Identifying High-Risk Bacteria with Active Nasal Swab Surveillance in Intensive Care Units to Prevent Ventilator-Associated Pneumonia
Abstract
1. Introduction
2. Methods
2.1. Study Cohort
2.2. ANSC Sample Collection and Processing
2.3. Definition of VAP
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Incidence of VAP and Antimicrobial Usage
3.3. Bacterial Species Diversity in ANSC
3.4. ANSC Bacteria Diversity and Relation to VAP
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torres, A.; Cilloniz, C.; Niederman, M.S.; Menéndez, R.; Chalmers, J.D.; Wunderink, R.G.; van der Poll, T. Pneumonia. Nat. Rev. Dis. Primers 2021, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Daghmouri, M.A.; Dudoignon, E.; Chaouch, M.A.; Baekgaard, J.; Bougle, A.; Leone, M.; Deniau, B.; Depret, F. Comparison of a short versus long-course antibiotic therapy for ventilator-associated pneumonia: A systematic review and meta-analysis of randomized controlled trials. eClinicalMedicine 2023, 58, 101880. [Google Scholar] [CrossRef] [PubMed]
- Ladbrook, E.; Khaw, D.; Bouchoucha, S.; Hutchinson, A. A systematic scoping review of the cost-impact of ventilator-associated pneumonia (VAP) intervention bundles in intensive care. Am. J. Infect. Control 2021, 49, 928–936. [Google Scholar] [CrossRef] [PubMed]
- Kalil, A.C.; Metersky, M.L.; Klompas, M.; Muscedere, J.; Sweeney, D.A.; Palmer, L.B.; Napolitano, L.M.; O’Grady, N.P.; Bartlett, J.G.; Carratalà, J.; et al. Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 2016, 63, e61–e111. [Google Scholar] [CrossRef]
- Melsen, W.G.; Rovers, M.M.; Groenwold, R.H.; Bergmans, D.C.; Camus, C.; Bauer, T.T.; Hanisch, E.W.; Klarin, B.; Koeman, M.; Krueger, W.A.; et al. Attributable mortality of ventilator-associated pneumonia: A meta-analysis of individual patient data from randomised prevention studies. Lancet Infect. Dis. 2013, 13, 665–671. [Google Scholar] [CrossRef]
- Gil-Perotin, S.; Ramirez, P.; Marti, V.; Sahuquillo, J.M.; Gonzalez, E.; Calleja, I.; Menendez, R.; Bonastre, J. Implications of endotracheal tube biofilm in ventilator-associated pneumonia response: A state of concept. Crit. Care 2012, 16, R93. [Google Scholar] [CrossRef]
- Shein, A.M.S.; Hongsing, P.; Smith, O.R.K.; Phattharapornjaroen, P.; Miyanaga, K.; Cui, L.; Ishikawa, H.; Amarasiri, M.; Monk, P.N.; Kicic, A.; et al. Current and novel therapies for management of Acinetobacter baumannii-associated pneumonia. Crit. Rev. Microbiol. 2024, 1–22. [Google Scholar] [CrossRef]
- Chirabhundhu, N.; Luk-In, S.; Phuadraksa, T.; Wichit, S.; Chatsuwan, T.; Wannigama, D.L.; Yainoy, S. Occurrence and mechanisms of tigecycline resistance in carbapenem- and colistin-resistant Klebsiella pneumoniae in Thailand. Sci. Rep. 2024, 14, 5215. [Google Scholar] [CrossRef]
