; Gómez-Ambrosi, J.; Rodríguez, A.; Fruhbeck, G. Mechanisms linking excess adiposity and carcinogenesis promotion. Front. Endocrinol. 2014, 5, 65. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, H.K.; Qu, Y.Y.; Ye, D.W. Prostate cancer in East Asia: Evolving trend over the last decade. Asian J. Androl. 2015, 17, 48–57. [Google Scholar] [CrossRef]
- Sfanos, K.S.; De Marzo, A. Prostate cancer and inflammation: The evidence. Histopathology 2012, 60, 199–215. [Google Scholar] [CrossRef]
- Desai, K.; McManus, J.M.; Sharifi, N. Hormonal therapy for prostate cancer. Endocr. Rev. 2021, 42, 354–373. [Google Scholar] [CrossRef]
- Vickers, A.J.; Cronin, A.M.; Roobol, M.J.; Hugosson, J.; Jones, J.S.; Kattan, M.W.; Klein, E.; Hamdy, F.; Neal, D.; Donovan, J.; et al. The relationship between prostate-specific antigen and prostate cancer risk: The Prostate Biopsy Collaborative Group. Clin. Cancer Res. 2010, 16, 4374–4381. [Google Scholar] [CrossRef]
- Rajaram, P.; Rivera, A.; Muthima, K.; Olveda, N.; Muchalski, H.; Chen, Q.-H. Second-generation androgen receptor antagonists as hormonal therapeutics for three forms of prostate cancer. Mol. J. 2020, 25, 2448. [Google Scholar] [CrossRef]
- Tan, E.; Li, J.; Xu, E.; Mecher, K.; Yong, E.-L. Androgen receptor: Structure, role in prostate cancer and drug discovery. Acta Pharmacol. Sin. 2015, 36, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Vral, A.; Magri, V.; Montanari, E.; Gazzano, G.; Gourvas, V.; Marras, E.; Perletti, G. Topographic and quantitative relationship between prostate inflammation, proliferative inflammatory atrophy and low/grade prostate intraepithelial neoplasia: A biopsy study in chronic prostatitis patients. Int. J. Oncol. 2012, 41, 1950–1958. [Google Scholar] [CrossRef] [PubMed]
- Rudzinski, J.; Govindasamy, N.P.; Lewis, J.D.; Jurasz, P. The role of the androgen receptor in prostate cancer-induce platelet aggregation and platelet-induced invasion. J. Thromb. Haemost. 2020, 18, 2976–2986. [Google Scholar] [CrossRef]
- Corti, M.; Lorenzetti, S.; Ubaldi, A.; Zilli, R.; Marcoccia, D. Endocrine Disruptors and Prostate Cancer. Int. J. Mol. Sci. 2022, 23, 1216. [Google Scholar] [CrossRef] [PubMed]
- Sánchez de Badajoz, E.; Lage-Sánchez, J.M.; Sánchez-Gallegos, P. Endocrine disruptors and prostate cancer. Arch. Esp. Urol. 2017, 70, 331–335. [Google Scholar] [PubMed]
- de Boussac, H.; Pommier, A.J.; Dufour, J.; Trousson, A.; Caira, F.; Volle, D.H.; Baron, S.; Lobaccaro, J.M. LXR, prostate cancer and cholesterol: The Good, the Bad and the Ugly. Am. J. Cancer Res. 2013, 3, 58–69. [Google Scholar]
- Xiao, H.M.; Cuoco, M.S.; Crowdis, J.; Bosma-Moody, A.; Zhang, Z.; Bi, K.; Kanodia, A.; Su, M.-J.; Garcia, M.M.; Sweet, A.R.; et al. Transcriptional Mediators of Treatment Resistance in Lethal Prostate Cancer. Nat. Med. 2021, 27, 426–433. Available online: https://www.nature.com/naturemedicine (accessed on 13 June 2023).
