Does Precision-Based Medicine Hold the Promise of a New Approach to Predicting and Treating Spontaneous Preterm Birth?
Abstract
:1. Introduction
2. Preterm Birth
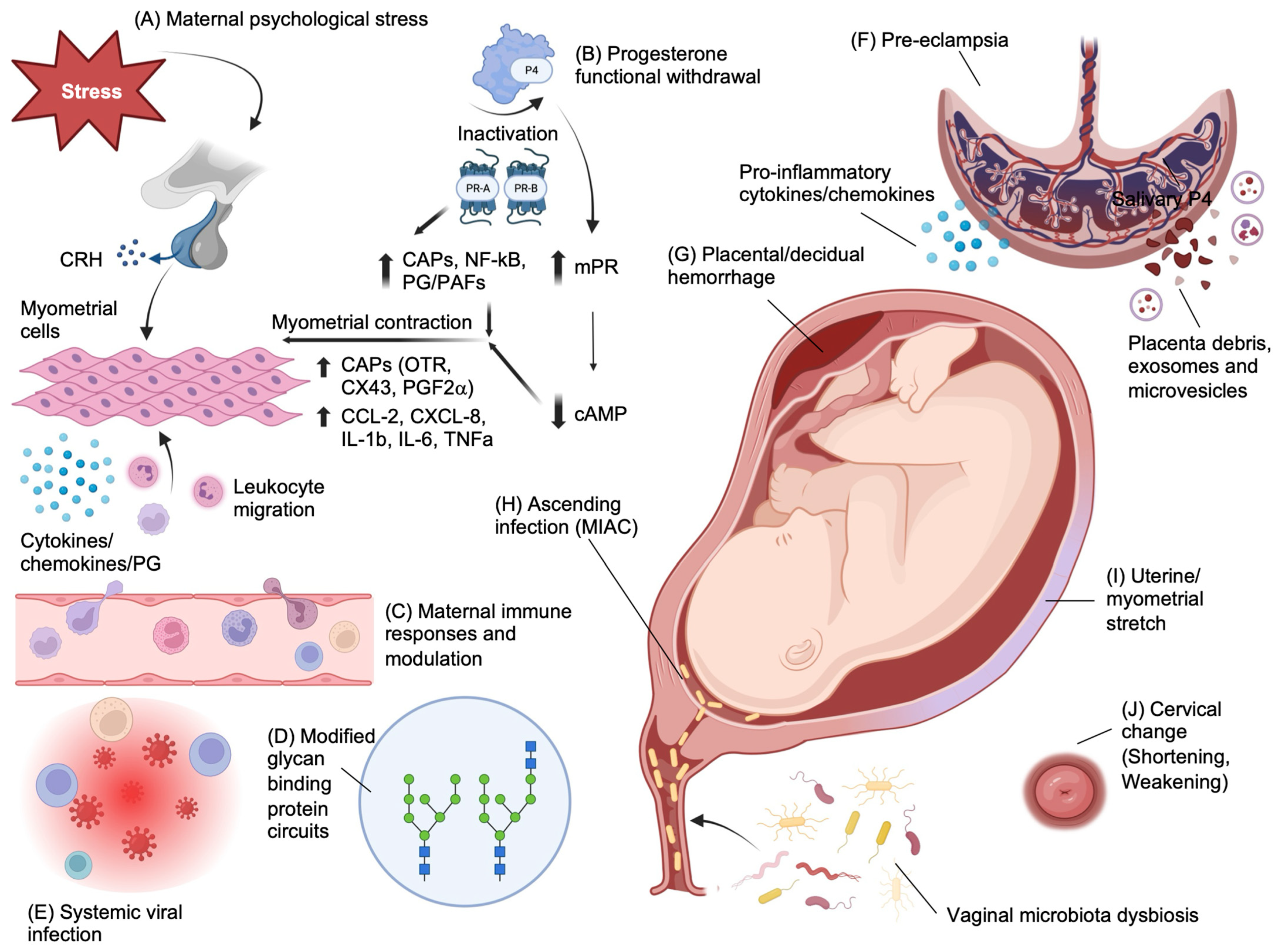
2.1. Risk Factors and Pathophysiology of Preterm Birth
- Offer antenatal corticosteroids for women with a high likelihood of preterm birth (PTB) from 24 weeks to 34 weeks of gestation when the following conditions are met:
- Gestational age assessment can be accurately undertaken.
- There is a high likelihood of preterm birth within 7 days of starting therapy.
- There is no clinical evidence of maternal infection.
- Adequate childbirth care is available (including the capacity to recognize and safely manage PTL and PTB).
- The preterm newborn can receive adequate care (including resuscitation, kangaroo mother care, thermal care, feeding support, infection treatment and respiratory support including continuous positive airway pressure (CPAP) as needed).
- Offer tocolysis in the form of nifedipine for acute and maintenance therapy for women with a high likelihood of PTB.
- For women at risk of PTL, vaginal progesterone and prophylactic cervical cerclage can be considered.
- To diagnose preterm, prelabor rupture of membranes (P-PROM), a speculum examination to look for pooling of amniotic fluid and an immunochromatographic binary point-of-care test to analyze amniotic fluid components such as insulin-like growth factor-binding protein-1 (ROMplus) or placental alpha macroglobulin-1 (PartoSure) if speculum examination is inconclusive. ROM plus is an immunochromatographic binary point-of-care test to identify two proteins found in amniotic fluid—insulin-like growth factor-binding protein-1 (IGFBP1) and alpha fetoprotein (aFP)—to determine rupture of fetal membranes. PartoSure helps to detect PTL in women with intact membranes through the identification of placental alpha macroglobulin-1 in the vaginal secretions of pregnant women.
- Transvaginal ultrasound to diagnose cervical competency and the ability to carry a fetus to term.
- If diagnosed with P-PROM, antenatal prophylactic antibiotics in the form of oral erythromycin QDS for 10 days or until established labor should be prescribed, and antenatal corticosteroids should be offered with counseling on the risks and benefits to both mother and baby.
- Emergency cervical cerclage can be considered for women between 16 + 0 and 27 + 6 weeks of gestation who have intact membranes and no uterine activity, signs of infection or vaginal bleeding.
- If membranes are intact, the use of fetal fibronectin for an understanding of delivery probability within the next 48 h.
- Nifedipine is the drug of choice for tocolysis in suspected or diagnosed PTL with intact membranes from 24 + 0 to 33 + 6 weeks of gestation.
- Women and family members should be counseled on the risks and benefits of antenatal corticosteroids, and steroids should be considered if the woman is in PTL from 22 + 0 to 35 + 6 weeks of gestation.
- The use of intravenous magnesium sulphate should also be considered for neuroprotection of the child and offered to women between 24 + 0 and 33 + 6 weeks of gestation who are planning to have or are already in PTL.
2.2. Current Clinical Tools to Predict Preterm Birth
3. Precision-Based Medicine
3.1. Biomarkers
3.1.1. Bacterial Biomarkers
- Targeting the vaginal microbiome
- 2.
- Targeting amniotic fluid microbial colonization
- 3.
- Targeting maternal infection
- 4.
- Targeting indicators of maternal immune response
3.1.2. Hormonal Markers
3.1.3. Genomic Markers
- Targeting cell-free RNA
- 2.
- Targeting maternal gene polymorphisms
- 3.