- Shein, A.M.S.; Wannigama, D.L.; Hurst, C.; Monk, P.N.; Amarasiri, M.; Badavath, V.N.; Phattharapornjaroen, P.; Ditcham, W.G.F.; Ounjai, P.; Saethang, T.; et al. Novel intranasal phage-CaEDTA-ceftazidime/avibactam triple combination therapy demonstrates remarkable efficacy in treating Pseudomonas aeruginosa lung infection. Biomed. Pharmacother. 2023, 168, 115793. [Google Scholar] [CrossRef]
- Srisakul, S.; Wannigama, D.L.; Higgins, P.G.; Hurst, C.; Abe, S.; Hongsing, P.; Saethang, T.; Luk-in, S.; Liao, T.; Kueakulpattana, N.; et al. Overcoming addition of phosphoethanolamine to lipid A mediated colistin resistance in Acinetobacter baumannii clinical isolates with colistin–sulbactam combination therapy. Sci. Rep. 2022, 12, 11390. [Google Scholar] [CrossRef]
- Shein, A.M.S.; Wannigama, D.L.; Higgins, P.G.; Hurst, C.; Abe, S.; Hongsing, P.; Chantaravisoot, N.; Saethang, T.; Luk-in, S.; Liao, T.; et al. High prevalence of mgrB-mediated colistin resistance among carbapenem-resistant Klebsiella pneumoniae is associated with biofilm formation, and can be overcome by colistin-EDTA combination therapy. Sci. Rep. 2022, 12, 12939. [Google Scholar] [CrossRef] [PubMed]
- Shein, A.M.S.; Wannigama, D.L.; Higgins, P.G.; Hurst, C.; Abe, S.; Hongsing, P.; Chantaravisoot, N.; Saethang, T.; Luk-in, S.; Liao, T.; et al. Novel colistin-EDTA combination for successful eradication of colistin-resistant Klebsiella pneumoniae catheter-related biofilm infections. Sci. Rep. 2021, 11, 21676. [Google Scholar] [CrossRef] [PubMed]
- Singkham-in, U.; Higgins, P.G.; Wannigama, D.L.; Hongsing, P.; Chatsuwan, T. Rescued chlorhexidine activity by resveratrol against carbapenem-resistant Acinetobacter baumannii via down-regulation of AdeB efflux pump. PLoS ONE 2020, 15, e0243082. [Google Scholar] [CrossRef]
- Wannigama, D.L.; Hurst, C.; Hongsing, P.; Pearson, L.; Saethang, T.; Chantaravisoot, N.; Singkham-in, U.; Luk-in, S.; Storer, R.J.; Chatsuwan, T. A rapid and simple method for routine determination of antibiotic sensitivity to biofilm populations of Pseudomonas aeruginosa. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 8. [Google Scholar] [CrossRef]
- Phuengmaung, P.; Somparn, P.; Panpetch, W.; Singkham-In, U.; Wannigama, D.L.; Chatsuwan, T.; Leelahavanichkul, A. Coexistence of Pseudomonas aeruginosa With Candida albicans Enhances Biofilm Thickness Through Alginate-Related Extracellular Matrix but Is Attenuated by N-acetyl-l-cysteine. Front. Cell Infect. Microbiol. 2020, 10, 594336. [Google Scholar] [CrossRef]
- Klompas, M.; Branson, R.; Cawcutt, K.; Crist, M.; Eichenwald, E.C.; Greene, L.R.; Lee, G.; Maragakis, L.L.; Powell, K.; Priebe, G.P.; et al. Strategies to prevent ventilator-associated pneumonia, ventilator-associated events, and nonventilator hospital-acquired pneumonia in acute-care hospitals: 2022 Update. Infect. Control Hosp. Epidemiol. 2022, 43, 687–713. [Google Scholar] [CrossRef]