- Lu, X.; Horner, J.W.; Paul, E.; Shang, X.; Tronoso, P.; Deng, P.; Jiang, S.; Chang, Q.; Spring, D.J.; Sharma, P.; et al. Effective combinatorial immunotherapy for castration resistant prostate cancer. Nat. J. 2017, 543, 728–732. [Google Scholar] [CrossRef]
- Cackpwski, F.C.; Heath, E.I. Prostate cancer dormancy and recurrence. Cancer Lett. 2022, 524, 103–108. [Google Scholar] [CrossRef]
- Van Poppel, H.; Albreht, T.; Basu, P.; Hogenhout, R.; Collen, S.; Roobol, M. Serum PSA-based early detection of prostate cancer in Europe and globally: Past, present and future. Nat. Rev. Urol. 2022, 19, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Kaochar, S.; Mitsiades, N. Glucocorticoids mediate adverse events of deep androgen receptor inhibition in prostate cancer patients. Ann. Oncol. 2020, 31, 323–325. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; He, Z.; Ma, S.; Liu, R. AST/ALT ratio as a significant predictor of the incidence prostate cancer. Cancer Med. 2020, 9, 5672–5677. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, M.; Zajac, J. Hematological change during androgen deprivation therapy. Asian J. Androl. 2012, 14, 187–192. [Google Scholar] [CrossRef]
- Michaelson, M.D.; Cotter, S.E.; Gargollo, P.C.; Zietman, A.L.; Dahl, D.M.; Smith, M.R. Management of complications of prostate cancer treatment. CA Cancer J. Clin. 2008, 58, 196–213. [Google Scholar] [CrossRef]
- Yue, S.; Li, J.; Lee, S.Y.; Lee, H.J.; Shao, T.; Song, B.; Cheng, L.; Masterson, T.A.; Liu, X.; Ratliff, T.L.; et al. Cholesteryl ester accumulation induced by PTEN loss and PI3K/AKT activation underlies human prostate cancer aggressiveness. Cell Metabol. 2014, 19, 393–406. [Google Scholar] [CrossRef]
- Lynch, M.; Sriprasad, S.; Subbramonian, K.; Thompson, P. Postoperative haemorrhage following transurethral resection of the prostate (TURP) and photoselective vaporization of the prostate (PVP). Ann. R. Coll. Surg. Engl. 2010, 92, 555–558. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, N.; Ridzwan, N.S.; Venalainen, E.; Haegert, A.; Parolia, A.; Xue, H.; Wang, Y.; Wu, R.; Dong, X.; Collins, C.; et al. miR-100-5p inhibition induces apoptosis in dormant prostate cancer cells and prevents the emergence of castration-resistant prostate cancer. Sci. Rep. 2017, 7, 4079. [Google Scholar] [CrossRef]
- Zaslavsky, A.; Gloeckner-Kalousek, A.; Adams, M.; Putluri, N.; Venghatakrishnan, H.; Li, H.; Morgan, T.M.; Feng, F.Y.; Tewari, M.; Sreekumar, A.; et al. Platelet-synthesized testosterone in men with prostate cancer induces androgen receptor signaling. Neoplasia 2015, 17, 490–496. [Google Scholar] [CrossRef]
- Green, D.; Karpatkin, S. Role of thrombin as a tumor growth factor. Cell Cycle 2010, 9, 656–661. [Google Scholar] [CrossRef]