- Targeting fetoplacental genes
3.2. Artificial Intelligence and Technology
3.2.1. Computational Modeling
3.2.2. Machine Learning
| Research Team | Model/Study Design | Methods | Sample Size (n) | Main Findings | Prediction/Diagnosis/Treatment of PTB |
|---|---|---|---|---|---|
| Computational | |||||
| Aslanidi et al., 2011 [144] | Computational/in vitro Experimental | Computational models were created from electrohysterogram data to understand the electrical activity in a pregnant uterus using data from various experiments on cells and tissues. Virtual tissue engineering of uterine tissue was developed using in vivo magnetic resonance imaging (MRI) and ex vivo diffusion tensor magnetic resonance imaging (DTI) to create these models, which aim to predict how the uterus contracts during labor. Similar tools are used for heart contraction modeling, where there is better data. | n/a |
| Diagnosis and treatment Using these models to study uterine activity during labor will advance understanding of the mechanisms underlying initiation and progression of labor with potential for direct diagnosis, management and treatment of PTL. |
| Tong et al., 2011 [145] | Computational/in vitro Mathematical modeling | The study employed a computational biology approach to develop a mathematical model describing the excitation-contraction (E-C) coupling of uterine smooth muscle cells (USMC). Fourteen ionic currents in USMCs were quantified using differential equations based on published and unpublished data, including maximal conductance, voltage-dependent gating variables and intracellular calcium changes. | n/a |
| Diagnosis This model can be used to investigate and predict myometrial electrogenesis at both the cellular and tissue levels, contributing to a better understanding of how to identify normal and dysfunctional labors. |
| Le et al., 2020 [150] | Computational and murine Observational: case control | The researchers used a rank-based pattern-matching approach to compare the differential gene expression signature for PTB with drug profiles in the Connectivity Map database. They assigned a reversal score to each PTB-drug pair to identify drugs with potential efficacy in preventing PTB. The drug lansoprazole was selected for further validation. | 30 |
| Treatment This study highlights the potential of computational drug repositioning to discover compounds that could be effective in preventing PTL. |
| Goldsztejn and Nehorai, 2020 [148] | Computational/in vitro Experimental | The researchers employed a computational model to study the relationship between electrical propagation, force development, intercellular coupling and cellular excitability in the myofiber. | n/a |
| Treatment This study used a computational model to understand how cellular functions like intercellular coupling and cellular excitability impact tissue properties such as electrical propagation and force development in the myometrium. This understanding is the start of developing advanced treatments for PTB. |
| Machine Learning | |||||
| Abraham et al., 2022 [151] | Machine learning Experimental | Machine-learning models were developed using billing codes and known risk factors from EHRs of 35,282 deliveries. The models were used to predict preterm birth risk at different gestational ages and compared with models based on known risk factors. The patterns learned by the model were examined to stratify deliveries into interpretable groups. | 35,282 |
| Prediction This study has shown that machine-learning models could be employed to identify patients at risk of preterm birth from as early as the booking appointment. |
| Zhou et al., 2022 [152] | Human Observational: cohort | A hospital-based cohort study using generalized additive models with penalized cubic regression spline was used to explore the non-linear association between maternal thyroid hormone (FT4) levels and the risk of PTD, including its subtypes. The time-to-event method and multivariable Cox proportional hazard model were applied to analyze the association of abnormally high and low maternal FT4 concentrations with the timing of PTD. | 65,565 |
| Prediction This study suggests that measurement of maternal FT4 levels could be used in the prediction and prevention of preterm birth. |
| Artificial Intelligence | |||||
| Maner et al., 2007 [153] | Human Observational: case control | Uterine EMG signals were measured trans-abdominally using surface electrodes. Bursts of elevated uterine EMG corresponding to uterine contractions were quantified using power spectrum peak frequency, burst duration, number of bursts per unit time and total burst activity. Artificial neural networks (ANN) were used to classify patients into labor and non-labor groups, and the percentage of correctly categorized patients was calculated. | 185 |
| Diagnosis Artificial neural networks, when used in conjunction with uterine EMG data, can effectively categorize patients into term or preterm and laboring or non-laboring, thus enabling diagnosis of PTL. |
| Most et al., 2008 [154] | Human Observational: cohort | Electrical uterine myography (EUM) was measured on 87 pregnant women with gestational age less than 35 weeks. The researchers developed an index score (1–5) for predicting preterm delivery (PTD) within 14 days of the test based on the period between contractions, power of contraction peaks and movement of the center of electrical activity (RMS). The EUM index score was compared with fetal fibronectin (fFN) and cervical length (CL) to assess its predictive ability. | 87 |
| Diagnosis Measuring myometrial electrical activity may enhance the identification of patients in true premature labor and rule out those who are not in labor. |
| Chen, L. and H. Xu. 2007 [155] | Human Observational: case-control | The study aimed to investigate the potential of a sparse autoencoder-based deep neural network (SAE-based DNN) in predicting preterm birth using ElectroHysteroGram (EHG) and Tocography (TOCO) signals, which are real-time and non-invasive technologies. The deep neural network (DNN) model was used to measure the bursts of uterine contraction intervals and non-contraction intervals (dummy intervals) from 26 recordings of the TPEHGT DS database that were manually segmented. The SSAE network was used to learn high-level features from these raw features through unsupervised learning. The proposed method was evaluated using 10-fold cross-validation and four performance indicators. | 26 |
| Diagnosis This method shows promise for the semi-automatic identification of term and preterm uterine recordings, offering a non-invasive approach for enhancing the diagnosis of PTL. |
| Anumba et al., 2021 [156] | Human Observational: cohort | The study aimed to evaluate the predictive performance of a cervical probe device based on electrical impedance spectroscopy (EIS) for preterm birth (PTB). The goal was to compare this method with the existing prediction methods—transvaginal ultrasound (TVS) cervical length (CL) measurement and fetal fibronectin (FFN)—in asymptomatic women during the mid-trimester. Multivariate linear and non-linear logistic regression analyses were used to assess the associations of cervical EIS, TVS-CL and FFN with spontaneous PTB before 37 weeks and before 32 weeks. Areas under the receiver operating characteristics curves (AUC) were calculated to compare the predictive performance of the parameters individually and in combination. | 365 |
| Prediction The mid-trimester assessment of the cervix using EIS is effective in predicting spontaneous PTB. |
| Tomialowicz et al., 2021 [157] | Human Observational: prospective cohort | Forty-five pregnant women with pregnancies ranging from 24 to 36 weeks of gestation and typical clinical symptoms of threatened preterm delivery were treated with tocolytic therapy. Bioelectric activity was recorded using electrohysterography simultaneously with mechanical activity recorded using tocography. | 45 |
| Diagnosis This study suggests bioelectric activity might be an early indicator, potentially preceding the mechanical activity of the uterus and therefore a mechanism for earlier diagnosis of PTL. |
3.2.3. Artificial Intelligence
3.3. Combining Methods
4. Precision-Based Medicine to Direct Treatments
4.1. Prevention Treatment
4.2. Acute Treatment
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chawanpaiboon, S.; Vogel, J.P.; Moller, A.-B.; Lumbiganon, P.; Petzold, M.; Hogan, D.; Landoulsi, S.; Jampathong, N.; Kongwattanakul, K.; Laopaiboon, M.; et al. Global, regional, and national estimates of levels of preterm birth in 2014: A systematic review and modelling analysis. Lancet Glob. Health 2019, 7, e37–e46. [Google Scholar] [CrossRef] [PubMed]
- Birth Characteristics in England and Wales: 2021. Released 19 January 2023, ONS Website, Statistical Bulletin. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/livebirths/bulletins/birthcharacteristicsinenglandandwales/2021 (accessed on 1 November 2023).
