Effects of Suckling on the Udder Health of Foster Cows
Abstract
:1. Introduction
2. Materials and Methods
2.1. Farm and Management
2.2. Study Design and Data Collection
2.3. Examination of the Udder
2.4. Quarter Milk Samples
2.5. Laboratory Analyses
2.6. Definitions
2.7. Statistical Analyses
3. Results
3.1. Numbers of Cows and Quarters
3.2. Bacteriological Examination
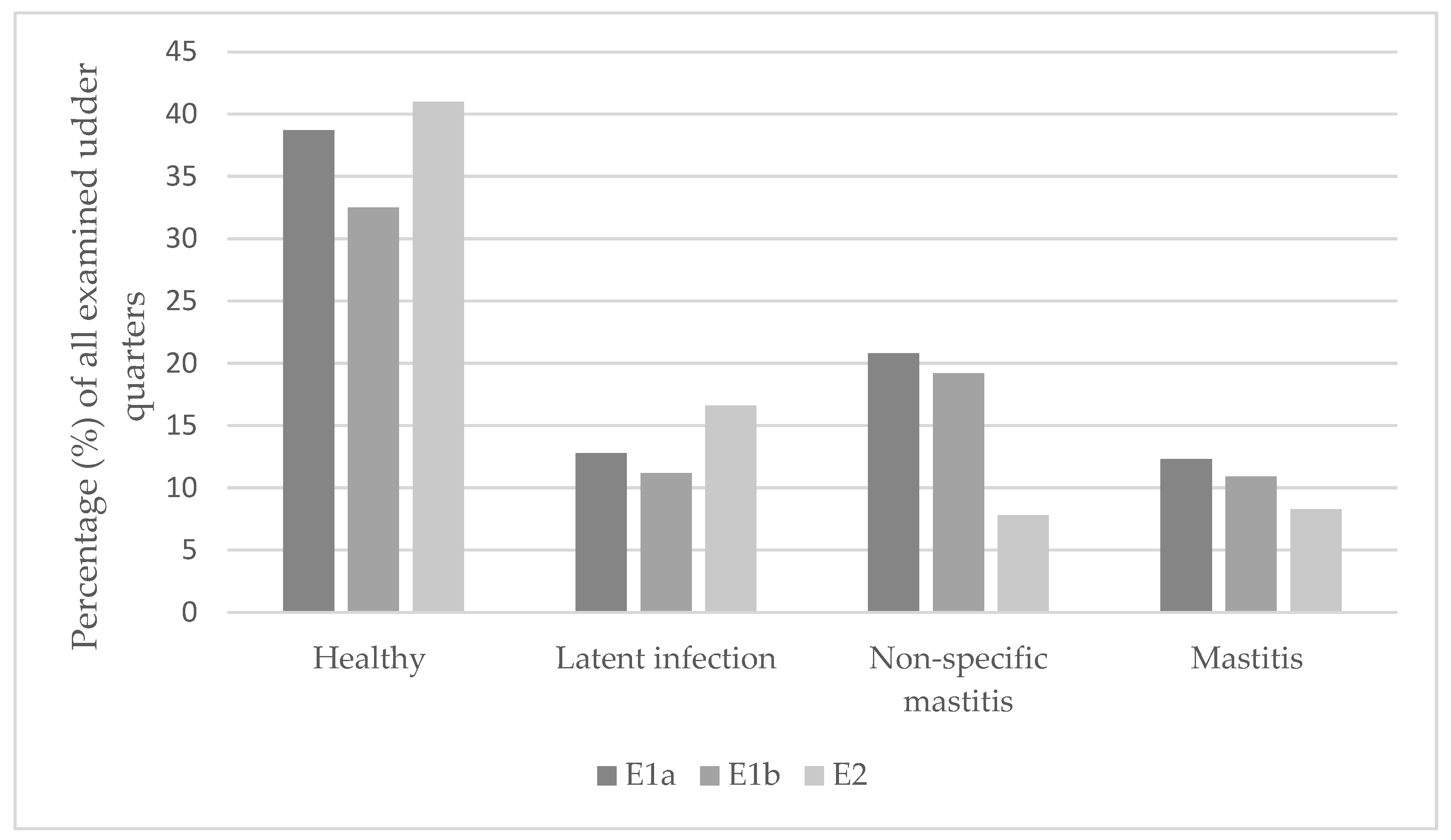
3.3. Classification of Udder Health
3.4. Bacteriological and Cytological Cure
3.5. New Subclinical Mastitis
3.6. Udder Examination
4. Discussion
4.1. Pathogen Distribution
4.2. Classification in Categories of Udder Health
4.3. Bacteriological and Cytological Cure Rate
4.4. Ratio of New Infections to Persistent Infections
4.5. Results of Udder Examination
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Newberry, R.C.; Swanson, J.C. Implications of breaking mother-young social bonds. Appl. Anim. Behav. Sci. 2008, 110, 3–23. [Google Scholar] [CrossRef]
- Stĕhulová, I.; Lidfors, L.; Špinka, M. Response of dairy cows and calves to early separation: Effect of calf age and visual and auditory contact after separation. Appl. Anim. Behav. Sci. 2008, 110, 144–165. [Google Scholar] [CrossRef]
- Kälber, T.; Barth, K. Practical implications of suckling systems for dairy calves in organic production systems—a review. Landbauforschung 2014, 64, 45–58. [Google Scholar] [CrossRef]
- Wagenaar, J.P.T.M.; Langhout, J. Practical implications of increasing ‘natural living’ through suckling systems in organic dairy calf rearing. NJAS Wagen. J. Life Sci. 2007, 54, 375–386. [Google Scholar] [CrossRef] [Green Version]
- Busch, G.; Weary, D.M.; Spiller, A.; von Keyserlingk, M.A.G. American and German attitudes towards cow-calf separation on dairy farms. PLoS ONE 2017, 12, e0174013. [Google Scholar] [CrossRef] [Green Version]
- Beaver, A.; Meagher, R.K.; von Keyserlingk, M.A.G.; Weary, D.M. Invited review: A systematic review of the effects of early separation on dairy cow and calf health. J Dairy Sci 2019, 102, 5784–5810. [Google Scholar] [CrossRef]
- Johnsen, J.F.; Zipp, K.; Kälber, T.; de Passillé, A.M.; Knierim, U.; Barth, K.; Mejdell, C.M. Is rearing calves with the dam a feasible option for dairy farms?—Current and future research. Appl. Anim. Behav. Sci. 2015, 181, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Loberg, J.; Lidfors, L. Effect of stage of lactation and breed on dairy cows’ acceptance of foster calves. Appl. Anim. Behav. Sci. 2001, 74, 97–108. [Google Scholar] [CrossRef]
- Kent, J. The cow-calf relationship: From maternal responsiveness to the maternal bond and the possibilities for fostering. J. Dairy Res. 2020, 87, 101–107. [Google Scholar] [CrossRef]
- Špinka, M.; Illmann, G. Suckling behaviour of young dairy calves with their own and alien mothers. Appl. Anim. Behav. Sci. 1992, 33, 165–173. [Google Scholar] [CrossRef]
