A Review of High-Intensity Focused Ultrasound
Abstract
1. Introduction
2. History of HIFU
3. The Technology
4. Applications of HIFU
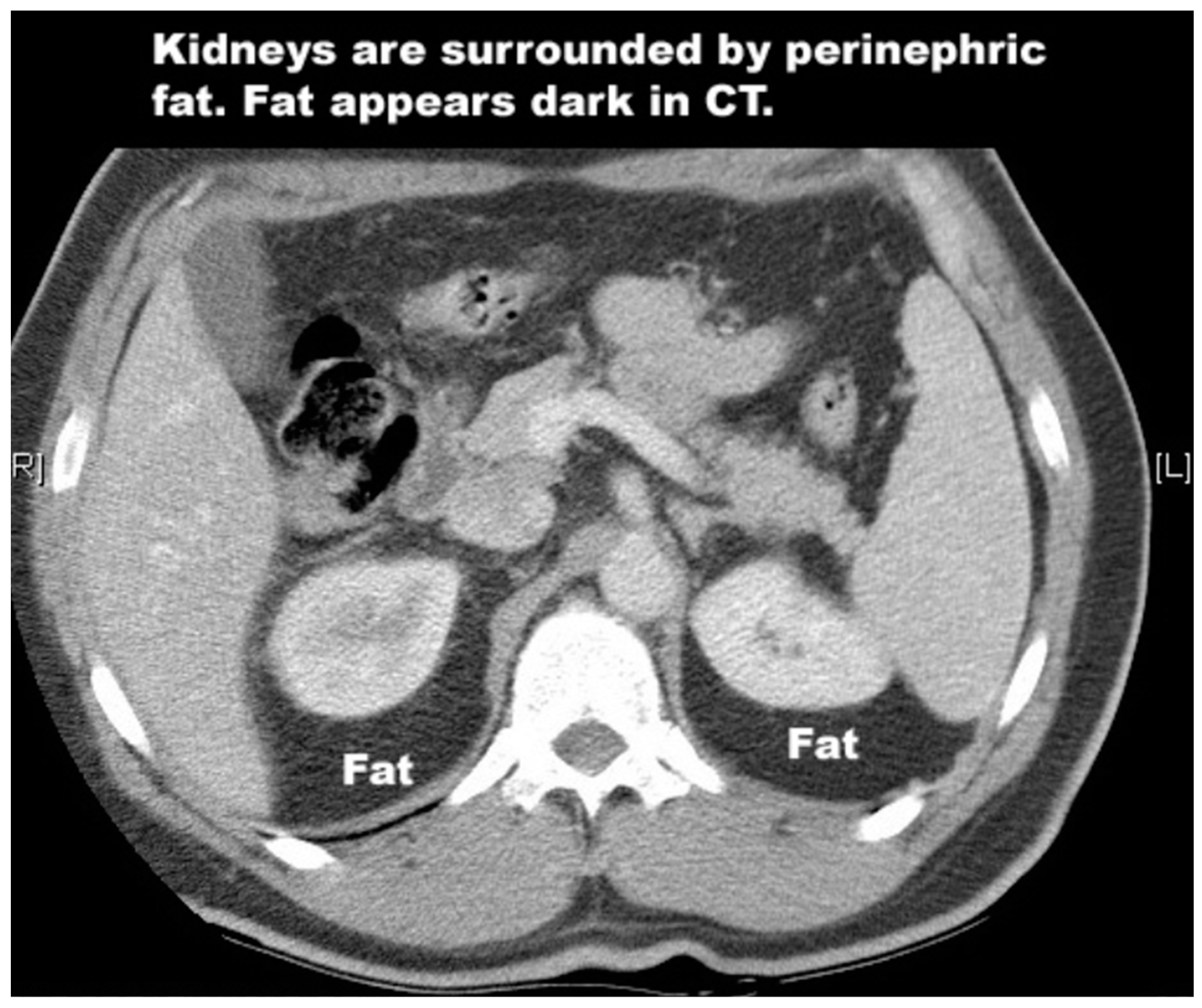
4.1. Kidney
4.2. Prostate
4.3. Liver
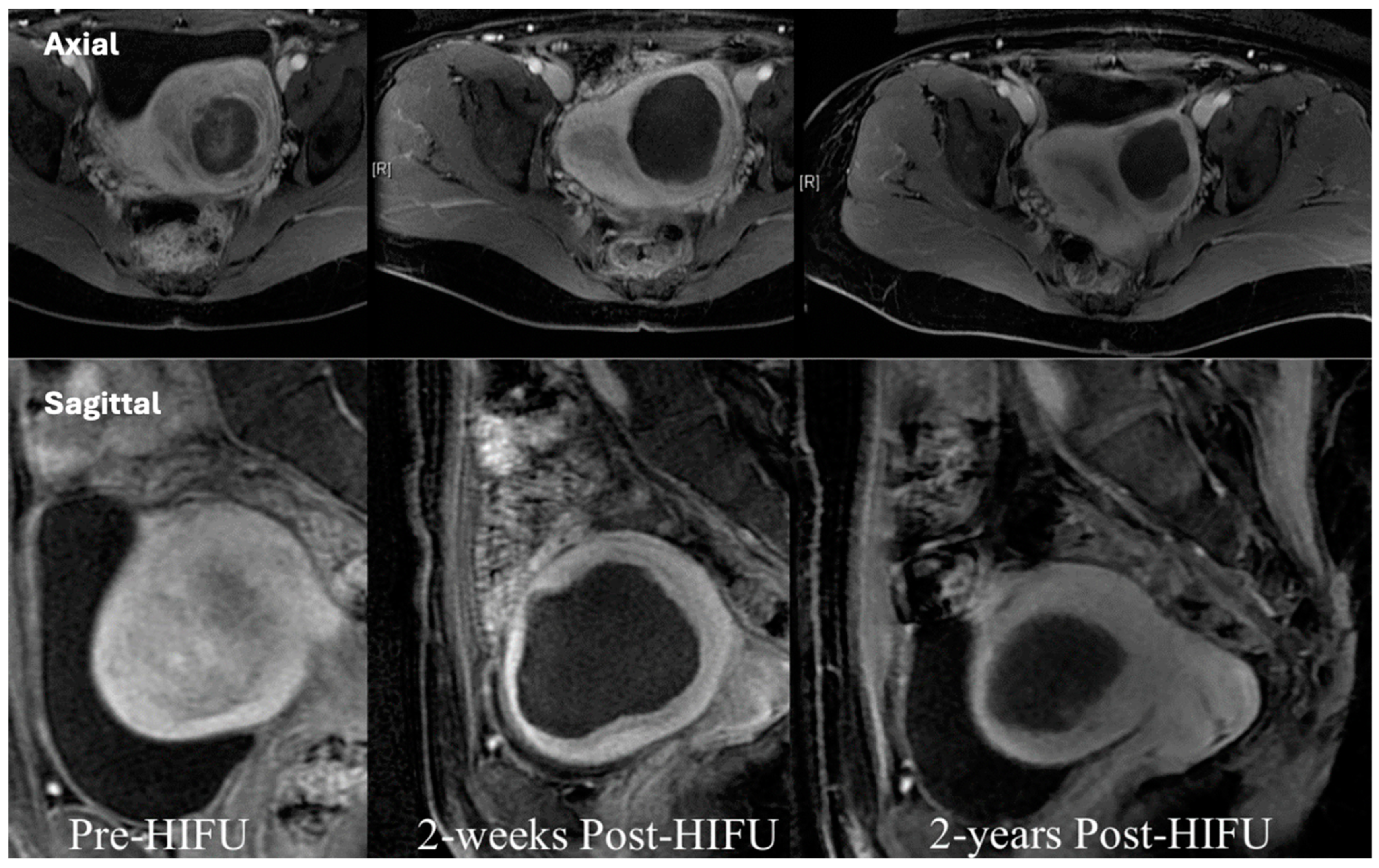
4.4. Uterine Fibroids
4.5. Chordomas
4.6. Pancreas and Breast Cancer
4.7. Ultrasound-Mediated Drug Delivery
4.8. Possible Immunological Benefits of HIFU
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kennedy, J.E.; ter Haar, G.R.; Cranston, D. High intensity focused ultrasound: Surgery of the future? Br. J. Radiol. 2003, 76, 590–599. [Google Scholar] [CrossRef]
- Xu, Z.; Hall, T.L.; Vlaisavljevich, E.; Lee, F.T. Histotripsy: The first noninvasive, non-ionizing, non-thermal ablation technique based on ultrasound. Int. J. Hyperth. 2021, 38, 561–575. [Google Scholar] [CrossRef]
- Kennedy, J.E. High-intensity focused ultrasound in the treatment of solid tumours. Nat. Rev. Cancer 2005, 5, 321–327. [Google Scholar] [CrossRef]
- Chen, L.; ter Haar, G.R.; Hill, C.R.; Dworkin, M.; Carnochan, P.; Young, H.; Bensted, J.F.M. Effect of blood perfusion on the ablation of liver parenchyma with high-intensity focused ultrasound. Phys. Med. Biol. 1993, 38, 1661–1673. [Google Scholar] [CrossRef]
- Hill, C.R.; Rivens, I.; Vaughan, M.G.; ter Haar, G. Lesion development in focused ultrasound surgery: A general model. Ultrasound Med. Biol. 1994, 20, 259–269. [Google Scholar] [CrossRef]
- Kepp, O.; Marabelle, A.; Zitvogel, L. Oncolysis without viruses—Inducing systemic anticancer immune responses with local therapies. Nat. Rev. Clin. Oncol. 2020, 17, 49–64. [Google Scholar] [CrossRef]
- Rückert, M.; Flohr, A.-S.; Hecht, M.; Gaipl, U.S. Radiotherapy and the immune system: More than just immune suppression. Stem Cells 2021, 39, 1155–1165. [Google Scholar] [CrossRef]
- Kennedy, J.; Wu, F.; ter Haar, G.; Gleeson, F.; Phillips, R.; Middleton, M.; Cranston, D. High-intensity focused ultrasound for the treatment of liver tumours. Ultrasonics 2004, 42, 931–935. [Google Scholar] [CrossRef] [PubMed]
- Izadifar, Z.; Izadifar, Z.; Chapman, D.; Babyn, P. An introduction to high intensity focused ultrasound: Systematic review on principles, devices, and clinical applications. J. Clin. Med. 2020, 9, 460. [Google Scholar] [CrossRef] [PubMed]