- Semet, C. The ongoing challenge of ventilator-associated pneumonia: Epidemiology, prevention, and risk factors for mortality in a secondary care hospital intensive care unit. Infect. Prev. Pract. 2023, 5, 100320. [Google Scholar] [CrossRef]
- Chaberny, I.F.; Schwab, F.; Ziesing, S.; Suerbaum, S.; Gastmeier, P. Impact of routine surgical ward and intensive care unit admission surveillance cultures on hospital-wide nosocomial methicillin-resistant Staphylococcus aureus infections in a university hospital: An interrupted time-series analysis. J. Antimicrob. Chemother. 2008, 62, 1422–1429. [Google Scholar] [CrossRef]
- Holzmann-Pazgal, G.; Monney, C.; Davis, K.; Wanger, A.; Strobel, N.; Zhong, F. Active surveillance culturing impacts methicillin-resistant Staphylococcus aureus acquisition in a pediatric intensive care unit. Pediatr. Crit. Care Med. 2011, 12, e171–e175. [Google Scholar] [CrossRef]
- Kondo, T.; Okabayashi, K.; Sugiura, K.; Obara, H.; Takeuchi, H.; Wada, N.; Takano, Y.; Iwata, S.; Hasegawa, N.; Kitagawa, Y. Effectiveness of active nasal surveillance culture for Methicillin-resistant Staphylococcus aureus in patients undergoing colorectal surgery. J. Infect. Chemother. 2020, 26, 1244–1248. [Google Scholar] [CrossRef]
- Murphy, E.; Spencer, S.J.; Young, D.; Jones, B.; Blyth, M.J. MRSA colonisation and subsequent risk of infection despite effective eradication in orthopaedic elective surgery. J. Bone Jt. Surg. Br. 2011, 93, 548–551. [Google Scholar] [CrossRef] [PubMed]
- Malde, D.J.; Hardern, L.; Welch, M. Is it possible to predict outcome in MRSA positive patients undergoing arterial reconstruction? Int. Angiol. 2006, 25, 78–83. [Google Scholar] [PubMed]
- Depuydt, P.; Benoit, D.; Vogelaers, D.; Decruyenaere, J.; Vandijck, D.; Claeys, G.; Verschraegen, G.; Blot, S. Systematic surveillance cultures as a toolto predict involvement of multidrug antibiotic resistant bacteria in ventilator-associated pneumonia. Intensive Care Med. 2008, 34, 675–682. [Google Scholar] [CrossRef]
- Depuydt, P.O.; Blot, S.I.; Benoit, D.D.; Claeys, G.W.; Verschraegen, G.L.; Vandewoude, K.H.; Vogelaers, D.P.; Decruyenaere, J.M.; Colardyn, F.A. Antimicrobial resistance in nosocomial bloodstream infection associated with pneumonia and the value of systematic surveillance cultures in an adult intensive care unit. Crit. Care Med. 2006, 34, 653–659. [Google Scholar] [CrossRef]
- Depuydt, P.; Benoit, D.; Vogelaers, D.; Claeys, G.; Verschraegen, G.; Vandewoude, K.; Decruyenaere, J.; Blot, S. Outcome in bacteremia associated with nosocomial pneumonia and the impact of pathogen prediction by tracheal surveillance cultures. Intensive Care Med. 2006, 32, 1773–1781. [Google Scholar] [CrossRef]
- Klompas, M. Prevention of Intensive Care Unit-Acquired Pneumonia. Semin. Respir. Crit. Care Med. 2019, 40, 548–557. [Google Scholar] [CrossRef]
- CDC. Ventilator-Associated Event (VAE). 2023. Available online: www.cdc.gov/nhsn/pdfs/pscmanual/10-vae_final.pdf (accessed on 25 April 2024).