- Moutquin, J.M. Classification and heterogeneity of preterm birth. BJOG 2003, 110 (Suppl. S20), 30–33. [Google Scholar] [CrossRef] [PubMed]
- Ohuma, E.O.; Moller, A.B.; Bradley, E.; Chakwera, S.; Hussain-Alkhateeb, L.; Lewin, A.; Okwaraji, Y.B.; Mahanani, W.R.; Johansson, E.W.; Lavin, T.; et al. National, regional, and global estimates of preterm birth in 2020, with trends from 2010: A systematic analysis. Lancet 2023, 402, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Vogel, J.P.; Oladapo, O.T.; Manu, A.; Gülmezoglu, A.M.; Bahl, R. New WHO recommendations to improve the outcomes of preterm birth. Lancet Glob. Health 2015, 3, e589–e590. [Google Scholar] [CrossRef] [PubMed]
- Department of Health and Social Care (Ed.) Safer Maternity Care; Department of Health and Social Care: London, UK, 2016. [Google Scholar]
- Department of Health and Social Care (Ed.) Safer Maternity Care—NHS England, Progress Report 2021: Safer Maternity Care; Department of Health and Social Care: London, UK, 2021. [Google Scholar]
- Blencowe, H.; Cousens, S.; Chou, D.; Oestergaard, M.; Say, L.; Moller, A.-B.; Kinney, M.; Lawn, J.; the Born Too Soon Preterm Birth Action Group. Born Too Soon: The global epidemiology of 15 million preterm births. Reprod. Health 2013, 10, S2. [Google Scholar] [CrossRef] [PubMed]
- Brew-Sam, N.; Parkinson, A.; Lueck, C.; Brown, E.; Brown, K.; Bruestle, A.; Chisholm, K.; Collins, S.; Cook, M.; Daskalaki, E.; et al. The current understanding of precision medicine and personalised medicine in selected research disciplines: Study protocol of a systematic concept analysis. BMJ Open 2022, 12, e060326. [Google Scholar] [CrossRef]
- Manuck, T.A.; Rice, M.M.; Bailit, J.L.; Grobman, W.A.; Reddy, U.M.; Wapner, R.J.; Thorp, J.M.; Caritis, S.N.; Prasad, M.; Tita, A.T.; et al. Preterm neonatal morbidity and mortality by gestational age: A contemporary cohort. Am. J. Obs. Gynecol. 2016, 215, 103.e101–103.e114. [Google Scholar] [CrossRef]
- Ward, R.M.; Beachy, J.C. Neonatal complications following preterm birth. BJOG 2003, 110 (Suppl. S20), 8–16. [Google Scholar] [CrossRef]
- Cao, G.; Liu, J.; Liu, M. Global, Regional, and National Incidence and Mortality of Neonatal Preterm Birth, 1990–2019. JAMA Pediatr. 2022, 176, 787–796. [Google Scholar] [CrossRef]
- Kurinczuk, J.J.; Draper, E.S.; Field, D.J.; Bevan, C.; Brocklehurst, P.; Gray, R.; Kenyon, S.; Manktelow, B.N.; Neilson, J.P.; Redshaw, M.; et al. Experiences with maternal and perinatal death reviews in the UK—The MBRRACE-UK programme. BJOG 2014, 121 (Suppl. S4), 41–46. [Google Scholar] [CrossRef]
- Glover Williams, A.; Tuvey, S.; McBain, H.; Menzies, N.; Hedge, S.; Bates, S.; Luyt, K. Perinatal excellence to reduce injury in preterm birth (PERIPrem) through quality improvement. BMJ Open Qual. 2022, 11, e001904. [Google Scholar] [CrossRef] [PubMed]
- Menon, R.; Behnia, F.; Polettini, J.; Richardson, L.S. Novel pathways of inflammation in human fetal membranes associated with preterm birth and preterm pre-labor rupture of the membranes. Semin. Immunopathol. 2020, 42, 431–450. [Google Scholar] [CrossRef] [PubMed]
- Cobo, T.; Vives, I.; Rodríguez-Trujillo, A.; Murillo, C.; Ángeles, M.A.; Bosch, J.; Vergara, A.; Gratacós, E.; Palacio, M. Impact of microbial invasion of amniotic cavity and the type of microorganisms on short-term neonatal outcome in women with preterm labor and intact membranes. Acta Obstet. Gynecol. Scand. 2017, 96, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Diemert, A.; Arck, P.C. Preterm birth: Pathogenesis and clinical consequences revisited. Semin. Immunopathol. 2020, 42, 375–376. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, P.D.; Culhane, J.F.; Rauh, V.; Barve, S.S.; Hogan, V.; Sandman, C.A.; Hobel, C.J.; Chicz-DeMet, A.; Dunkel-Schetter, C.; Garite, T.J.; et al. Stress, infection and preterm birth: A biobehavioural perspective. Paediatr. Perinat. Epidemiol. 2001, 15 (Suppl. S2), 17–29. [Google Scholar] [CrossRef] [PubMed]
- Blois, S.M.; Verlohren, S.; Wu, G.; Clark, G.; Dell, A.; Haslam, S.M.; Barrientos, G. Role of galectin-glycan circuits in reproduction: From healthy pregnancy to preterm birth (PTB). Semin. Immunopathol. 2020, 42, 469–486. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.; Bennett, P.R.; Lee, Y.S.; Kundu, S.; Teoh, T.G.; Adan, M.; Ahmed, S.; Brown, R.G.; David, A.L.; Lewis, H.V.; et al. Microbial-driven preterm labour involves crosstalk between the innate and adaptive immune response. Nat. Commun. 2022, 13, 975. [Google Scholar] [CrossRef] [PubMed]
- Vrachnis, N.; Malamas, F.M.; Sifakis, S.; Tsikouras, P.; Iliodromiti, Z. Immune aspects and myometrial actions of progesterone and CRH in labor. Clin. Dev. Immunol. 2012, 2012, 937618. [Google Scholar] [CrossRef]
- Brown, A.G.; Leite, R.S.; Strauss, J.F., 3rd. Mechanisms underlying “functional” progesterone withdrawal at parturition. Ann. N. Y. Acad. Sci. 2004, 1034, 36–49. [Google Scholar] [CrossRef]
- Nadeem, L.; Shynlova, O.; Matysiak-Zablocki, E.; Mesiano, S.; Dong, X.; Lye, S. Molecular evidence of functional progesterone withdrawal in human myometrium. Nat. Commun. 2016, 7, 11565. [Google Scholar] [CrossRef]
- Shah, N.M.; Imami, N.; Johnson, M.R. Progesterone Modulation of Pregnancy-Related Immune Responses. Front. Immunol. 2018, 9, 1293. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, S.A.; Lavery, J.A.; Ananth, C.V.; Vintzileos, A. Placental implantation abnormalities and risk of preterm delivery: A systematic review and metaanalysis. Am. J. Obstet. Gynecol. 2015, 213, S78–S90. [Google Scholar] [CrossRef] [PubMed]
- Yart, L.; Roset Bahmanyar, E.; Cohen, M.; Martinez de Tejada, B. Role of the Uteroplacental Renin-Angiotensin System in Placental Development and Function, and Its Implication in the Preeclampsia Pathogenesis. Biomedicines 2021, 9, 1332. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Dey, S.K.; Fisher, S.J. Preterm labor: One syndrome, many causes. Science 2014, 345, 760–765. [Google Scholar] [CrossRef]
- Di Renzo, G.C.; Tosto, V.; Giardina, I. The biological basis and prevention of preterm birth. Best Pr. Res. Clin. Obs. Gynaecol. 2018, 52, 13–22. [Google Scholar] [CrossRef]
- Salafia, C.M.; Vogel, C.A.; Vintzileos, A.M.; Bantham, K.F.; Pezzullo, J.; Silberman, L. Placental pathologic findings in preterm birth. Am. J. Obs. Gynecol. 1991, 165, 934–938. [Google Scholar] [CrossRef]
- Min, A.M.; Saito, M.; Simpson, J.A.; Kennedy, S.H.; Nosten, F.H.; McGready, R. Placental histopathology in preterm birth with confirmed maternal infection: A systematic literature review. PLoS ONE 2021, 16, e0255902. [Google Scholar] [CrossRef]
- Adams Waldorf, K.M.; Singh, N.; Mohan, A.R.; Young, R.C.; Ngo, L.; Das, A.; Tsai, J.; Bansal, A.; Paolella, L.; Herbert, B.R.; et al. Uterine overdistention induces preterm labor mediated by inflammation: Observations in pregnant women and nonhuman primates. Am. J. Obs. Gynecol. 2015, 213, 830.e1–830.e19. [Google Scholar] [CrossRef]
- Vidal, M.S., Jr.; Lintao, R.C.V.; Severino, M.E.L.; Tantengco, O.A.G.; Menon, R. Spontaneous preterm birth: Involvement of multiple feto-maternal tissues and organ systems, differing mechanisms, and pathways. Front. Endocrinol. 2022, 13, 1015622. [Google Scholar] [CrossRef]
- Terzidou, V.; Sooranna, S.R.; Kim, L.U.; Thornton, S.; Bennett, P.R.; Johnson, M.R. Mechanical stretch up-regulates the human oxytocin receptor in primary human uterine myocytes. J. Clin. Endocrinol. Metab. 2005, 90, 237–246. [Google Scholar] [CrossRef]
- Ou, C.W.; Orsino, A.; Lye, S.J. Expression of connexin-43 and connexin-26 in the rat myometrium during pregnancy and labor is differentially regulated by mechanical and hormonal signals. Endocrinology 1997, 138, 5398–5407. [Google Scholar] [CrossRef] [PubMed]
- Roman, A.; Suhag, A.; Berghella, V. Overview of Cervical Insufficiency: Diagnosis, Etiologies, and Risk Factors. Clin. Obs. Gynecol. 2016, 59, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Vink, J.; Feltovich, H. Cervical etiology of spontaneous preterm birth. Semin. Fetal. Neonatal. Med. 2016, 21, 106–112. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Progress Report on HIV, Viral Hepatitis and Sexually Transmitted Infections, 2021: Accountability for the Global Health Sector Strategies 2016–2021: Actions for Impact; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Elenga, N.; Djossou FÉ, L.; Nacher, M. Association between maternal human immunodeficiency virus infection and preterm birth: A matched case-control study from a pregnancy outcome registry. Medicine 2021, 100, e22670. [Google Scholar] [CrossRef] [PubMed]
- Albert, A.Y.K.; Elwood, C.; Wagner, E.C.; Pakzad, Z.; Chaworth-Musters, T.; Berg, K.; Van Schalkwyk, J.; Maan, E.J.; Azampanah, A.; McClymont, E.; et al. Investigation of factors associated with spontaneous preterm birth in pregnant women living with HIV. AIDS 2020, 34, 719–727. [Google Scholar] [CrossRef]