- Everitt, G.C.; Philips, D.S.M. Calf rearing by multiple suckling and the effects of lactation performance of the cow. NZ Soc. Anim. Prod. Proc. 1971, 31, 22–40. [Google Scholar]
- Brouĉek, J.; Mihina, Š.; Uhrinčat, M.; Tančin, V.; Harcek, L.; Hetényi, L. Effect of suckling several calves on milk yield and reproduction of dairy cows. Zivocisna Vyrob. 1995, 40, 59–64. [Google Scholar]
- Margerison, J.K.; Preston, T.R.; Philips, C.J.C. Restricted suckling of tropical dairy cows by their own calf or other cow’s calves. J. Anim. Sci. 2002, 80, 1663–1670. [Google Scholar] [CrossRef]
- Rigby, C.; Ugarte, J.; Boucourt, R. Rearing dairy calves by restricted suckling. VII. Effect on mastitis development caused by Staphylococcus aureus. Cuba. J. Agric. Sci. 1976, 10, 35–40. [Google Scholar]
- Krohn, C.C.; Jonasen, B.; Munksgaard, L. Cow-Calf Relations II. The Effect of 0 Versus 5 Days Suckling on Behaviour, Milk Production and Udder Health of Cows in Different Stabling; Report No. 678; National Institute of Animal Science: Foulum, Denmark, 1990. [Google Scholar]
- González-Sedano, M.; Marin-Mejia, B.; Maranto, M.I.; Leme de Magalhães-Labarthe, A.C.; Alonso-Diaz, M.A. Effect of residual calf suckling on clinical and sub-clinical infections of mastitis in dual-purpose cows: Epidemiological measurements. Res. Vet. Sci. 2010, 89, 362–366. [Google Scholar] [CrossRef]
- Boonbrahm, N.; Peters, K.J.; Intisang, W. The influence of calf rearing methods and milking methods on performance traits of crossbred dairy cattle in Thailand. 1. Milk yield and udder health. Arch. Anim. Breed 2004, 47, 405–414. [Google Scholar] [CrossRef] [Green Version]
- Andrade, R.; Pulido, M.; Molano, D. Effect of restricted suckling on somatic cell counts in cross holstein cows. Rev. MVZ Córdoba 2010, 15, 2095–2101. [Google Scholar]
- Walsh, J.P. Milk secretion in machine-milked and suckled cows. Isr. J. Agric Res. 1974, 13, 77–89. [Google Scholar]
- Krömker, V.; Zinke, C.; Paduch, J.H.; Klocke, D.; Reimann, A.; Eller, G. Evaluation of increased milking frequency as an additional treatment for cows with clinical mastitis. J. Dairy Res. 2010, 77, 90–94. [Google Scholar] [CrossRef]
- Fulkerson, W.J.; Hooley, R.D.; Findlay, J.K. Improvement in milk production of first calf heifers by multiple suckling. Aust. J. Agric. Res. 1978, 29, 351–357. [Google Scholar] [CrossRef]
- Thomas, G.W.; Spiker, S.A.; Mickan, F.J. Influence of suckling by Friesian cows on milk production and anoestrus (meat calf rearing). Aust. J. Exp. Agric. 1981, 21, 5–11. [Google Scholar] [CrossRef]
- Kilgour, R. Some observations on the suckling activity of calves on nurse cows. Proc. N. Z. Soc. Anim. Prod. 1972, 32, 132–136. [Google Scholar]