- Wolfram, F.; Lesser, T.G. A simulation study of the HIFU ablation process on lung tumours, showing consequences of atypical acoustic properties in flooded lung. Z. Med. Phys. 2019, 29, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Wood, R.; Loomis, A.L. The physical and biological effects of high-frequency sound-waves of great intensity. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1927, 4, 417–436. [Google Scholar] [CrossRef]
- Lynn, J.G.; Zwemer, R.L.; Chick, A.J.; Miller, A.E. A new method for the generation and use of focused ultrasound in experimental biology. J. Gen. Physiol. 1942, 26, 179–193. [Google Scholar] [CrossRef]
- Fry, W.J.; Barnard, J.W.; Fry, F.J.; Krumins, R.F.; Brennan, J.F. Ultrasonic lesions in the mammalian central nervous system. Science 1955, 122, 517–518. [Google Scholar] [CrossRef]
- Fry, F.J. Precision high intensity focusing ultrasonic machines for surgery. Am. J. Phys. Med. Rehabil. 1958, 37, 152–156. [Google Scholar] [CrossRef]
- Burov, A.K. High-intensity ultrasonic vibrations for action on animal and human malignant tumours. Dokl Akad. Nauk SSSR 1956, 106, 239–241. [Google Scholar]
- Lee, K.W. The Asian perspective on Hifu. Int. J. Hyperth. 2021, 38, 5–8. [Google Scholar] [CrossRef]
- ter Haar, G.; Sinnett, D.; Rivens, I. High intensity focused ultrasound-a surgical technique for the treatment of discrete liver tumours. Phys. Med. Biol. 1989, 34, 1743–1750. [Google Scholar] [CrossRef] [PubMed]
- Bachu, V.S.; Kedda, J.; Suk, I.; Green, J.J.; Tyler, B. High-intensity focused ultrasound: A review of mechanisms and clinical applications. Ann. Biomed. Eng. 2021, 49, 1975–1991. [Google Scholar] [CrossRef]
- Koerhrman, K. High intensity focused ultrasound for non-invasive tissue ablation in the kidney, prostate and uterus. J. Urol. 2000, 163, 698. [Google Scholar]
- Ritchie, R.W.; Leslie, T.; Phillips, R.; Wu, F.; Illing, R.; Ter Haar, G.; Protheroe, A.; Cranston, D. Extracorporeal high intensity focused ultrasound for renal tumours: A 3-year follow-up. BJU Int. 2010, 106, 1004–1009. [Google Scholar] [CrossRef] [PubMed]
- Mancini, M.; Righetto, M.; Baggio, G. Gender-related approach to kidney cancer management: Moving forward. Int. J. Mol. Sci. 2020, 21, 3378. [Google Scholar] [CrossRef] [PubMed]