- Marcolino, E.; Siddiqui, Y.H.; van den Bosch, M.; Poole, A.W.; Jayaraman, P.S.; Gaston, K. Blood platelets stimulate cancer extravasation through TGFβ-mediated downregulation of PRH/HHEX. Oncogenesis 2020, 9, 10. [Google Scholar] [CrossRef]
- Zaslavky, A.; Adams, M.; Cao, X.; Yamaguchi, A.; Henderson, J.; Busch-Ostergren Udager, A.; Pitchiaya, S.; Palapattu, S. Antisense oligonucleotides and nucleic acids generate hypersensitive platelets. Thromb. Res. 2021, 200, 64–71. [Google Scholar] [CrossRef]
- Weinstein, S.J.; Mackrain, K.; Stolzenberg-Solomon, R.Z.; Selhub, J.; Virtamo, J.; Albanes, D. Serum creatinine and prostate cancer risk in a prospective study. Cancer Epidemiol. Biomark. Prev. 2009, 18, 2643–2649. [Google Scholar] [CrossRef] [PubMed]
- Mollinedo, F.; Gajate, C. Lipid rafts as signaling hubs in cancer cell survival/death and invasion: Implications in tumor progression and therapy. J. Lipid Res. 2020, 61, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Van den Broeck, T.; van den Bergh, R.; Briers, E.; Cornford, P.; Cumberbatch, M.; Tilki, D.; De Santis, M.; Fanti, S.; Fossati, N.; Gillessen, S.; et al. Biochemical Recurrence in Prostate Cancer: The Eur. Ass.Urol. Prostate Cancer Guidelines Panel Recommendations. Eur. Urol. Focus 2020, 6, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Kalogirou, C.; Linxweiler, J.; Schmucker, P.; Snaebjornsson, M.T.; Schmitz, W.; Wach, S.; Krebs, M.; Hartmann, E.; Puhr, M.; Müller, A.; et al. MiR-205-driven downregulation of cholesterol biosynthesis through SQLE-inhibition identifies therapeutic vulnerability in aggressive prostate cancer. Nat. Commun. 2021, 12, 5066. [Google Scholar] [CrossRef] [PubMed]
- Rendón-Ramírez, A.L.; Maldonado-Vega, M.; Quintanar-Escorza, M.A.; Hernández, G.; Arévalo-Rivas, B.I.; Zentella-Dehesa, A.; Calderón-Salinas, J.V. Effect of vitamin E and C supplementation on oxidative damage and total antioxidant capacity in lead-exposed workers. Environ. Toxicol. Pharmacol. 2014, 37, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Dorado, I.C.; Hernández, G.; Quintanar-Escorza, M.A.; Maldonado-Vega, M.; Rosas-Flores, M.; Calderón-Salinas, J.V. Eryptosis in lead-exposed workers. Toxicol. Appl. Pharmacol. 2014, 281, 195–202. [Google Scholar] [CrossRef]
- Osuchowski, M.; Aebisher, D.; Bartusik-Aebisher, D.; Krupka-Olek, M.; Dynarowicz, K.; Przygoda, M.; Kawczyk-Krupka, A. Photodynamic Therapy-Adjunctive Therapy in the Treatment of Prostate Cancer. Diagnostics 2022, 12, 1113. [Google Scholar] [CrossRef]
- Gu, X.; Wu, J.; Liu, X.; Hong, Y.; Wu, Y.; Tian, Y. Role of Serum Creatinine Levels in Prognostic Risk Stratification of Prostate Cancer Patients. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2022, 28, e937100-1–e937100-7. [Google Scholar] [CrossRef]