- Rasmussen, M.D.; Larsen, H.D. The Effect of Post Milking Teat Dip and Suckling on Teat Skin Condition, Bacterial Colonisation, and Udder Health. Acta. Vet. Scand. 1998, 39, 443–452. [Google Scholar] [CrossRef]
- Grunert, E. Weiblicher Geschlechtsapparat und Euter. In Die Klinische Untersuchung des Rindes, 4th ed.; Gründer, G., Stöber, M., Eds.; Enke-Verlag: Stuttgart, Germany, 2012; pp. 472–548. [Google Scholar]
- Haverkamp, H.; Paduch, J.-H.; Klocke, D.; Hoedemaker, M.; Krömker, V. Prevalence of teat end hyperkeratosis in lactating dairy cattle and their association with animal variables. Int. J. Environ. Agric Res. 2017, 3. [Google Scholar] [CrossRef]
- Mein, G.A.; Neijenhuis, F.; Morgan, W.F.; Reinemann, D.J.; Hillerton, J.E.; Baines, J.R.; Ohnstad, L.; Rasmussen, M.D.; Timms, L.; Britt, J.S.; et al. Evaluation of bovine teat condition in commercial dairy herds: 1 Non-infectious factors. National Mastitis Council Inc. In Proceedings of the 2nd International Symposium on Mastitis and Milk Quality, Vancouver, BC, Canada, 13–15 September 2001; National Mastitis Council Inc.: Madison, WI, USA, 2001; pp. 344–351. [Google Scholar]
- German Veterinary Medical Association. Leitlinien zur Labordiagnostik der Mastitis—Probenahme und Mikrobiologische Untersuchung, 3rd ed.; German Veterinary Medical Association: Gießen, Germany, 2018. [Google Scholar]
- Theel, E.S.; Schmitt, B.H.; Hall, L.; Cunningham, S.A.; Walchak, R.C.; Patel, R.; Wengenack, N.L. Formic Acid-Based Direct, On-Plate Testing of Yeast and Corynebacterium Species by Bruker Biotyper Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry. J. Clin. Microbiol. 2012, 50, 3093–3095. [Google Scholar] [CrossRef] [Green Version]
- German Veterinary Medical Association. Leitlinien zur Bekämpfung der Mastitis als Bestandsproblem, 5th ed.; German Veterinary Medical Association: Gießen, Germany, 2012. [Google Scholar]
- Kiesner, K.; Wente, N.; Volling, O.; Krömker, V. Selection of cows for treatment at dry-off on organic farms. J. Dairy Res. 2016, 83, 468–475. [Google Scholar] [CrossRef]
- Nitz, J.; Wente, N.; Zhang, Y.; Klocke, D.; tho Seeth, M.; Krömker, V. Dry Period or Early Lactation—Time of Onset and Associated Risk Factors for Intramammary Infections in Dairy Cows. Pathogens 2020, 10, 224. [Google Scholar] [CrossRef]
- Nitz, J.; Krömker, V.; Klocke, D.; Wente, N.; Zhang, Y.; tho Seeth, M. Intramammary Infections in Heifers—Time of Onset and Associated Risk Factors. Animals 2020, 10, 1053. [Google Scholar] [CrossRef]
- Benites, N.R.; Melville, P.A.; Costa, E.O. Evaluation of the Microbiological Status of Milk and Various Structures in Mammary Glands from Naturally Infected Dairy Cows. Trop. Anim. Health Prod. 2003, 35, 301–307. [Google Scholar] [CrossRef]