- Boyd, M.A.A.; van Bockel, D.; Munier, C.M.L.; Kelleher, A.D. Navigating the complexity of chronic HIV-1 associated immune dysregulation. Curr. Opin. Immunol. 2022, 76, 102186. [Google Scholar] [CrossRef]
- Ikumi, N.M.; Matjila, M. Preterm Birth in Women with HIV: The Role of the Placenta. Front. Glob. Women’s Health 2022, 3, 820759. [Google Scholar] [CrossRef]
- Cocker, A.T.H.; Shah, N.M.; Raj, I.; Dermont, S.; Khan, W.; Mandalia, S.; Imami, N.; Johnson, M.R. Pregnancy Gestation Impacts on HIV-1-Specific Granzyme B Response and Central Memory CD4 T Cells. Front. Immunol. 2020, 11, 153. [Google Scholar] [CrossRef]
- Mesfin, Y.M.; Kibret, K.T.; Taye, A. Is protease inhibitors based antiretroviral therapy during pregnancy associated with an increased risk of preterm birth? Systematic review and a meta-analysis. Reprod. Health 2016, 13, 30. [Google Scholar] [CrossRef]
- Lorenzi, P.; Spicher, V.M.; Laubereau, B.; Hirschel, B.; Kind, C.; Rudin, C.; Irion, O.; Kaiser, L. Antiretroviral therapies in pregnancy: Maternal, fetal and neonatal effects. Swiss HIV Cohort Study, the Swiss Collaborative HIV and Pregnancy Study, and the Swiss Neonatal HIV Study. AIDS 1998, 12, F241–F247. [Google Scholar] [CrossRef]
- Combination antiretroviral therapy and duration of pregnancy. AIDS 2000, 14, 2913–2920. [CrossRef] [PubMed]
- Cowdell, I.; Beck, K.; Portwood, C.; Sexton, H.; Kumarendran, M.; Brandon, Z.; Kirtley, S.; Hemelaar, J. Adverse perinatal outcomes associated with protease inhibitor-based antiretroviral therapy in pregnant women living with HIV: A systematic review and meta-analysis. EClinicalMedicine 2022, 46, 101368. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Shapiro, D.E.; Brogly, S.B.; Livingston, E.G.; Stek, A.M.; Bardeguez, A.D.; Tuomala, R.E. Prenatal protease inhibitor use and risk of preterm birth among HIV-infected women initiating antiretroviral drugs during pregnancy. J. Infect. Dis. 2010, 201, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.; Holland, B.; Bozdogan, U.; Alvarez, J.; Apuzzio, J.; Bardeguez, A. 410: Do protease inhibitors increase preterm births in HIV-infected patients? Am. J. Obstet. Gynecol. 2008, 199, S124. [Google Scholar] [CrossRef]
- Favarato, G.; Townsend, C.L.; Bailey, H.; Peters, H.; Tookey, P.A.; Taylor, G.P.; Thorne, C. Protease inhibitors and preterm delivery: Another piece in the puzzle. AIDS 2018, 32, 243–252. [Google Scholar] [CrossRef]
- Dunk, C.E.; Serghides, L. Protease inhibitor-based antiretroviral therapy in pregnancy: Effects on hormones, placenta, and decidua. Lancet HIV 2022, 9, e120–e129. [Google Scholar] [CrossRef]
- Siou, K.; Walmsley, S.L.; Murphy, K.E.; Raboud, J.; Loutfy, M.; Yudin, M.H.; Silverman, M.; Ladhani, N.N.; Serghides, L. Progesterone supplementation for HIV-positive pregnant women on protease inhibitor-based antiretroviral regimens (the ProSPAR study): A study protocol for a pilot randomized controlled trial. Pilot Feasibility Stud. 2016, 2, 49. [Google Scholar] [CrossRef]
- Papp, E.; Mohammadi, H.; Loutfy, M.R.; Yudin, M.H.; Murphy, K.E.; Walmsley, S.L.; Shah, R.; MacGillivray, J.; Silverman, M.; Serghides, L. HIV Protease Inhibitor Use during Pregnancy Is Associated with Decreased Progesterone Levels, Suggesting a Potential Mechanism Contributing to Fetal Growth Restriction. J. Infect. Dis. 2015, 211, 10–18. [Google Scholar] [CrossRef]
- Mohammadi, H.; Papp, E.; Cahill, L.; Rennie, M.; Banko, N.; Pinnaduwage, L.; Lee, J.; Kibschull, M.; Dunk, C.; Sled, J.G.; et al. HIV antiretroviral exposure in pregnancy induces detrimental placenta vascular changes that are rescued by progesterone supplementation. Sci. Rep. 2018, 8, 6552. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Preterm Labour and Birth: Guidance; NICE: London, UK, 2022. [Google Scholar]
- Darmstadt, G.L.; Al Jaifi, N.H.; Arif, S.; Bahl, R.; Blennow, M.; Cavallera, V.; Chou, D.; Chou, R.; Comrie-Thomson, L.; Edmond, K.; et al. New World Health Organization recommendations for care of preterm or low birth weight infants: Health policy. eClinicalMedicine 2023, 63, 102155. [Google Scholar] [CrossRef]
- Barton, J.R.; Woelkers, D.A.; Newman, R.B.; Combs, C.A.; How, H.Y.; Boggess, K.A.; Martin, J.N., Jr.; Kupfer, K.; Sibai, B.M. Placental growth factor predicts time to delivery in women with signs or symptoms of early preterm preeclampsia: A prospective multicenter study. Am. J. Obs. Gynecol. 2020, 222, 259.e1–259.e11. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, F.P.; Gill, C.; Seed, P.T.; Bramham, K.; Chappell, L.C.; Shennan, A.H. Comparison of three commercially available placental growth factor-based tests in women with suspected preterm pre-eclampsia: The COMPARE study. Ultrasound Obs. Gynecol. 2019, 53, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.P.H.; Feng, Q.; Chaemsaithong, P.; Sahota, D.S.; Lau, Y.Y.; Yeung, Y.K.; Yim, L.W.; Chung, J.P.W.; Poon, L.C. Prediction of spontaneous preterm birth and preterm prelabor rupture of membranes using maternal factors, obstetric history and biomarkers of placental function at 11–13 weeks. Ultrasound Obs. Gynecol. 2022, 60, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, C.M.; Sotiriadis, A.; Odibo, A.; Khalil, A.; D’Antonio, F.; Feltovich, H.; Salomon, L.J.; Sheehan, P.; Napolitano, R.; Berghella, V.; et al. ISUOG Practice Guidelines: Role of ultrasound in the prediction of spontaneous preterm birth. Ultrasound Obs. Gynecol. 2022, 60, 435–456. [Google Scholar] [CrossRef] [PubMed]
- Honest, H.; Bachmann, L.M.; Gupta, J.K.; Kleijnen, J.; Khan, K.S. Accuracy of cervicovaginal fetal fibronectin test in predicting risk of spontaneous preterm birth: Systematic review. BMJ 2002, 325, 301. [Google Scholar] [CrossRef] [PubMed]
- Carlisle, N.; Watson, H.A.; Seed, P.T.; Carter, J.; Kuhrt, K.; Tribe, R.M.; Shennan, A.H. Impact of a medical mobile phone app (QUiPP) for predicting preterm birth on the anxiety and decisional conflicts faced by women in threatened preterm labour. Midwifery 2021, 92, 102864. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.; Anumba, D.; Brigante, L.; Burden, C.; Draycott, T.; Gillespie, S.; Harlev-Lam, B.; Judge, A.; Lenguerrand, E.; Sheehan, E.; et al. The Tommy’s Clinical Decision Tool, a device for reducing the clinical impact of placental dysfunction and preterm birth: Protocol for a mixed- methods early implementation evaluation study. BMC Pregnancy Childbirth 2022, 22, 639. [Google Scholar] [CrossRef]
- Watson, H.A.; Seed, P.T.; Carter, J.; Hezelgrave, N.L.; Kuhrt, K.; Tribe, R.M.; Shennan, A.H. Development and validation of predictive models for QUiPP App v.2: Tool for predicting preterm birth in asymptomatic high-risk women. Ultrasound Obs. Gynecol. 2020, 55, 348–356. [Google Scholar] [CrossRef]
- Watson, H.A.; Carlisle, N.; Seed, P.T.; Carter, J.; Kuhrt, K.; Tribe, R.M.; Shennan, A.H. Evaluating the use of the QUiPP app and its impact on the management of threatened preterm labour: A cluster randomised trial. PLoS Med. 2021, 18, e1003689. [Google Scholar] [CrossRef]
- Watson, H.A.; Carter, J.; Seed, P.T.; Tribe, R.M.; Shennan, A.H. The QUiPP App: A safe alternative to a treat—All strategy for threatened preterm labor. Ultrasound Obs. Gynecol. 2017, 50, 342–346. [Google Scholar] [CrossRef]
- Ashley, E.A. Towards precision medicine. Nat. Rev. Genet. 2016, 17, 507–522. [Google Scholar] [CrossRef] [PubMed]
- Xiao, P.-L.; Zhou, Y.-B.; Chen, Y.; Yang, M.-X.; Song, X.-X.; Shi, Y.; Jiang, Q.-W. Association between maternal HIV infection and low birth weight and prematurity: A meta-analysis of cohort studies. BMC Pregnancy Childbirth 2015, 15, 246. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Liu, B.; Yang, W.; Wu, Y.; Wang, B.; Santillan, M.K.; Ryckman, K.; Santillan, D.A.; Bao, W. Association of Maternal Sexually Transmitted Infections with Risk of Preterm Birth in the United States. JAMA Netw. Open 2021, 4, e2133413. [Google Scholar] [CrossRef] [PubMed]
- Screening for Hepatitis B, HIV and Syphilis. Available online: https://www.nhs.uk/pregnancy/your-pregnancy-care/screening-for-hepatitis-b-hiv-and-syphilis/ (accessed on 30 August 2023).
- Public Health England (Ed.) Infectious Diseases in Pregnancy Screening (IDPS): Programme Overview; PHE: London, UK, 2021. [Google Scholar]
- National Institute for Health and Care Excellence. When Should I Screen for Chlamydia in Primary Care; NICE: London, UK, 2022. [Google Scholar]
- Adhikari, E.H.; Roberts, S. Sexually Transmitted Infections and Preterm Birth-Attempting to Pin Down Targets for Intervention from Population-Level Observational Data. JAMA Netw. Open 2021, 4, e2134459. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.M.; Xu, J.X.; Jiang, L.X.; Deng, L.R.; Gu, Z.M.; Xie, X.Y.; Ji, H.C.; Wang, W.H.; Li, L.M.; Tian, C.N.; et al. Design and Evaluation of a Novel Multiplex Real-Time PCR Melting Curve Assay for the Simultaneous Detection of Nine Sexually Transmitted Disease Pathogens in Genitourinary Secretions. Front. Cell. Infect. Microbiol. 2019, 9, 382. [Google Scholar] [CrossRef] [PubMed]