- Chakera, A.; Leslie, T.; Roberts, I.; O’Callaghan, C.A.; Cranston, D. A lucky fall? Case report. Transplant. Proc. 2010, 42, 3883–3886. [Google Scholar] [CrossRef] [PubMed]
- Klingler, H.C.; Susani, M.; Seip, R.; Mauermann, J.; Sanghvi, N.; Marberger, M.J. A novel approach to energy ablative therapy of small renal tumours: Laparoscopic high-intensity focused ultrasound. Eur. Urol. 2008, 53, 810–816. [Google Scholar] [CrossRef]
- Nakamura, K. Clinical application of high-intensity focused ultrasound for benign prostatic hyperplasia. In Treatment of Benign Prostatic Hyperplasia; Springer: Tokyo, Japan, 2000; pp. 123–132. [Google Scholar] [CrossRef]
- Madersbacher, S.; Pedevilla, M.; Vingers, L.; Susani, M.; Marberger, M. Effect of High-Intensity Focused Ultrasound on human prostate cancer in vivo. Cancer Res. 1995, 55, 3346–3351. [Google Scholar]
- National Prostate Cancer Audit. NPCA Annual Report 2019. 2020. Available online: https://www.npca.org.uk/reports/npca-annual-report-2019/ (accessed on 25 January 2024).
- McCulloch, P.; Altman, D.G.; Campbell, W.B.; Flum, D.R.; Glasziou, P.; Marshall, J.C.; Nicholl, J. No surgical intervention without evaluation. The IDEAL recommendations. Lancet 2009, 374, 1105–1112. [Google Scholar] [CrossRef]
- Rischmann, P.; Gelet, A.; Riche, B.; Villers, A.; Pasticier, G.; Bondil, P.; Jung, J.L.; Bugel, H.; Petit, J.; Toledano, H.; et al. Focal High Intensity Focused Ultrasound of Unilateral Localized Prostate Cancer: A Prospective Multicentric Hemiablation Study of 111 Patients. Eur. Urol. 2017, 71, 267–273. [Google Scholar] [CrossRef]
- Cooperberg, M.R. RE: Fifteen-year outcomes after monitoring, surgery, or radiotherapy for prostate cancer. Eur. Urol. 2023, 84, 435–436. [Google Scholar] [CrossRef]
- Dickinson, L.; Arya, M.; Afzal, N.; Cathcart, P.; Charman, S.C.; Cornaby, A.; Hindley, R.G.; Lewi, H.; McCartan, N.; Moore, C.M.; et al. Medium-term Outcomes After Whole-gland High-intensity Focused Ultrasound for the Treatment of Nonmetastatic Prostate Cancer from a Multicentre Registry Cohort. Eur. Urol. 2016, 70, 668–674. [Google Scholar] [CrossRef]