- Milanov, D.; Aleksić, N.; Todorović, D.; Bugarski, D. Pasteurella multocida mastitis in cow: Case report. Vet. Glas. 2017, 71, 117–122. [Google Scholar] [CrossRef] [Green Version]
- Barnum, D.A. A herd outbreak of mastitis caused by Pasteurella Multocida. Can. J. Comp. Med. 1954, 18, 113–119. [Google Scholar]
- Hansmann, V.K.; Volling, O.; Krömker, V. Udder health in organic dairy herds in Northern Germany. Milchwiss. Milk Sci. Int. 2019, 72, 6–23. [Google Scholar] [CrossRef]
- Alvarez, F.J.; Saucedo, G.; Arriaga, A.; Preston, T.R. Effect on milk production and calf performance of milking crossbred European/Zebu cattle in the absence or presence of the calf, and of rearing their calves artificially. Trop. Anim. Prod. 1980, 5, 25–27. [Google Scholar]
- Schalm, O.W. Streptococcus agalactiae in the udders of heifers at parturition traced to sucking among calves. Cornell Vet. 1942, 32, 49–60. [Google Scholar]
- Fröberg, S.; Gratte, E.; Svennersten-Sjaunja, K.; Olsson, I.; Berg, C.; Orihuela, A.; Galina, G.S.; García, B.; Lidfors, L. Effect of suckling (‘restricted suckling’) on dairy cows’ udder health and milk let-down and their calves’ weight gain, feed intake and behaviour. Appl. Anim. Behav. Sci. 2008, 113, 1–14. [Google Scholar] [CrossRef]
- Barth, K. Effect of suckling on milk yield and milk composition of dairy cows in cow-calf contact systems. J. Dairy Res. 2020, 87, 133–137. [Google Scholar] [CrossRef]
- Waller, K.P.; Persson, Y.; Nyman, A.-K.; Stengärde, L. Udder health in beef cows and its association with calf growth. Acta Vet. Scand 2014, 56, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Wente, N.; Grieger, A.S.; Klocke, D.; Paduch, J.-H.; Zhang, Y.; Leimbach, S.; tho Seeth, M.; Mansion-De Vries, E.M.; Mohr, E.; Kroemker, V. Recurrent mastitis- persistent or new infections? Vet. Microbiol. 2020, 244. [Google Scholar] [CrossRef]
- Vaarst, M. Mastitis in Danish organic dairying. Proc. Br. Mastit. Conf. 2001, 1–12. [Google Scholar]
- Ceyhan, A.; Cinar, M.; Serbeser, U. Milk Yield, Somatic Cell Count, and Udder Measurements in Holstein Cows at Different Lactation Number and Month. Media Peternak. 2015, 38, 118–122. [Google Scholar] [CrossRef]
- Akdag, F.; Ugurlu, M.; Gurler, H.; Teke, B.; Kocak, O. The relationships between udder traits and milk yield, milk composition, and subclinical mastitis in Jersey cows. Large Anim. Rev. 2017, 23, 203–209. [Google Scholar]
- Hamann, J.; Stanitzke, U. Studies on pathogenesis of bovine mastitis by comparison of milking conditions as calf suckling, hand milking and machine milking. Milchwissenschaft 1990, 45, 632–637. [Google Scholar]
- Olde Riekerink, R.G.M.; van Amersfort, K.; Sampimon, O.C.; Hoojer, G.A.; Lam, T.J.G.M. Short communication: Prevalence, risk factors, and a field scoring system for udder cleft dermatitis in Dutch dairy herds. J. Dairy Sci. 2014, 97, 5007–5011. [Google Scholar] [CrossRef] [Green Version]