- RcoreTeam. R, a Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Wannigama, D.L.; Hurst, C.; Monk, P.N.; Hartel, G.; Ditcham, W.G.F.; Hongsing, P.; Phattharapornjaroen, P.; Ounjai, P.; Torvorapanit, P.; Jutivorakool, K.; et al. tesG expression as a potential clinical biomarker for chronic Pseudomonas aeruginosa pulmonary biofilm infections. BMC Med. 2025, 23, 191. [Google Scholar] [CrossRef]
- Abe, S.; Wannigama, D.L. Quick Sequential Organ Failure Assessment (qSOFA) and Performance Status Scoring Systems as Prognostic Predictors in Pneumococcal Community-Acquired Pneumonia. Cureus 2024, 16, e73201. [Google Scholar] [CrossRef]
- Maertens, B.; Blot, S.; Veld, D.H.I.; Blot, K.; Koch, A.; Mignolet, K.; Pannier, E.; Sarens, T.; Temmerman, W.; Swinnen, W. Stepwise implementation of prevention strategies and their impact on ventilator-associated pneumonia incidence: A 13-Year observational surveillance study. Intensive Crit. Care Nurs. 2025, 86, 103769. [Google Scholar] [CrossRef]
- Motowski, H.; Ilges, D.; Hampton, N.; Kollef, M.H.; Micek, S.T. Determinants of Mortality for Ventilated Hospital-Acquired Pneumonia and Ventilator-Associated Pneumonia. Crit. Care Explor. 2023, 5, e0867. [Google Scholar] [CrossRef]
- Rocha, L.A.; Marques Ribas, R.; da Costa Darini, A.L.; Gontijo Filho, P.P. Relationship between nasal colonization and ventilator-associated pneumonia and the role of the environment in transmission of Staphylococcus aureus in intensive care units. Am. J. Infect. Control 2013, 41, 1236–1240. [Google Scholar] [CrossRef] [PubMed]
- Tsay, T.-B.; Jiang, Y.-Z.; Hsu, C.-M.; Chen, L.-W. Pseudomonas aeruginosa colonization enhances ventilator-associated pneumonia-induced lung injury. Respir. Res. 2016, 17, 101. [Google Scholar] [CrossRef]
- Spoto, S.; Daniel Markley, J.; Valeriani, E.; Abbate, A.; Argemi, J.; Markley, R.; Fogolari, M.; Locorriere, L.; Anguissola, G.B.; Battifoglia, G.; et al. Active Surveillance Cultures and Procalcitonin in Combination With Clinical Data to Guide Empirical Antimicrobial Therapy in Hospitalized Medical Patients With Sepsis. Front. Microbiol. 2022, 13, 797932. [Google Scholar] [CrossRef]
- Brusselaers, N.; Labeau, S.; Vogelaers, D.; Blot, S. Value of lower respiratory tract surveillance cultures to predict bacterial pathogens in ventilator-associated pneumonia: Systematic review and diagnostic test accuracy meta-analysis. Intensive Care Med. 2013, 39, 365–375. [Google Scholar] [CrossRef]