- Hyman, R.W.; Fukushima, M.; Jiang, H.; Fung, E.; Rand, L.; Johnson, B.; Vo, K.C.; Caughey, A.B.; Hilton, J.F.; Davis, R.W.; et al. Diversity of the vaginal microbiome correlates with preterm birth. Reprod. Sci. 2014, 21, 32–40. [Google Scholar] [CrossRef]
- DiGiulio, D.B.; Callahan, B.J.; McMurdie, P.J.; Costello, E.K.; Lyell, D.J.; Robaczewska, A.; Sun, C.L.; Goltsman, D.S.; Wong, R.J.; Shaw, G.; et al. Temporal and spatial variation of the human microbiota during pregnancy. Proc. Natl. Acad. Sci. USA 2015, 112, 11060–11065. [Google Scholar] [CrossRef]
- Gudnadottir, U.; Debelius, J.W.; Du, J.; Hugerth, L.W.; Danielsson, H.; Schuppe-Koistinen, I.; Fransson, E.; Brusselaers, N. The vaginal microbiome and the risk of preterm birth: A systematic review and network meta-analysis. Sci. Rep. 2022, 12, 7926. [Google Scholar] [CrossRef]
- Fettweis, J.M.; Serrano, M.G.; Brooks, J.P.; Edwards, D.J.; Girerd, P.H.; Parikh, H.I.; Huang, B.; Arodz, T.J.; Edupuganti, L.; Glascock, A.L.; et al. The vaginal microbiome and preterm birth. Nat. Med. 2019, 25, 1012–1021. [Google Scholar] [CrossRef]
- Miller, E.A.; Beasley, D.E.; Dunn, R.R.; Archie, E.A. Lactobacilli Dominance and Vaginal pH: Why Is the Human Vaginal Microbiome Unique? Front. Microbiol. 2016, 7, 1936. [Google Scholar] [CrossRef]
- Walther-Antonio, M.R.; Jeraldo, P.; Berg Miller, M.E.; Yeoman, C.J.; Nelson, K.E.; Wilson, B.A.; White, B.A.; Chia, N.; Creedon, D.J. Pregnancy’s stronghold on the vaginal microbiome. PLoS ONE 2014, 9, e98514. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaei, N.; Eren, A.M.; Barreiro, L.B.; Yotova, V.; Dumaine, A.; Allard, C.; Fraser, W.D. Vaginal microbiome in early pregnancy and subsequent risk of spontaneous preterm birth: A case-control study. BJOG 2019, 126, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Z.; Ma, X.; Du, L.; Jia, Z.; Cui, X.; Yu, L.; Yang, J.; Xiao, L.; Zhang, B.; et al. Translocation of vaginal microbiota is involved in impairment and protection of uterine health. Nat. Commun. 2021, 12, 4191. [Google Scholar] [CrossRef] [PubMed]
- Stinson, L.; Hallingström, M.; Barman, M.; Viklund, F.; Keelan, J.; Kacerovsky, M.; Payne, M.; Jacobsson, B. Comparison of Bacterial DNA Profiles in Mid-Trimester Amniotic Fluid Samples from Preterm and Term Deliveries. Front. Microbiol. 2020, 11, 415. [Google Scholar] [CrossRef] [PubMed]
- Combs, C.A.; Gravett, M.; Garite, T.J.; Hickok, D.E.; Lapidus, J.; Porreco, R.; Rael, J.; Grove, T.; Morgan, T.K.; Clewell, W.; et al. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am. J. Obstet. Gynecol. 2014, 210, 125.e1–125.e15. [Google Scholar] [CrossRef]
- Urushiyama, D.; Suda, W.; Ohnishi, E.; Araki, R.; Kiyoshima, C.; Kurakazu, M.; Sanui, A.; Yotsumoto, F.; Murata, M.; Nabeshima, K.; et al. Microbiome profile of the amniotic fluid as a predictive biomarker of perinatal outcome. Sci. Rep. 2017, 7, 12171. [Google Scholar] [CrossRef]
- Kenyon, S.L.; Taylor, D.J.; Tarnow-Mordi, W. Broad-spectrum antibiotics for preterm, prelabour rupture of fetal membranes: The ORACLE I randomised trial. ORACLE Collaborative Group. Lancet 2001, 357, 979–988. [Google Scholar] [CrossRef]
- Kenyon, S.L.; Taylor, D.J.; Tarnow-Mordi, W. Broad-spectrum antibiotics for spontaneous preterm labour: The ORACLE II randomised trial. Lancet 2001, 357, 989–994. [Google Scholar] [CrossRef]
- Kenyon, S.; Taylor, D.J.; Tarnow-Mordi, W.O. ORACLE—Antibiotics for preterm prelabour rupture of the membranes: Short-term and long-term outcomes. Acta Paediatr. Suppl. 2002, 91, 12–15. [Google Scholar] [CrossRef]
- Russell, A.R.; Steer, P.J. Antibiotics in preterm labour—The ORACLE speaks. Lancet 2008, 372, 1276–1278. [Google Scholar] [CrossRef]
- Mendz, G.L.; Kaakoush, N.O.; Quinlivan, J.A. Bacterial aetiological agents of intra-amniotic infections and preterm birth in pregnant women. Front. Cell. Infect. Microbiol. 2013, 3, 58. [Google Scholar] [CrossRef] [PubMed]
- Motomura, K.; Romero, R.; Xu, Y.; Theis, K.R.; Galaz, J.; Winters, A.D.; Slutsky, R.; Garcia-Flores, V.; Zou, C.; Levenson, D.; et al. Intra-Amniotic Infection with Ureaplasma parvum Causes Preterm Birth and Neonatal Mortality That Are Prevented by Treatment with Clarithromycin. mBio 2020, 11, e00797-20. [Google Scholar] [CrossRef] [PubMed]
- Kacerovsky, M.; Romero, R.; Stepan, M.; Stranik, J.; Maly, J.; Pliskova, L.; Bolehovska, R.; Palicka, V.; Zemlickova, H.; Hornychova, H.; et al. Antibiotic administration reduces the rate of intraamniotic inflammation in preterm prelabor rupture of the membranes. Am. J. Obstet. Gynecol. 2020, 223, 114.e111–114.e120. [Google Scholar] [CrossRef] [PubMed]
- Galaz, J.; Romero, R.; Arenas-Hernandez, M.; Farias-Jofre, M.; Motomura, K.; Liu, Z.; Kawahara, N.; Demery-Poulos, C.; Liu, T.N.; Padron, J.; et al. Clarithromycin prevents preterm birth and neonatal mortality by dampening alarmin-induced maternal-fetal inflammation in mice. BMC Pregnancy Childbirth 2022, 22, 503. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Luo, F.; Hu, W.; Han, Y.; Wang, Y.; Zheng, H.; Guo, X.; Qin, J. Bacterial Communities in the Womb during Healthy Pregnancy. Front. Microbiol. 2018, 9, 2163. [Google Scholar] [CrossRef] [PubMed]
- Stinson, L.F.; Boyce, M.C.; Payne, M.S.; Keelan, J.A. The Not-so-Sterile Womb: Evidence That the Human Fetus Is Exposed to Bacteria Prior to Birth. Front. Microbiol. 2019, 10, 1124. [Google Scholar] [CrossRef]
- Pustotina, O. Effects of antibiotic therapy in women with the amniotic fluid “sludge” at 15-24 weeks of gestation on pregnancy outcomes. J. Matern. Fetal Neonatal Med. 2020, 33, 3016–3027. [Google Scholar] [CrossRef]
- Tsunoda, Y.; Fukami, T.; Yoneyama, K.; Kawabata, I.; Takeshita, T. The presence of amniotic fluid sludge in pregnant women with a short cervix: An independent risk of preterm delivery. J. Matern. Fetal Neonatal Med. 2020, 33, 920–923. [Google Scholar] [CrossRef]
- Yoo, I.Y.; Huh, K.; Shim, H.J.; Yun, S.A.; Chung, Y.N.; Kang, O.K.; Huh, H.J.; Lee, N.Y. Evaluation of the BioFire FilmArray Pneumonia Panel for rapid detection of respiratory bacterial pathogens and antibiotic resistance genes in sputum and endotracheal aspirate specimens. Int. J. Infect. Dis. 2020, 95, 326–331. [Google Scholar] [CrossRef]
- Peri, A.M.; Bauer, M.J.; Bergh, H.; Butkiewicz, D.; Paterson, D.L.; Harris, P.N. Performance of the BioFire Blood Culture Identification 2 panel for the diagnosis of bloodstream infections. Heliyon 2022, 8, e09983. [Google Scholar] [CrossRef]
- Trotter, A.J.; Aydin, A.; Strinden, M.J.; O’Grady, J. Recent and emerging technologies for the rapid diagnosis of infection and antimicrobial resistance. Curr. Opin. Microbiol. 2019, 51, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Taxt, A.M.; Avershina, E.; Frye, S.A.; Naseer, U.; Ahmad, R. Rapid identification of pathogens, antibiotic resistance genes and plasmids in blood cultures by nanopore sequencing. Sci. Rep. 2020, 10, 7622. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.P.; Burnham, P.; Lee, J.R.; Cheng, M.P.; Suthanthiran, M.; Dadhania, D.; De Vlaminck, I. A cell-free DNA metagenomic sequencing assay that integrates the host injury response to infection. Proc. Natl. Acad. Sci. USA 2019, 116, 18738–18744. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Guo, W.; Shen, H.; Guo, J.; Wen, D.; Yu, Y.; Wu, W. Plasma Microbial Cell-Free DNA Sequencing Technology for the Diagnosis of Sepsis in the ICU. Front. Mol. Biosci. 2021, 8, 659390. [Google Scholar] [CrossRef] [PubMed]
- Witt, R.G.; Blair, L.; Frascoli, M.; Rosen, M.J.; Nguyen, Q.H.; Bercovici, S.; Zompi, S.; Romero, R.; Mackenzie, T.C. Detection of microbial cell-free DNA in maternal and umbilical cord plasma in patients with chorioamnionitis using next generation sequencing. PLoS ONE 2020, 15, e0231239. [Google Scholar] [CrossRef]
- Frascoli, M.; Coniglio, L.; Witt, R.; Jeanty, C.; Fleck-Derderian, S.; Myers, D.E.; Lee, T.H.; Keating, S.; Busch, M.P.; Norris, P.J.; et al. Alloreactive fetal T cells promote uterine contractility in preterm labor via IFN-gamma and TNF-alpha. Sci. Transl. Med. 2018, 10, eaan2263. [Google Scholar] [CrossRef]
- Shah, N.M.; Edey, L.F.; Imami, N.; Johnson, M.R. Human labour is associated with altered regulatory T cell function and maternal immune activation. Clin. Exp. Immunol. 2020, 199, 182–200. [Google Scholar] [CrossRef]
- Okeke, E.B.; Okwor, I.; Mou, Z.; Jia, P.; Uzonna, J.E. CD4+CD25+ regulatory T cells attenuate lipopolysaccharide-induced systemic inflammatory responses and promotes survival in murine Escherichia coli infection. Shock 2013, 40, 65–73. [Google Scholar] [CrossRef]