- Agger, J.F.; Willeberg, P. Epidemiology of teat lesions in a dairy herd. II. Associations with subclinical mastitis. Nord. Vet.-Med. 1986, 38, 220–232. [Google Scholar]
| Parameter | Recording Method | Score/Category |
|---|---|---|
| Udder shape | Visual | Belly-thigh udder versus milking-machine udder versus other |
| Teat shape | Visual | Normal versus other |
| Teat length (mm) | Measuring | From the base of the teat to the teat tip |
| Teat diameter (mm) | Measuring | 1 cm above the teat tip |
| Teat end position | Visual | In relation to the ankle: higher versus same height versus lower |
| Teat end shape | Visual | Normal versus other |
| Teat end hyperkeratosis | Visual | 1 = no ring 2 = smooth ring 3 = rough ring 4 = very rough ring |
| Surplus teats | Visual | Yes versus no |
| Incontinentia lactis | Visual | Yes versus no |
| Colour of the udder skin | Visual | Normal versus other |
| Udder cleft dermatitis (UCD) | Visual | 0 = no signs of UCD 1 = reddened skin 2 = skin abrasion or scabs 3 = wound4 = wound larger than a hen’s egg |
| Temperature of udder skin | Manual | Normal versus other |
| Udder oedema | Manual | Yes versus no |
| Consistency of the mammary gland tissue | Manual | Normal versus partly knotted versus coarse knotted to hardened |
| Cistern grip | Manual | Normal versus other |
| Roll grip | Manual | Normal versus other |
| Udder lymph nodes | Visual | Normal versus other |
| Pathogen | Number (%) of Isolated Pathogens | ||||
|---|---|---|---|---|---|
| E1a 1 | E1b 2 | E2 | Persistent Infections 3 | New Infections 4 | |
| Streptococcus uberis | 1 (0.2) | 1 (0.3) | 2 (0.5) | 0 (0) | 2 (100) |
| NaS 5 | 34 (7.0) | 29 (7.5) | 24 (6.2) | 3 (12.5) | 20 (83.3) |
| Staphylococcus aureus | 0 (0) | 0 (0) | 3 (0.8) | 0 (0) | 3 (100) |
| Streptococcus dysgalactiae | 0 (0) | 0 (0) | 2 (0.5) | 0 (0) | 1 (50) |
| Trueperella pyogenes | 0 (0) | 0 (0) | 2 (0.5) | 0 (0) | 2 (100) |
| Escherichia coli | 8 (1.6) | 8 (2.1) | 0 (0) | 0 (0) | 0 (0) |
| Coliforms | 8 (1.6) | 5 (1.3) | 0 (0) | 0 (0) | 0 (0) |
| Klebsiella spp. | 2 (0.4) | 2 (0.5) | 1 (0.3) | 0 (0) | 1 (100) |
| Bacillus spp. | 3 (0.6) | 3 (0.8) | 0 (0) | 0 (0) | 0 (0) |
| Corynebacterium spp. | 44 (9.1) | 37 (9.6) | 50 (13) | 20 (40) | 27 (54) |
| Enterococcus spp. | 4 (0.8) | 4 (1.0) | 2 (0.5) | 0 (0) | 2 (100) |
| Pseudomonas spp. | 2 (0.4) | 2 (0.5) | 0 (0) | 0 (0) | 0 (0) |
| Other streptococci | 6 (1.2) | 3 (0.8) | 4 (1.0) | 0 (0) | 3 (75) |
| Lactic acid bacteria | 9 (1.9) | 6 (1.6) | 0 (0) | 0 (0) | 0 (0) |
| Pasteurella spp. | 0 (0) | 0 (0) | 13 (3.4) | 0 (0) | 13 (100) |
| Others 6 | 3 (0.6) | 2 (0.5) | 7 (1.9) | 0 (0) | 7 (100) |
| Mixed | 21 (4.3) | 19 (4.9) | 12 (3.1) | 0 (0) | 8 (66.7) |