- Righi, E.; Aggazzotti, G.; Ferrari, E.; Giovanardi, C.; Busani, S.; Rinaldi, L.; Girardis, M. Trends in ventilator-associated pneumonia: Impact of a ventilator care bundle in an Italian tertiary care hospital intensive care unit. Am. J. Infect. Control 2014, 42, 1312–1316. [Google Scholar] [CrossRef]
- Vacheron, C.H.; Lepape, A.; Savey, A.; Machut, A.; Timsit, J.F.; Comparot, S.; Courno, G.; Vanhems, P.; Landel, V.; Lavigne, T.; et al. Attributable Mortality of Ventilator-associated Pneumonia Among Patients with COVID-19. Am. J. Respir. Crit. Care Med. 2022, 206, 161–169. [Google Scholar] [CrossRef]
- Ippolito, M.; Misseri, G.; Catalisano, G.; Marino, C.; Ingoglia, G.; Alessi, M.; Consiglio, E.; Gregoretti, C.; Giarratano, A.; Cortegiani, A. Ventilator-Associated Pneumonia in Patients with COVID-19: A Systematic Review and Meta-Analysis. Antibiotics 2021, 10, 545. [Google Scholar] [CrossRef]
- Wicky, P.H.; Niedermann, M.S.; Timsit, J.F. Ventilator-associated pneumonia in the era of COVID-19 pandemic: How common and what is the impact? Crit. Care 2021, 25, 153. [Google Scholar] [CrossRef]
- Wannigama, D.L.; Jacquet, A. NOD2-dependent BCG-induced trained immunity: A way to regulate innate responses to SARS-CoV-2? Int. J. Infect. Dis. 2020, 101, 52–55. [Google Scholar] [CrossRef]
- Rad, S.M.A.H.; Wannigama, D.L.; Hirankarn, N.; McLellan, A.D. The impact of non-synonymous mutations on miRNA binding sites within the SARS-CoV-2 NSP3 and NSP4 genes. Sci. Rep. 2023, 13, 16945. [Google Scholar] [CrossRef]
- Laing, A.G.; Lorenc, A.; del Molino del Barrio, I.; Das, A.; Fish, M.; Monin, L.; Muñoz-Ruiz, M.; McKenzie, D.R.; Hayday, T.S.; Francos-Quijorna, I.; et al. A dynamic COVID-19 immune signature includes associations with poor prognosis. Nat. Med. 2020, 26, 1623–1635. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, R.S.; Monneret, G.; Payen, D. Immunosuppression in sepsis: A novel understanding of the disorder and a new therapeutic approach. Lancet Infect. Dis. 2013, 13, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Higginson, E.; Pereira-Dias, J.; Curran, M.D.; Parmar, S.; Khokhar, F.; Cuchet-Lourenço, D.; Lux, J.; Sharma-Hajela, S.; Ravenhill, B.; et al. Ventilator-associated pneumonia in critically ill patients with COVID-19. Crit. Care 2021, 25, 25. [Google Scholar] [CrossRef]
- Ayzac, L.; Girard, R.; Baboi, L.; Beuret, P.; Rabilloud, M.; Richard, J.C.; Guérin, C. Ventilator-associated pneumonia in ARDS patients: The impact of prone positioning. A secondary analysis of the PROSEVA trial. Intensive Care Med. 2016, 42, 871–878. [Google Scholar] [CrossRef]