| Characteristics | Median (Percentile 25–75) | Range |
|---|---|---|
| Age (years) | 69 (63–75) | 43–95 |
| BMI: n (%) | ||
| normal weight | 241 (27) | |
| underweight | 20 (2) | |
| overweight | 289 (32) | |
| obesity | 157 (17) | |
| Hemoglobin baseline (g/dL) | 14 (12–15) | 3.5–20 |
| Hemoglobin after (gr/dL) | 12.4 (10–14) * | 2.5–20 |
| Index L/L baseline | 4.94 (1.3–50) | 1.3–50 |
| Index L/L after | 6.72 (0.86–50) * | 0.89–50 |
| Platelets baseline (cel/uL) | 236 ± 126 | 9–719 |
| Platelets after (cel/uL) | 237 ± 127 | 9–719 |
| INR baseline (second) | 1.06 ± 0.63 | 0.75–10.93 |
| INR after (second) | 1.08 ± 0.56 | 0.76–4.19 |
| Creatinine baseline (mg/dL) | 0.9 (0.8–1.10) | 0.3–46 |
| Creatinine after (mg/dL) | 0.9 (0.8–1.30) | 0.2–13 |
| Total, cholesterol baseline (mg/dL) | 180 (148–204) | 40–298 |
| Total, cholesterol after (mg/dL) | 157 (120–200) | 180–450 |
| (%) | ||
| Survival | 617 (68) | |
| Smoking | 520 (57) | |
| Ethylisms | 508 (56) | |
| Hypertension | 370 (41) | |
| Diabetes mellitus | 222 (24) | |
| Infections | 259 (29) | |
| Kidney disease | 80 (8) | |
| Clinical Parameters | Hormonal Response (n = 466) 51% (TSPC) | Treatment Discontinuation (n = 60) 7% (AT) | Biochemical Recurrence (n = 384) 42% (TRPC) |
|---|---|---|---|
| PSA (ng/mL) | 16 ** | 32 ** | 85 ** |
| Hemoglobin baseline (g/dL) | 14.5 | 14.3 * | 13.2 * |
| Hemoglobin after (g/dL) | 13.0 | 14.0 | 11.0 |
| L/L index baseline | 4.84 * | 5.00 | 5.06 * |
| L/L index after | 6.73 * | 4.21 * | 6.89 * |
| Platelets (cells/µL) Baseline min–max | 229 ± 122 [10–662] | 236 ± 137 [92–618] | 244 ± 120 * [9–719] |
| Platelets (cells/µL) after min–max | 221 ± 125 [6–606] | 206 ± 120 [9–719] | 221 ± 137 [1–681] |
| INR (seconds) Baseline min–max | 1.01 ± 0.51 [0.75–2.26] | 1.10 ± 0.55 [0.86–2.01] | 1.11 ± 0.77 [0.76–10.93] |
| INR (seconds) after min–max | 1.00 ± 0.49 [0.76–1.70] | 1.12 ± 0.34 [0.86–2.01] | 1.17 ± 0.64 * [0.76–4.19] |
| Creatinine baseline (mg/dL) | 1.20 ± 0.9 [0.5–8.4] | 0.90 ± 0.5 [0.5–1.8] | 1.40 ± 2.7 [0.3–4.6] |
| Creatinine after (mg/dL) | 1.40 ± 1.5 [0.5–13] | 1.10 ± 0.5 [0.4–3.4] | 1.40 ± 1 [0.2–12.5] |
| Total cholesterol baseline (mg/dL) | 174 | 150 | 184 |
| Total Cholesterol (mg/dL) | 162 | 182 | 156 |
| Metastasis frequency n * (%) | 108 (23) | 22 (37) | 301 (78) |
| Group | Age Range | Hb (mg/dL) | L/L Index | Platelets | Infections | ||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | After | Baseline | After | Baseline | After | Baseline | After | ||
| 1 | <50 | 14 ± 6 [9–16] | 12 ± 7 [6–17] | 4.5 ± 2 [2.4–8] | 6.9 ± 2.8 * [3.8–13] | 237 ± 107 [119–323] | 235 ± 112 [131–360] | 1 | 2 |
| 51–70 | 15 ± 7 [6–19] | 13 ± 7 * [8–17] | 4.7 ± 23 [1.3–17] | 6.75 ± 6.0 * [2.0–33] | 230 ± 11 [36–662] | 223 ± 127 [24–606] | 29 | 35 | |