| Contaminated 7 | 31 (6.4) | 22 (5.7) | 38 (9.9) | 0 (0) | 0 (0) |
| In total | 145 (29.8) | 121 (31.4) | 122 (31.7) | 23 (18.9) | 89 (73) |
| No specific growth 8 | 310 (63.8) | 242 (62.9) | 225 (58.4) | ||
| Pathogen | Number (%) of Isolated Pathogens | |||
|---|---|---|---|---|
| E1 1 | E2 | Persistent Infections 2 | New Infections 3 | |
| Streptococcus uberis | 1 (2.3) | 0 (0) | 0 (0) | 0 (0) |
| NaS 4 | 8 (18.6) | 15 (23.4) | 2 (13.3) | 13 (86.7) |
| Staphylococcus aureus | 0 (0) | 1 (1.6) | 0 (0) | 1 (100) |
| Streptococcus dysgalactiae | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Trueperella pyogenes | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Escherichia coli | 1 (2.3) | 0 (0) | 0 (0) | 0 (0) |
| Coliforms | 1 (2.3) | 0 (0) | 0 (0) | 0 (0) |
| Klebsiella spp. | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Bacillus spp. | 3 (7) | 0 (0) | 0 (0) | 0 (0) |
| Corynebacterium spp. | 16 (37.2) | 36 (56.3) | 15 (41.7) | 21 (58.3) |
| Enterococcus spp. | 1 (2.3) | 1 (1.6) | 0 (0) | 1 (100) |
| Pseudomonas spp. | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Other streptococci | 1 (2.3) | 1 (1.6) | 0 (0) | 1 (100) |
| Lactic acid bacteria | 3 (7) | 0 (0) | 0 (0) | 0 (0) |
| Pasteurella spp. | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Others 5 | 2 (4.7) | 7 (11) | 0 (0) | 7 (100) |
| Mixed | 6 (14) | 3 (4.7) | 0 (0) | 3 (100) |
| In total | 43 (100) | 64 (100) | 17 (26.6) | 47 (73.4) |
| Pathogen | Number (%) of Isolated Pathogens | |||
|---|---|---|---|---|
| E1 1 | E2 | Persistent Infections 2 | New Infections 3 | |
| Streptococcus uberis | 0 (0) | 1 (3.1) | 0 (0) | 1 (100) |
| NaS 4 | 15 (35.7) | 6 (18.8) | 1 (16.7) | 5 (83.3) |
| Staphylococcus aureus | 0 (0) | 2 (6.3) | 0 (0) | 2 (100) |
| Streptococcus dysgalactiae | 0 (0) | 1 (3.1) | 0 (0) | 1 (100) |
| Trueperella pyogenes | 0 (0) | 2 (6.3) | 0 (0) | 2 (100) |
| Escherichia coli | 5 (11.9) | 0 (0) | 0 (0) | 0 (0) |
| Coliforms | 2 (4.8) | 0 (0) | 0 (0) | 0 (0) |
| Klebsiella spp. | 1 (2.4) | 1 (3.1) | 0 (0) | 1 (100) |
| Bacillus spp. | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Corynebacterium spp. | 12 (28.6) | 4 (12.5) | 2 (50) | 2 (50) |
| Enterococcus spp. | 2 (4.8) | 1 (3.1) | 0 (0) | 1 (100) |
| Pseudomonas spp. | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Other streptococci | 1 (2.4) | 2 (6.3) | 0 (0) | 2 (100) |
| Lactic acid bacteria | 1 (2.4) | 0 (0) | 0 (0) | 0 (0) |
| Pasteurella spp. | 0 (0) | 9 (28.1) | 0 (0) | 9 (100) |
| Others 5 | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Mixed | 3 (7.1) | 3 (9.4) | 0 (0) | 3 (100) |
| In total | 42 (100) | 32 (100) | 3 (9.4) | 29 (90.6) |
| Pathogen | Bacteriological Cure | Cytological Cure | ||||
|---|---|---|---|---|---|---|
| n | % 1 | % 2 | n | % 1 | % 2 | |