- Vermeulen, H.; Hens, N.; Catteau, L.; Catry, B.; Coenen, S. Impact of the COVID-19 pandemic on community antibiotic consumption in the EU/European Economic Area: A changepoint analysis. J. Antimicrob. Chemother. 2023, 78, 2572–2580. [Google Scholar] [CrossRef]
- Önal, U.; Tüzemen, Ü.; Kazak, E.; Gençol, N.; Souleiman, E.; İmer, H.; Heper, Y.; Yılmaz, E.; Özakın, C.; Ener, B.; et al. Effects of COVID-19 pandemic on healthcare-associated infections, antibiotic resistance and consumption rates in intensive care units. Le Infez. Med. 2023, 31, 195–203. [Google Scholar] [CrossRef]
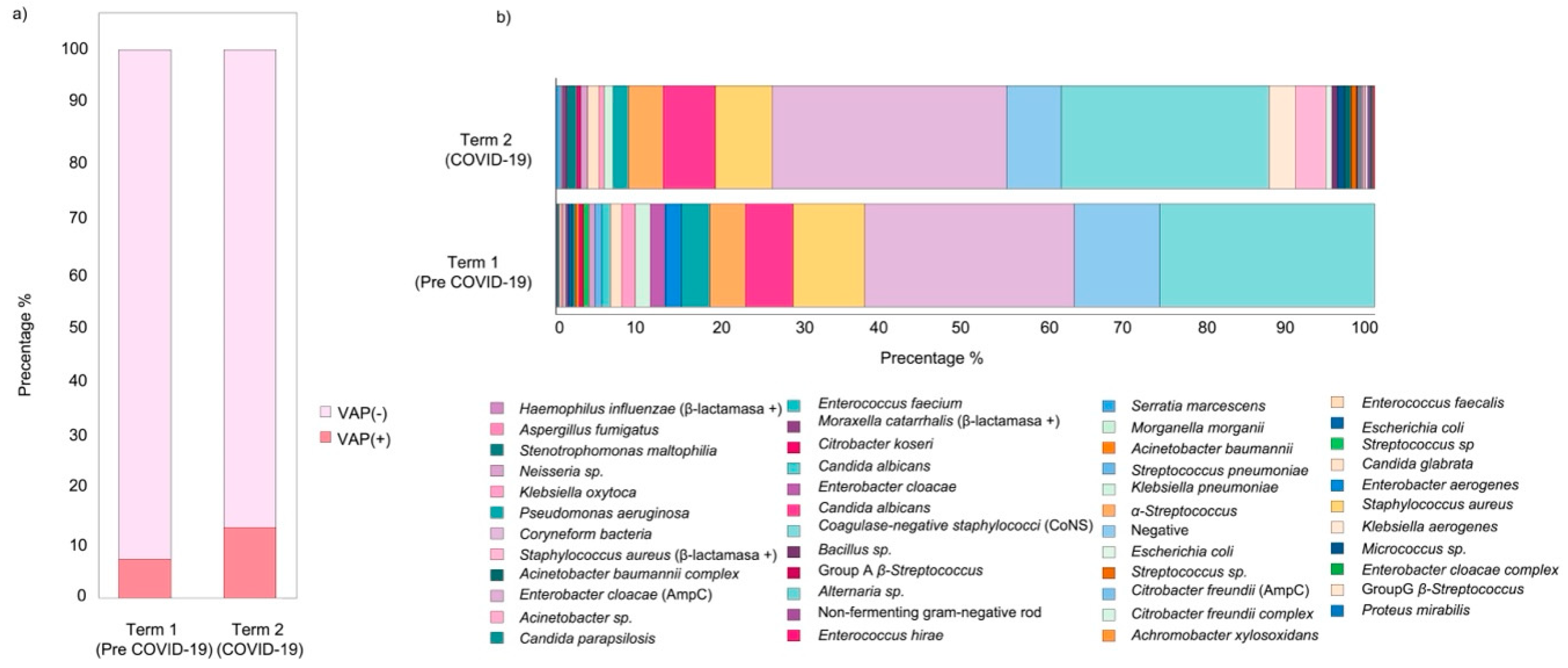

| Characteristic | VAP (n = 73) | Non-VAP (n = 706) | p-Value |
|---|---|---|---|
| Median age—year | 66 | 67 | 0.17 |
| Male sex—number (%) | 52 (71) | 458 (65) | 0.28 |
| Median body–mass index | 23.4 | 22.9 | 0.38 |
| Smoking (%) | 55% | 69% | 0.29 |
| Primary reason for ICU admission | |||
| State after surgical operation | 13 | 314 | <0.0001 |
| Sepsis | 4 | 87 | 0.08 |
| Heart failure | 8 | 80 | 0.92 |
| Trauma/Poisoning | 16 | 39 | <0.0001 |
| CPR recovery | 10 | 13 | <0.0001 |
| COVID-19 | 10 | 4 | <0.0001 |
| Others | 12 | 169 | |
| Duration of hospital stay—days | 64.3 | 32 | <0.0001 |
| Mortality (%) | 19% | 8% | <0.0002 |
| SOFA SCORE | |||
| Respiration | 2.49 | 2.08 | <0.0001 |
| Coagulation | 0.90 | 1.01 | 0.32 |
| Liver function | 0.50 | 0.52 | 0.98 |
| Circulation | 2.57 | 2.11 | 0.01 |
| CNS | 1.79 | 0.94 | <0.0001 |
| Renal function | 0.91 | 0.70 | 0.095 |
| Total | 9.14 | 7.36 | <0.0001 |
| Antimicrobials—no. (%) | |||
| Cefazolin (CEZ) | 45 (62) | 516 (73) | 0.038 |
| Meropenem (MEM) | 19 (27) | 54 (8) | <0.0001 |
| Tazobactam/piperacillin (TZP) | 20 (27) | 32 (5) | <0.0001 |
| Cefepime (FEP) or cefozopran (CZOP) | 21 (28) | 15 (2) | <0.0001 |
| Vancomycin (VAN) | 16 (22) | 15 (2) | <0.0001 |
| Others | 46 (63) | 161 (23) | <0.0001 |
| Characteristic | ||||||
|---|---|---|---|---|---|---|
| Antimicrobialsno (%) | non-VAP | VAP | ||||
| Term 1 (Pre COVID-19) | Term 2 (COVID-19) | p-Value | Term 1 (Pre COVID-19) | Term 2 (COVID-19) | p-Value | |
| Cefazolin (CEZ) | 322 (74) | 194 (71) | 0.49 | 21 (64) | 24 (60) | 0.75 |
| Meropenem (MEM) | 49 (11) | 39 (14) | 0.22 | 7 (21) | 12 (30) | 0.39 |
| Piperacillin–Tazobactam (TZP) | 29 (6.6) | 23 (8.5) | 0.37 | 10 (30) | 10 (25) | 0.61 |
| Cefepime (FEP)/Cefoperazone (CZOP) | 11 (2.5) | 14 (5.2) | 0.065 | 5 (15) | 16 (40) | 0.020 |
| Vancomycin (VAN) | 14 (3.2) | 13 (4.8) | 0.29 | 5 (15) | 11 (28) | 0.20 |
| Others | 156 (36) | 45 (17) | <0.0001 | 24 (72) | 22 (55) | 0.12 |