| >71 | 14 ± 7 [6–20] | 13 ± 7 * [6–18] | 5.0 ± 4 [2.0–20] | 7.1 ± 7 * [2.1–50] | 230 ± 127 [10–500] | 220 ± 122 [6–495] | 42 | 18 | |
| 2 | 51–70 | 13 ± 7 [4–17] | 14 ± 6 * [9–20] | 4.0 ± 2 [2.0–20] | 4.0 ± 2.1 [2.7–10] | 233 ± 149 [92–618] | 212 ± 97 * [79–279] | 3 | 2 |
| >71 | 14 ± 7 [8–16] | 13 ± 5 [6–14] | 4.5 ± 2.5 [2.0–14] | 3.9 ± 2.3 [1.6–10] | 241 ± 150 [143–445] | 186 ± 111 [123–237] | 3 | 1 | |
| 3 | <50 | 12 ± 5 [9–15] | 8 ± 4 ** [6–11] | 5.3 ± 4.9 [3.1–8.0] | 6.7 ± 5.0 * [5.0–33] | 320 ± 150 [226–526] | 243 ± 200 [123–681] | 3 | 3 |
| 51–70 | 13 ± 4 [5–20] | 11 ± 5 ** [4–17] | 5.2 ± 4 [1.7–20] | 7.1 ± 7.0 * [0.9–33] | 249 ± 110 [30–616] | 236 ± 144 * [1–680] | 34 | 36 | |
| >71 | 13 ± 6 [4–16] | 11 ± 5 ** [3–17] | 5.0 ± 4.5 [2–0–33] | 6.7 ± 7.0 * [1.4–50] | 238 ± 120 [9–719] | 209 ± 123 * [27–520] | 24 | 23 | |
| Gleason Grade | Age Range (Years) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| <50 | 51–70 | >70 | |||||||
| Patients | Survival | Survival (%) | Patients | Survival | Survival (%) | Patients | Survival | Survival (%) | |
| 1 | 2 | 2 | 100 | 132 | 117 | 89 | 98 | 83 | 85 |
| 2 | 3 | 3 | 100 | 119 | 84 | 70 | 80 | 49 | 61 |
| 3 | 1 | 0 | 0 | 72 | 53 | 74 * | 55 | 42 | 76 * |
| 4 | 10 | 4 | 40 | 78 | 58 | 81 * | 63 | 40 | 64 * |
| 5 | 1 | 0 | 0 | 76 | 28 | 37 * | 67 | 35 | 52 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cejudo-Arteaga, S.; Ramírez-Reyes, M.A.; Badillo-Santoyo, M.A.; Martínez-Cordero, E.; Farías-Serratos, F.; Maldonado-Vega, M. Prostate Cancer, Treatment and Response of the Hematological System in Mexican Population. Int. J. Transl. Med. 2023, 3, 286-298. https://doi.org/10.3390/ijtm3030020
Cejudo-Arteaga S, Ramírez-Reyes MA, Badillo-Santoyo MA, Martínez-Cordero E, Farías-Serratos F, Maldonado-Vega M. Prostate Cancer, Treatment and Response of the Hematological System in Mexican Population. International Journal of Translational Medicine. 2023; 3(3):286-298. https://doi.org/10.3390/ijtm3030020
Chicago/Turabian StyleCejudo-Arteaga, Shaila, Marco Antonio Ramírez-Reyes, Marco Antonio Badillo-Santoyo, Erika Martínez-Cordero, Felipe Farías-Serratos, and María Maldonado-Vega. 2023. "Prostate Cancer, Treatment and Response of the Hematological System in Mexican Population" International Journal of Translational Medicine 3, no. 3: 286-298. https://doi.org/10.3390/ijtm3030020
APA StyleCejudo-Arteaga, S., Ramírez-Reyes, M. A., Badillo-Santoyo, M. A., Martínez-Cordero, E., Farías-Serratos, F., & Maldonado-Vega, M. (2023). Prostate Cancer, Treatment and Response of the Hematological System in Mexican Population. International Journal of Translational Medicine, 3(3), 286-298. https://doi.org/10.3390/ijtm3030020