- Stabile, A.; Orczyk, C.; Hosking-Jervis, F.; Giganti, F.; Arya, M.; Hindley, R.G.; Dickinson, L.; Allen, L.; Punwani, S.; Jameson, C.; et al. Medium-term Oncological Outcomes in a Large Cohort of Men Treated with Either Focal or Hemi-Ablation Using High-Intensity Focused Ultrasonography for Primary Localized Prostate Cancer. BJU Int. 2019, 124, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Abreu, A.L.; Peretsman, S.; Iwata, A.; Shakir, A.; Iwata, T.; Brooks, J.; Tafuri, A.; Ashrafi, A.; Park, D.; Cacciamani, G.E.; et al. High Intensity Focused Ultrasound Hemi-gland Ablation for Prostate Cancer: Initial Outcomes of a United States Series. J. Urol. 2020, 204, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Hostiou, T.; Gelet, A.; Chapelon, J.Y.; Rouviere, O.; Mege-Lechevalier, F.; Lafon, C.; Tonoli-Catez, H.; Badet, L.; Crouzet, S. Salvage High-Intensity Focused Ultrasound for Locally Recurrent Prostate Cancer After Low-Dose-Rate Brachytherapy: Oncological and Functional Outcomes. BJU Int. 2019, 124, 746–757. [Google Scholar] [CrossRef] [PubMed]
- Crouzet, S.; Blana, A.; Murat, F.J.; Pasticier, G.; Brown, S.C.; Conti, G.N.; Ganzer, R.; Chapet, O.; Gelet, A.; Chaussy, C.G.; et al. Salvage High-Intensity Focused Ultrasound (Hifu) For Locally Recurrent Prostate Cancer After Failed Radiation Therapy: Multi-Institutional Analysis Of 418 Patients. BJU Int. 2017, 119, 896–904. [Google Scholar] [CrossRef]
- Illing, R.O.; Kennedy, J.E.; Wu, F.; Ter Haar, G.R.; Protheroe, A.S.; Friend, P.J.; Gleeson, F.V.; Cranston, D.W.; Phillips, R.R.; Middleton, M.R. The safety and feasibility of extracorporeal high-intensity focused ultrasound (HIFU) for the treatment of liver and kidney tumours in a Western population. Br. J. Cancer 2005, 93, 890–895. [Google Scholar] [CrossRef] [PubMed]
- Yang, T. HIFU for the treatment of difficult colorectal liver metastases with unsuitable indications for resection and radiofrequency ablation: A phase I clinical trial. J. Clin. Oncol. 2020, 38 (Suppl. 15), e16715. [Google Scholar] [CrossRef]
- Rees, M.; Tekkis, P.P.; Welsh, F.K.; O’rourke, T.; John, T.G. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: A multifactorial model of 929 patients. Ann. Surg. 2008, 247, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Pawlik, T.M.; Scoggins, C.R.; Zorzi, D.; Abdalla, E.K.; Andres, A.; Eng, C.; Vauthey, J.N. Efect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann. Surg. 2005, 241, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Nordlinger, B.; Sorbye, H.; Glimelius, B.; Poston, G.J.; Schlag, P.M.; Rougier, P.; Gruenberger, T. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): Long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013, 14, 1208–1215. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Wang, Z.; McCulloch, P.; Hu, L.; Chen, W.; Liu, G.; Li, J.; Lang, J. Evaluation of high-intensity focused ultrasound ablation for uterine fibroids: An ideal prospective exploration study. BJOG Int. J. Obstet. Gynaecol. 2017, 125, 354–364. [Google Scholar] [CrossRef]
- Lyon, P.C.; Rai, V.; Price, N.; Shah, A.; Wu, F.; Cranston, D. Ultrasound-guided high intensity focused ultrasound ablation for symptomatic uterine fibroids: Preliminary clinical experience. Ultraschall Med.-Eur. J. Ultrasound 2019, 41, 550–556. [Google Scholar] [CrossRef]
- Yan, L.; Huang, H.; Lin, J.; Yu, R. High-intensity focused ultrasound treatment for symptomatic uterine fibroids: A systematic review and meta-analysis. Int. J. Hyperth. 2022, 39, 230–238. [Google Scholar] [CrossRef]
- Yakkioui, Y.; van Overbeeke, J.J.; Santegoeds, R.; van Engeland, M.; Temel, Y. Chordoma: The entity. Biochim. Biophys. Acta 2014, 1846, 655–669. [Google Scholar] [CrossRef]
- Chetan, M.R.; Lyon, P.C.; Wu, F.; Phillips, R.; Cranston, D.; Gillies, M.J.; Bojanic, S. Role of diffusion-weighted imaging in monitoring treatment response following high-intensity focused ultrasound ablation of recurrent sacral chordoma. Radiol. Case Rep. 2019, 14, 1197–1201. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Niu, X.; Li, Y.; Liu, W.; Xu, H. Recurrence and survival factors analysis of 171 cases of sacral chordoma in a single institute. Eur. Spine J. 2017, 26, 1910–1916. [Google Scholar] [CrossRef] [PubMed]
- Radaelli, S.; Stacchiotti, S.; Ruggieri, P.; Donati, D.; Casali, P.; Palmerini, E.; Collini, P.; Gambarotti, M.; Porcu, L.; Boriana, S.; et al. Sacral chordoma: Long-term outcome of a large series of patients surgically treated at two reference centres. Spine. 2016, 41, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Gillies, M.J.; Lyon, P.C.; Wu, F.; Leslie, T.; Chung, D.Y.; Gleeson, F.; Cranston, D.; Bojanic, S. High-intensity focused ultrasonic ablation of sacral chordoma is feasible: A series of four cases and details of a national clinical trial. Br. J. Neurosurg. 