| ANSC | VAP(+) | VAP(−) | Incidences | Match with ANSC and Sputum Culture | Match % | |
|---|---|---|---|---|---|---|
| Haemophilus influenz | 1 | 1 | 0 | 100% | 1 | 100% |
| Serratia marcescens | 4 | 2 | 2 | 50% | 2 | 100% |
| Moraxella catarrhalis | 8 | 1 | 7 | 13% | 0 | 0% |
| Klebsiella spp. | 75 | 8 | 67 | 10.7% | 5 | 63% |
| Staphylococcus aureus | 176 | 18 | 158 | 10.2% | 15 | 83% |
| Streptococcus pneumoniae | 10 | 1 | 9 | 10.0% | 1 | 100% |
| Escherichia coli | 10 | 1 | 9 | 10% | 0 | 0% |
| Enterobacter spp. | 46 | 4 | 42 | 8.7% | 1 | 25% |
| Acinetobacter sp. | 13 | 1 | 12 | 8% | 0 | 0% |
| Stenotrophomonas maltophilia | 13 | 1 | 13 | 8% | 1 | 100% |
| Pseudomonas aeruginosa | 53 | 3 | 50 | 6% | 3 | 100% |
| Coagulase-negative staphylococci | 302 | 25 | 456 | 5.2% | 0 | 0% |
| Coryneform bacteria | 295 | 5 | 494 | 1.0% | 0 | 0% |
| Negative | 120 | 2 | 118 | 1.7% | 0 | 0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuramasu, Y.; Suzuki, Y.; Akaneya, D.; Okuma, Y.; Tsujimoto, Y.; Ishizawa, D.; Moriya, K.; Hongsing, P.; Amarasiri, M.; Hurst, C.; et al. Identifying High-Risk Bacteria with Active Nasal Swab Surveillance in Intensive Care Units to Prevent Ventilator-Associated Pneumonia. Int. J. Transl. Med. 2025, 5, 17. https://doi.org/10.3390/ijtm5020017
Kuramasu Y, Suzuki Y, Akaneya D, Okuma Y, Tsujimoto Y, Ishizawa D, Moriya K, Hongsing P, Amarasiri M, Hurst C, et al. Identifying High-Risk Bacteria with Active Nasal Swab Surveillance in Intensive Care Units to Prevent Ventilator-Associated Pneumonia. International Journal of Translational Medicine. 2025; 5(2):17. https://doi.org/10.3390/ijtm5020017
Chicago/Turabian StyleKuramasu, Yu, Yu Suzuki, Daisuke Akaneya, Yoshikazu Okuma, Yuta Tsujimoto, Daisuke Ishizawa, Kazunori Moriya, Parichart Hongsing, Mohan Amarasiri, Cameron Hurst, and et al. 2025. "Identifying High-Risk Bacteria with Active Nasal Swab Surveillance in Intensive Care Units to Prevent Ventilator-Associated Pneumonia" International Journal of Translational Medicine 5, no. 2: 17. https://doi.org/10.3390/ijtm5020017
APA StyleKuramasu, Y., Suzuki, Y., Akaneya, D., Okuma, Y., Tsujimoto, Y., Ishizawa, D., Moriya, K., Hongsing, P., Amarasiri, M., Hurst, C., Higgins, P. G., Shibuya, K., Kicic, A., Shimotai, Y., Hamamoto, H., Wannigama, D. L., & Abe, S. (2025). Identifying High-Risk Bacteria with Active Nasal Swab Surveillance in Intensive Care Units to Prevent Ventilator-Associated Pneumonia. International Journal of Translational Medicine, 5(2), 17. https://doi.org/10.3390/ijtm5020017