- Denney, J.M.; Nelson, E.; Wadhwa, P.; Waters, T.; Mathew, L.; Goldenberg, R.L.; Culhane, J.F. Cytokine profiling: Variation in immune modulation with preterm birth vs. uncomplicated term birth identifies pivotal signals in pathogenesis of preterm birth. J. Perinat. Med. 2021, 49, 299–309. [Google Scholar] [CrossRef]
- Deng, W.; Yuan, J.; Cha, J.; Sun, X.; Bartos, A.; Yagita, H.; Hirota, Y.; Dey, S.K. Endothelial Cells in the Decidual Bed Are Potential Therapeutic Targets for Preterm Birth Prevention. Cell Rep. 2019, 27, 1755–1768.e1754. [Google Scholar] [CrossRef]
- Xiao, H.; Siddiqui, J.; Remick, D.G. Mechanisms of mortality in early and late sepsis. Infect. Immun. 2006, 74, 5227–5235. [Google Scholar] [CrossRef] [PubMed]
- Kacerovsky, M.; Musilova, I.; Hornychova, H.; Kutova, R.; Pliskova, L.; Kostal, M.; Jacobsson, B. Bedside assessment of amniotic fluid interleukin-6 in preterm prelabor rupture of membranes. Am. J. Obs. Gynecol. 2014, 211, 385.e1–385.e9. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Park, K.H.; Kim, Y.M.; Kook, S.Y.; Jeon, S.J.; Yoo, H.-N. Plasma inflammatory and immune proteins as predictors of intra-amniotic infection and spontaneous preterm delivery in women with preterm labor: A retrospective study. BMC Pregnancy Childbirth 2018, 18, 146. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, M.; Boucard, C.; Di Nicuolo, F.; Haesler, V.; Castellani, R.; Pontecorvi, A.; Scambia, G.; Granieri, C.; Barnea, E.R.; Surbek, D.; et al. Synthetic PreImplantation Factor (sPIF) reduces inflammation and prevents preterm birth. PLoS ONE 2020, 15, e0232493. [Google Scholar] [CrossRef] [PubMed]
- Chaemsaithong, P.; Romero, R.; Korzeniewski, S.J.; Martinez-Varea, A.; Dong, Z.; Yoon, B.H.; Hassan, S.S.; Chaiworapongsa, T.; Yeo, L. A point of care test for interleukin-6 in amniotic fluid in preterm prelabor rupture of membranes: A step toward the early treatment of acute intra-amniotic inflammation/infection. J. Matern. Fetal Neonatal Med. 2016, 29, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Tian, J.; Liu, C.; Long, Y.; Cao, D.; Wei, L.; Zhu, X.; Tang, R.; Liu, W.; Zeng, D.; et al. Elevated C-reactive protein and complement C3 levels are associated with preterm birth: A nested case-control study in Chinese women. BMC Pregnancy Childbirth 2020, 20, 131. [Google Scholar] [CrossRef] [PubMed]
- Cetin, O.; Karaman, E.; Alisik, M.; Erel, O.; Kolusari, A.; Sahin, H.G. The evaluation of maternal systemic thiol/disulphide homeostasis for the short-term prediction of preterm birth in women with threatened preterm labour: A pilot study. J. Obstet. Gynaecol. J. Inst. Obstet. Gynaecol. 2022, 42, 1972–1977. [Google Scholar] [CrossRef] [PubMed]
- Jaju, P.B. Effectiveness and Safety of Isoxsuprine Hydrochloride as Tocolytic Agent in Arresting Active/Threatened Preterm Labor and Its Role in Maintenance Tocolysis: A Prospective, Open-Label Study. Am. J. Perinatol. 2021, 38, 291–295. [Google Scholar] [CrossRef]
- Munoz-Perez, V.M.; Ortiz, M.I.; Salas-Casa, A.; Perez-Guerrero, J.; Castillo-Pacheco, N.; Barragan-Ramirez, G.; Hernandes-Alejandro, M. In vitro effects of citral on the human myometrium: Potential adjunct therapy to prevent preterm births. Birth Defects Res. 2021, 113, 613–622. [Google Scholar] [CrossRef]
- Khanam, R.; Fleischer, T.C.; Boghossian, N.S.; Nisar, I.; Dhingra, U.; Rahman, S.; Fox, A.C.; Ilyas, M.; Dutta, A.; Naher, N.; et al. Performance of a validated spontaneous preterm delivery predictor in South Asian and Sub-Saharan African women: A nested case control study. J. Matern. Fetal Neonatal Med. 2022, 35, 8878–8886. [Google Scholar] [CrossRef]
- Peltier, M.R.; Fassett, M.J.; Arita, Y.; Chiu, V.Y.; Shi, J.M.; Takhar, H.S.; Mahfuz, A.; Garcia, G.S.; Menon, R.; Getahun, D. Women with high plasma levels of PBDE-47 are at increased risk of preterm birth. J. Perinat. Med. 2021, 49, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Chen, Y.; Wan, S.; Zhou, T.; Chang, Y.S.; Zhao, X.; Hua, X. Predictors for histological chorioamnionitis among women with preterm prelabour rupture of membranes after dexamethasone treatment: A retrospective study. BJOG 2023, 130, 1072–1079. [Google Scholar] [CrossRef] [PubMed]
- Splichal, I.; Splichalova, A. High Mobility Group Box 1 in Pig Amniotic Membrane Experimentally Infected with E. coli O55. Biomolecules 2021, 11, 1146. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Lopez, N.; Romero, R.; Plazyo, O.; Panaitescu, B.; Furcron, A.E.; Miller, D.; Roumayah, T.; Flom, E.; Hassan, S.S. Intra-Amniotic Administration of HMGB1 Induces Spontaneous Preterm Labor and Birth. Am. J. Reprod. Immunol. 2016, 75, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Buhimschi, C.S.; Baumbusch, M.A.; Dulay, A.T.; Oliver, E.A.; Lee, S.; Zhao, G.; Bhandari, V.; Ehrenkranz, R.A.; Weiner, C.P.; Madri, J.A.; et al. Characterization of RAGE, HMGB1, and S100beta in inflammation-induced preterm birth and fetal tissue injury. Am. J. Pathol. 2009, 175, 958–975. [Google Scholar] [CrossRef] [PubMed]
- Abuelghar, W.M.; Ellaithy, M.I.; Swidan, K.H.; Allam, I.S.; Haggag, H.M. Prediction of spontaneous preterm birth: Salivary progesterone assay and transvaginal cervical length assessment after 24 weeks of gestation, another critical window of opportunity. J. Matern. Fetal Neonatal Med. 2019, 32, 3847–3858. [Google Scholar] [CrossRef] [PubMed]
- Vogel, I.; Glavind-Kristensen, M.; Thorsen, P.; Armbruster, F.P.; Uldbjerg, N. S-relaxin as a predictor of preterm delivery in women with symptoms of preterm labour. BJOG 2002, 109, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.; Goldsmith, L.T. Mechanisms of relaxin-mediated premature birth. Ann. N. Y. Acad. Sci. 2005, 1041, 345–350. [Google Scholar] [CrossRef]
- Figueiredo, K.A.; Mui, A.L.; Nelson, C.C.; Cox, M.E. Relaxin stimulates leukocyte adhesion and migration through a relaxin receptor LGR7-dependent mechanism. J. Biol. Chem. 2006, 281, 3030–3039. [Google Scholar] [CrossRef]
- Goldsmith, L.T.; Weiss, G.; Palejwala, S.; Plant, T.M.; Wojtczuk, A.; Lambert, W.C.; Ammur, N.; Heller, D.; Skurnick, J.H.; Edwards, D.; et al. Relaxin regulation of endometrial structure and function in the rhesus monkey. Proc. Natl. Acad. Sci. USA 2004, 101, 4685–4689. [Google Scholar] [CrossRef]
- Goldsmith, L.T.; Weiss, G. Relaxin in human pregnancy. Ann. N. Y. Acad. Sci. 2009, 1160, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Bullerwell, C.E.; Robichaud, P.P.; Deprez, P.M.L.; Joy, A.P.; Wajnberg, G.; D’Souza, D.; Chacko, S.; Fournier, S.; Crapoulet, N.; Barnett, D.A.; et al. EBF1 drives hallmark B cell gene expression by enabling the interaction of PAX5 with the MLL H3K4 methyltransferase complex. Sci. Rep. 2021, 11, 1537. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Holzman, C.; Heng, Y.J.; Kibschull, M.; Lye, S.J.; Vazquez, A. EBF1 Gene mRNA Levels in Maternal Blood and Spontaneous Preterm Birth. Reprod. Sci. 2020, 27, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Holzman, C.; Heng, Y.J.; Kibschull, M.; Lye, S.J. Maternal blood EBF1-based microRNA transcripts as biomarkers for detecting risk of spontaneous preterm birth: A nested case-control study. J. Matern. Fetal Neonatal Med. 2022, 35, 1239–1247. [Google Scholar] [CrossRef]
- Weiner, C.P.; Dong, Y.; Zhou, H.; Cuckle, H.; Ramsey, R.; Egerman, R.; Buhimschi, I.; Buhimschi, C. Early pregnancy prediction of spontaneous preterm birth before 32 completed weeks of pregnancy using plasma RNA: Transcriptome discovery and initial validation of an RNA panel of markers. BJOG 2021, 128, 1870–1880. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.K.; Care, A.; Goodfellow, L.; Alfirevic, Z.; Muller-Myhsok, B.; Alfirevic, A. Genome and transcriptome profiling of spontaneous preterm birth phenotypes. Sci. Rep. 2022, 12, 1003. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, E.; Thiruvengadam, R.; Ayushi; Das, C.; Bal, V.; Bhatnagar, S.; Das, B.; Desiraju, B.K.; Kshetrapal, P.; Misra, S.; et al. Genetic variants associated with spontaneous preterm birth in women from India: A prospective cohort study. Lancet Reg. Health Southeast Asia 2023, 14, 100190. [Google Scholar] [CrossRef]
- Rocha, F.G.; Slavin, T.P.; Li, D.; Tiirikainen, M.I.; Bryant-Greenwood, G.D. Genetic associations of relaxin: Preterm birth and premature rupture of fetal membranes. Am. J. Obs. Gynecol. 2013, 209, 258.e1–258.e8. [Google Scholar] [CrossRef]
- Wang, C.; White, M.; Penova-Veselinovic, B.; Ang, Q.; Williams, S.; Pennell, C. Preterm Birth Genome Project (PGP) Phase Iii Development of a Bespoke Ptb Array; Sage Publications: Thousand Oaks, CA, USA, 2018; Volume 54. [Google Scholar]
- Gupta, J.K.; Care, A.; Goodfellow, L.; Alfirevic, Z.; Lian, L.Y.; Muller-Myhsok, B.; Alfirevic, A.; Phelan, M.M. Metabolic profiling of maternal serum of women at high-risk of spontaneous preterm birth using NMR and MGWAS approach. Biosci. Rep. 2021, 41, BSR20210759. [Google Scholar] [CrossRef]