2016, 31, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Prachee, I.; Wu, F.; Cranston, D. Oxford’s clinical experience in the development of high intensity focused ultrasound therapy. Int. J. Hyperth. 2021, 38, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Maloney, E.; Hwang, J.H. Emerging HIFU applications in cancer therapy. Int. J. Hyperth. 2015, 31, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Yu, A.C. Ultrasound-mediated drug delivery: Sonoporation Mechanisms, biophysics, and critical factors. BME Front. 2022, 2022, 9807347. [Google Scholar] [CrossRef] [PubMed]
- Lyon, P.C.; Gray, M.D.; Mannaris, C.; Folkes, L.K.; Stratford, M.; Campo, L.; Chung, D.Y.F.; Scott, S.; Anderson, M.; Goldin, R.; et al. Safety and feasibility of ultrasound-triggered targeted drug delivery of doxorubicin from thermosensitive liposomes in liver tumours (TARDOX): A single-centre, open-label, phase 1 trial. Lancet Oncol. 2018, 19, 1027–1039. [Google Scholar] [CrossRef]
- den Brok, M.H.; Sutmuller, R.P.M.; van der Voort, R.; Bennink, E.J.; Figdor, C.G.; Ruers, T.J.M.; Adema, G.J. In situ tumor ablation creates an antigen source for the generation of antitumor immunity. Cancer Res. 2004, 64, 4024–4029. [Google Scholar] [CrossRef]
- Schueller, G.; Kettenbach, J.; Sedivy, R.; Bergmeister, H.; Stift, A.; Fried, J.; Gnant, M.; Lammer, J. Expression of heat shock proteins in human hepatocellular carcinoma after radiofrequency ablation in an animal model. Oncol. Rep. 2004, 12, 495–499. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turner, B.; Cranston, D. A Review of High-Intensity Focused Ultrasound. Int. J. Transl. Med. 2024, 4, 197-207. https://doi.org/10.3390/ijtm4010011
Turner B, Cranston D. A Review of High-Intensity Focused Ultrasound. International Journal of Translational Medicine. 2024; 4(1):197-207. https://doi.org/10.3390/ijtm4010011
Chicago/Turabian StyleTurner, Ben, and David Cranston. 2024. "A Review of High-Intensity Focused Ultrasound" International Journal of Translational Medicine 4, no. 1: 197-207. https://doi.org/10.3390/ijtm4010011
APA StyleTurner, B., & Cranston, D. (2024). A Review of High-Intensity Focused Ultrasound. International Journal of Translational Medicine, 4(1), 197-207. https://doi.org/10.3390/ijtm4010011