| Streptococcus uberis | 1/1 | 100 | 1.2 | 0/1 | 0 | 0 |
| NaS 3 | 25/29 | 86.2 | 29.8 | 13/29 | 44.8 | 12 |
| Staphylococcus aureus | 0/0 | 0 | 0 | 0/0 | 0 | 0 |
| Streptococcus dysgalactiae | 0/0 | 0 | 0 | 0/0 | 0 | 0 |
| Trueperella pyogenes | 0/0 | 0 | 0 | 0/0 | 0 | 0 |
| Escherichia coli | 8/8 | 100 | 9.5 | 4/8 | 50 | 3.7 |
| Coliforms | 5/5 | 100 | 6 | 2/5 | 40 | 1.9 |
| Klebsiella spp. | 2/2 | 100 | 2.4 | 1/2 | 50 | 0.9 |
| Bacillus spp. | 3/3 | 100 | 3.6 | 0/3 | 0 | 0 |
| Corynebacterium spp. | 16/37 | 43.2 | 19 | 9/37 | 24.3 | 8.3 |
| Enterococcus spp. | 3/4 | 23.1 | 3.6 | 1/4 | 25 | 0.9 |
| Pseudomonas spp. | 1/2 | 50 | 1.2 | 0/2 | 0 | 0 |
| Other streptococci | 3/3 | 100 | 3.6 | 1/3 | 33.3 | 0.9 |
| Lactic acid bacteria | 5/6 | 83.3 | 6 | 1/6 | 16.7 | 0.9 |
| Pasteurella spp. | 0/0 | 0 | 0 | 0/0 | 0 | 0 |
| Others 4 | 2/2 | 100 | 2.4 | 0/2 | 0 | 0 |
| Mixed | 10/19 | 52.6 | 11.9 | 5/19 | 26.3 | 4.6 |
| Contaminated 5 | 6/22 | 27.3 | 5.6 | |||
| No specific growth 6 | 65/242 | 26.9 | 60.2 | |||
| In total | 84/121 | 69.4 | 100 | 108/385 | 28.1 | 100 |
| Pathogen | New Subclinical Mastitis | ||
|---|---|---|---|
| n | % 1 | % 2 | |
| Streptococcus uberis | 0/2 | 0 | 0 |
| NaS 3 | 5/24 | 20.8 | 12.8 |
| Staphylococcus aureus | 1/3 | 33.3 | 2.6 |
| Streptococcus dysgalactiae | 0/2 | 0 | 0 |
| Trueperella pyogenes | 0/2 | 0 | 0 |
| Escherichia coli | 0/0 | 0 | 0 |
| Coliforms | 0/0 | 0 | 0 |
| Klebsiella spp. | 1/1 | 100 | 2.6 |
| Bacillus spp. | 0/0 | 0 | 0 |
| Corynebacterium spp. | 2/50 | 4 | 5.1 |
| Enterococcus spp. | 1/2 | 50 | 2.6 |
| Pseudomonas spp. | 0/0 | 0 | 0 |
| Other streptococci | 2/4 | 50 | 5.1 |
| Lactic acid bacteria | 0/0 | 0 | 0 |
| Pasteurella spp. | 4/13 | 31 | 10.3 |
| Others 4 | 0/7 | 0 | 0 |
| Mixed | 1/12 | 8 | 2.6 |
| Contaminated 5 | 1/38 | 2.6 | 2.6 |
| No specific growth 6 | 21/229 | 9.3 | 53.8 |
| In total | 39/385 | 10.1 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Köllmann, K.; Zhang, Y.; Wente, N.; Lücken, A.; Leimbach, S.; Krömker, V. Effects of Suckling on the Udder Health of Foster Cows. Ruminants 2021, 1, 100-117. https://doi.org/10.3390/ruminants1020008
Köllmann K, Zhang Y, Wente N, Lücken A, Leimbach S, Krömker V. Effects of Suckling on the Udder Health of Foster Cows. Ruminants. 2021; 1(2):100-117. https://doi.org/10.3390/ruminants1020008
Chicago/Turabian StyleKöllmann, Katharina, Yanchao Zhang, Nicole Wente, Anneke Lücken, Stefanie Leimbach, and Volker Krömker. 2021. "Effects of Suckling on the Udder Health of Foster Cows" Ruminants 1, no. 2: 100-117. https://doi.org/10.3390/ruminants1020008
APA StyleKöllmann, K., Zhang, Y., Wente, N., Lücken, A., Leimbach, S., & Krömker, V. (2021). Effects of Suckling on the Udder Health of Foster Cows. Ruminants, 1(2), 100-117. https://doi.org/10.3390/ruminants1020008