- Lee, N.; Yoon, H.Y.; Park, J.Y.; Kim, Y.J.; Hwang, H.S.; Yee, J.; Gwak, H.S. Association between ADCY9 Gene Polymorphisms and Ritodrine Treatment Outcomes in Patients with Preterm Labor. Pharmaceutics 2021, 13, 1653. [Google Scholar] [CrossRef]
- Li, J.; Oehlert, J.; Snyder, M.; Stevenson, D.K.; Shaw, G.M. Fetal de novo mutations and preterm birth. PLoS Genet. 2017, 13, e1006689. [Google Scholar] [CrossRef] [PubMed]
- Chien, C.W.; Lo, Y.S.; Wu, H.Y.; Hsuan, Y.; Lin, C.K.; Chen, Y.J.; Lin, W.; Han, C.L. Transcriptomic and Proteomic Profiling of Human Mesenchymal Stem Cell Derived from Umbilical Cord in the Study of Preterm Birth. Proteom. Clin. Appl. 2020, 14, e1900024. [Google Scholar] [CrossRef] [PubMed]
- Bhavnani, S.K.; Dang, B.; Kilaru, V.; Caro, M.; Visweswaran, S.; Saade, G.; Smith, A.K.; Menon, R. Methylation differences reveal heterogeneity in preterm pathophysiology: Results from bipartite network analyses. J. Perinat. Med. 2018, 46, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Ran, Y.; He, J.; Peng, W.; Liu, Z.; Mei, Y.; Zhou, Y.; Yin, N.; Qi, H. Development and validation of a transcriptomic signature-based model as the predictive, preventive, and personalized medical strategy for preterm birth within 7 days in threatened preterm labor women. EPMA J. 2022, 13, 87–106. [Google Scholar] [CrossRef] [PubMed]
- Aslanidi, O.; Atia, J.; Benson, A.P.; van den Berg, H.A.; Blanks, A.M.; Choi, C.; Gilbert, S.H.; Goryanin, I.; Hayes-Gill, B.R.; Holden, A.V.; et al. Towards a computational reconstruction of the electrodynamics of premature and full term human labour. Prog. Biophys. Mol. Biol. 2011, 107, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.C.; Choi, C.Y.; Kharche, S.; Holden, A.V.; Zhang, H.; Taggart, M.J. A computational model of the ionic currents, Ca2+ dynamics and action potentials underlying contraction of isolated uterine smooth muscle. PLoS ONE 2011, 6, e18685. [Google Scholar] [CrossRef]
- Bastos, L.F.; Lobo, M.F.; van Meurs, W.L.; Ayres-de-Campos, D. An intrauterine pressure generator for educational simulation of labour and delivery. Med. Eng. Phys. 2010, 32, 740–745. [Google Scholar] [CrossRef]
- Lobo, M.F.; Bastos, L.F.; van Meurs, W.L.; Ayres-de-Campos, D. A model for educational simulation of the effect of oxytocin on uterine contractions. Med. Eng. Phys. 2013, 35, 524–531. [Google Scholar] [CrossRef]
- Goldsztejn, U.; Nehorai, A. A myofibre model for the study of uterine excitation-contraction dynamics. Sci. Rep. 2020, 10, 16221. [Google Scholar] [CrossRef]
- Lamb, J.; Crawford, E.D.; Peck, D.; Modell, J.W.; Blat, I.C.; Wrobel, M.J.; Lerner, J.; Brunet, J.-P.; Subramanian, A.; Ross, K.N.; et al. The Connectivity Map: Using Gene-Expression Signatures to Connect Small Molecules, Genes, and Disease. Science 2006, 313, 1929–1935. [Google Scholar] [CrossRef]
- Le, B.L.; Iwatani, S.; Wong, R.J.; Stevenson, D.K.; Sirota, M. Computational discovery of therapeutic candidates for preventing preterm birth. JCI Insight 2020, 5, e133761. [Google Scholar] [CrossRef] [PubMed]
- Abraham, A.; Le, B.; Kosti, I.; Straub, P.; Velez-Edwards, D.R.; Davis, L.K.; Newton, J.M.; Muglia, L.J.; Rokas, A.; Bejan, C.A.; et al. Dense phenotyping from electronic health records enables machine learning-based prediction of preterm birth. BMC Med. 2022, 20, 333. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, Y.; Zhang, Y.; Zhang, Y.; Wu, W.; Fan, J. Identifying Non-Linear Association between Maternal Free Thyroxine and Risk of Preterm Delivery by a Machine Learning Model. Front. Endocrinol. 2022, 13, 817595. [Google Scholar] [CrossRef] [PubMed]
- Maner, W.L.; Garfield, R.E. Identification of human term and preterm labor using artificial neural networks on uterine electromyography data. Ann. Biomed. Eng. 2007, 35, 465–473. [Google Scholar] [CrossRef]
- Most, O.; Langer, O.; Kerner, R.; David, G.B.; Calderon, I. Can myometrial electrical activity identify patients in preterm labor? Am. J. Obs. Gynecol. 2008, 199, 378.e1–378.e6. [Google Scholar] [CrossRef]
- Chen, L.; Xu, H. Deep neural network for semi-automatic classification of term and preterm uterine recordings. Artif. Intell. Med. 2020, 105, 101861. [Google Scholar] [CrossRef]
- Anumba, D.O.C.; Stern, V.; Healey, J.T.; Dixon, S.; Brown, B.H. Value of cervical electrical impedance spectroscopy to predict spontaneous preterm delivery in asymptomatic women: The ECCLIPPx prospective cohort study. Ultrasound Obs. Gynecol. 2021, 58, 293–302. [Google Scholar] [CrossRef]
- Tomialowicz, M.; Zimmer, M.; Fuchs, T.; Matonia, A. The assessment of selected parameters of bioelectric and mechanical activity of the uterus during pharmacologic treatment of threatening preterm delivery. Ginekol. Pol. 2021, 92, 183–187. [Google Scholar] [CrossRef]
- Reoma, J.L.; Rojas, A.; Kim, A.C.; Khouri, J.S.; Boothman, E.; Brown, K.; Grotberg, J.; Cook, K.E.; Bartlett, R.H.; Hirschl, R.B.; et al. Development of an artificial placenta I: Pumpless arterio-venous extracorporeal life support in a neonatal sheep model. J. Pediatr. Surg. 2009, 44, 53–59. [Google Scholar] [CrossRef]
- Miura, Y.; Matsuda, T.; Usuda, H.; Watanabe, S.; Kitanishi, R.; Saito, M.; Hanita, T.; Kobayashi, Y. A Parallelized Pumpless Artificial Placenta System Significantly Prolonged Survival Time in a Preterm Lamb Model. Artif. Organs 2016, 40, E61–E68. [Google Scholar] [CrossRef]
- Spencer, B.L.; Mychaliska, G.B. Milestones for clinical translation of the artificial placenta. Semin. Fetal Neonatal Med. 2022, 27, 101408. [Google Scholar] [CrossRef] [PubMed]
- Olbrich, P.; Pavón, A.; Rosso, M.L.; Molinos, A.; de Felipe, B.; Sanchez, B.; Praena-Fernández, J.M.; Jimenez, F.; Obando, I.; Neth, O. Association of human beta-defensin-2 serum levels and sepsis in preterm neonates. Pediatr. Crit. Care Med. 2013, 14, 796–800. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, R.L.; Andrews, W.W.; Mercer, B.M.; Moawad, A.H.; Meis, P.J.; Iams, J.D.; Das, A.; Caritis, S.N.; Roberts, J.M.; Miodovnik, M.; et al. The preterm prediction study: Granulocyte colony-stimulating factor and spontaneous preterm birth. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am. J. Obs. Gynecol. 2000, 182, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, R.L.; Iams, J.D.; Mercer, B.M.; Meis, P.J.; Moawad, A.; Das, A.; Miodovnik, M.; Vandorsten, P.J.; Caritis, S.N.; Thurnau, G.; et al. The Preterm Prediction Study: Toward a multiple-marker test for spontaneous preterm birth. Am. J. Obs. Gynecol. 2001, 185, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, N.; Fouzas, S.; Kritikou, D.; Dimitriou, G.; Chrysis, D.; Karatza, A.A. Preterm newborns exposed to early-onset preeclampsia have altered postnatal Tumor Necrosis Factor-related apoptosis-inducing ligand trends versus controls. Pediatr. Res. 2023, 93, 1955–1958. [Google Scholar] [CrossRef]
- Huang, L.; Hou, Q.; Huang, Y.; Ye, J.; Huang, S.; Tian, J.; Tang, R.; Liu, C.; Long, Y.; Qin, X.; et al. Serum multiple cytokines for the prediction of spontaneous preterm birth in asymptomatic women: A nested case-control study. Cytokine 2019, 117, 91–97. [Google Scholar] [CrossRef]
- Kindschuh, W.F.; Baldini, F.; Liu, M.C.; Liao, J.; Meydan, Y.; Lee, H.H.; Heinken, A.; Thiele, I.; Thaiss, C.A.; Levy, M.; et al. Preterm birth is associated with xenobiotics and predicted by the vaginal metabolome. Nat. Microbiol. 2023, 8, 246–259. [Google Scholar] [CrossRef]
- Radan, A.P.; Aleksandra Polowy, J.; Heverhagen, A.; Simillion, C.; Baumann, M.; Raio, L.; Schleussner, E.; Mueller, M.; Surbek, D. Cervico-vaginal placental alpha-macroglobulin-1 combined with cervical length for the prediction of preterm birth in women with threatened preterm labor. Acta Obs. Gynecol. Scand. 2020, 99, 357–363. [Google Scholar] [CrossRef]
- Hu, S.; Zhong, H.; Huang, W.; Zhan, W.; Yang, X.; Tang, B.; Chen, K.; Wang, J.; Hu, T.; Zhang, C.; et al. Rapid and visual detection of Group B streptococcus using recombinase polymerase amplification combined with lateral flow strips. Diagn. Microbiol. Infect. Dis. 2019, 93, 9–13. [Google Scholar] [CrossRef]
- Liang, Y.; Jin, X.; Yuan, F.; Li, Z.; Chen, S. Comparison of rRNA-based and DNA-based nucleic acid amplifications for detection of Chlamydia trachomatis, Neisseria gonorrhoeae, and Ureaplasma urealyticum in urogenital swabs. BMC Infect. Dis. 2018, 18, 651. [Google Scholar] [CrossRef]
- Golparian, D.; Unemo, M. Antimicrobial resistance prediction in Neisseria gonorrhoeae: Current status and future prospects. Expert Rev. Mol. Diagn. 2022, 22, 29–48. [Google Scholar] [CrossRef] [PubMed]
- Vasala, A.; Hytönen, V.P.; Laitinen, O.H. Modern Tools for Rapid Diagnostics of Antimicrobial Resistance. Front. Cell. Infect. Microbiol. 2020, 10, 308. [Google Scholar] [CrossRef] [PubMed]
- Routine Testing for Group B Strep: The GBS3 Trial. Available online: https://www.gbs3trial.ac.uk/home.aspx (accessed on 20 November 2023).
- Gonçalves, B.P.; Procter, S.R.; Paul, P.; Chandna, J.; Lewin, A.; Seedat, F.; Koukounari, A.; Dangor, Z.; Leahy, S.; Santhanam, S.; et al. Group B streptococcus infection during pregnancy and infancy: Estimates of regional and global burden. Lancet Glob. Health 2022, 10, e807–e819. [Google Scholar] [CrossRef] [PubMed]
- Carreras-Abad, C.; Ramkhelawon, L.; Heath, P.T.; Le Doare, K. A Vaccine Against Group B Streptococcus: Recent Advances. Infect. Drug Resist. 2020, 13, 1263–1272. [Google Scholar] [CrossRef] [PubMed]
- Brokaw, A.; Nguyen, S.; Quach, P.; Orvis, A.; Furuta, A.; Johansson-Lindbom, B.; Fischer, P.B.; Rajagopal, L. A Recombinant Alpha-Like Protein Subunit Vaccine (GBS-NN) Provides Protection in Murine Models of Group B Streptococcus Infection. J. Infect. Dis. 2022, 226, 177–187. [Google Scholar] [CrossRef]
- Grammenıatıs, A.; Burrıel, A.R.; Pappa, K.; Ioannıdıs, A. A Review of Current Knowledge on the Development of a Group B Streptococcus Vaccine for Pregnant Women and the Protection of Neonates: Advances in Diagnosis and Treatment. J. Clin. Obstet. Gynecol. 2022, 32, 56–66. [Google Scholar] [CrossRef]
- Procter, S.R.; Gonçalves, B.P.; Paul, P.; Chandna, J.; Seedat, F.; Koukounari, A.; Hutubessy, R.; Trotter, C.; Lawn, J.E.; Jit, M. Maternal immunisation against Group B Streptococcus: A global analysis of health impact and cost-effectiveness. PLoS Med. 2023, 20, e1004068. [Google Scholar] [CrossRef]
- Cappelletti, M.; Presicce, P.; Feiyang, M.; Senthamaraikannan, P.; Miller, L.A.; Pellegrini, M.; Sim, M.S.; Jobe, A.H.; Divanovic, S.; Way, S.S.; et al. The induction of preterm labor in rhesus macaques is determined by the strength of immune response to intrauterine infection. PLoS Biol. 2021, 19, e3001385. [Google Scholar] [CrossRef]
- Presicce, P.; Park, C.W.; Senthamaraikannan, P.; Bhattacharyya, S.; Jackson, C.; Kong, F.; Rueda, C.M.; DeFranco, E.; Miller, L.A.; Hildeman, D.A.; et al. IL-1 signaling mediates intrauterine inflammation and chorio-decidua neutrophil recruitment and activation. JCI Insight 2018, 3, e98306. [Google Scholar] [CrossRef]
- Fenlon, S.N.; Chee, Y.C.; Chee, J.L.Y.; Choy, Y.H.; Khng, A.J.; Liow, L.T.; Mehershahi, K.S.; Ruan, X.; Turner, S.W.; Yao, F.; et al. Sequencing of E. coli strain UTI89 on multiple sequencing platforms. BMC Res. Notes 2020, 13, 487. [Google Scholar] [CrossRef]
- Zaga-Clavellina, V.; Garcia-Lopez, G.; Flores-Herrera, H.; Espejel-Nuñez, A.; Flores-Pliego, A.; Soriano-Becerril, D.; Maida-Claros, R.; Merchant-Larios, H.; Vadillo-Ortega, F. In vitro secretion profiles of interleukin (IL)-1beta, IL-6, IL-8, IL-10, and TNF alpha after selective infection with Escherichia coli in human fetal membranes. Reprod. Biol. Endocrinol. 2007, 5, 46. [Google Scholar] [CrossRef] [PubMed]
- Watt, S.; Lanotte, P.; Mereghetti, L.; Moulin-Schouleur, M.; Picard, B.; Quentin, R. Escherichia coli strains from pregnant women and neonates: Intraspecies genetic distribution and prevalence of virulence factors. J. Clin. Microbiol. 2003, 41, 1929–1935. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.M.; Charani, E.; Ming, D.; Cheah, F.-C.; Johnson, M.R. Antimicrobial stewardship and targeted therapies in the changing landscape of maternal sepsis. J. Intensive Med. 2023. [Google Scholar] [CrossRef]
- Stinson, L.F.; Payne, M.S. Infection-mediated preterm birth: Bacterial origins and avenues for intervention. Aust. N. Z. J. Obs. Gynaecol. 2019, 59, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Romero, R.; Kim, S.M.; Chaemsaithong, P.; Park, C.W.; Park, J.S.; Jun, J.K.; Yoon, B.H. A new anti-microbial combination prolongs the latency period, reduces acute histologic chorioamnionitis as well as funisitis, and improves neonatal outcomes in preterm PROM. J. Matern. Fetal Neonatal Med. 2016, 29, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.H.; Romero, R.; Park, J.Y.; Oh, K.J.; Lee, J.; Conde-Agudelo, A.; Hong, J.-S. Antibiotic administration can eradicate intra-amniotic infection or intra-amniotic inflammation in a subset of patients with preterm labor and intact membranes. Am. J. Obstet. Gynecol. 2019, 221, 142.e1–142.e22. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.H.; Salami, O.O.; Koya, J.; Munnangi, S.; Pekson, R.; Ashby, C.R., Jr.; Reznik, S.E. N,N-Dimethylformamide Delays LPS-Induced Preterm Birth in a Murine Model by Suppressing the Inflammatory Response. Reprod. Sci. 2022, 29, 2894–2907. [Google Scholar] [CrossRef] [PubMed]
- von Hehn, C.; Howard, J.; Liu, S.; Meka, V.; Pultz, J.; Mehta, D.; Prada, C.; Ray, S.; Edwards, M.R.; Sheikh, S.I. Immune response to vaccines is maintained in patients treated with dimethyl fumarate. Neurol. Neuroimmunol. Neuroinflamm. 2018, 5, e409. [Google Scholar] [CrossRef]
- Longbrake, E.E.; Mao-Draayer, Y.; Cascione, M.; Zielinski, T.; Bame, E.; Brassat, D.; Chen, C.; Kapadia, S.; Mendoza, J.P.; Miller, C.; et al. Dimethyl fumarate treatment shifts the immune environment toward an anti-inflammatory cell profile while maintaining protective humoral immunity. Mult. Scler. 2021, 27, 883–894. [Google Scholar] [CrossRef]
- Suff, N.; Karda, R.; Diaz, J.A.; Ng, J.; Baruteau, J.; Perocheau, D.; Taylor, P.W.; Alber, D.; Buckley, S.M.K.; Bajaj-Elliott, M.; et al. Cervical Gene Delivery of the Antimicrobial Peptide, Human beta-Defensin (HBD)-3, in a Mouse Model of Ascending Infection-Related Preterm Birth. Front. Immunol. 2020, 11, 106. [Google Scholar] [CrossRef]
- Hoover, D.M.; Wu, Z.; Tucker, K.; Lu, W.; Lubkowski, J. Antimicrobial characterization of human beta-defensin 3 derivatives. Antimicrob. Agents Chemother. 2003, 47, 2804–2809. [Google Scholar] [CrossRef] [PubMed]




| Past Medical History | Pregnancy Complications |
| Previous preterm birth Short cervix < 25 mm Early cervical dilatation Past procedures on the cervix (LLETZ) Injury during a past delivery | Carrying more than one fetus Vaginal bleeding during pregnancy Infections during pregnancy |
| Lifestyle | Other |
| Low pre-pregnancy weight Smoking during pregnancy Dietary deficiencies Injury during a past delivery | Younger than 17 or older than 35 years |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, H.; Singh, N.; Yovera Leyva, L.; Malawana, J.; Shah, N.M. Does Precision-Based Medicine Hold the Promise of a New Approach to Predicting and Treating Spontaneous Preterm Birth? Int. J. Transl. Med. 2024, 4, 15-52. https://doi.org/10.3390/ijtm4010002
Khan H, Singh N, Yovera Leyva L, Malawana J, Shah NM. Does Precision-Based Medicine Hold the Promise of a New Approach to Predicting and Treating Spontaneous Preterm Birth? International Journal of Translational Medicine. 2024; 4(1):15-52. https://doi.org/10.3390/ijtm4010002
Chicago/Turabian StyleKhan, Hiba, Natasha Singh, Luis Yovera Leyva, Johann Malawana, and Nishel M. Shah. 2024. "Does Precision-Based Medicine Hold the Promise of a New Approach to Predicting and Treating Spontaneous Preterm Birth?" International Journal of Translational Medicine 4, no. 1: 15-52. https://doi.org/10.3390/ijtm4010002
APA StyleKhan, H., Singh, N., Yovera Leyva, L., Malawana, J., & Shah, N. M. (2024). Does Precision-Based Medicine Hold the Promise of a New Approach to Predicting and Treating Spontaneous Preterm Birth? International Journal of Translational Medicine, 4(1), 15-52. https://doi.org/10.3390/ijtm4010002






