Physiological and Behavioral Reactions of Simmental Dairy Cows to Increasing Heat Load on Pasture
Abstract
1. Introduction
2. Materials and Methods
2.1. Farm Management and Animals
2.2. Data Collection and Sampling
2.2.1. Weather Conditions
2.2.2. Behavior Data (Monitoring System)
2.2.3. Milk Yield and Milk Composition
2.2.4. Respiration Rate
2.2.5. Fecal Cortisol
2.3. Statistical Analysis
3. Results
3.1. Weather Data
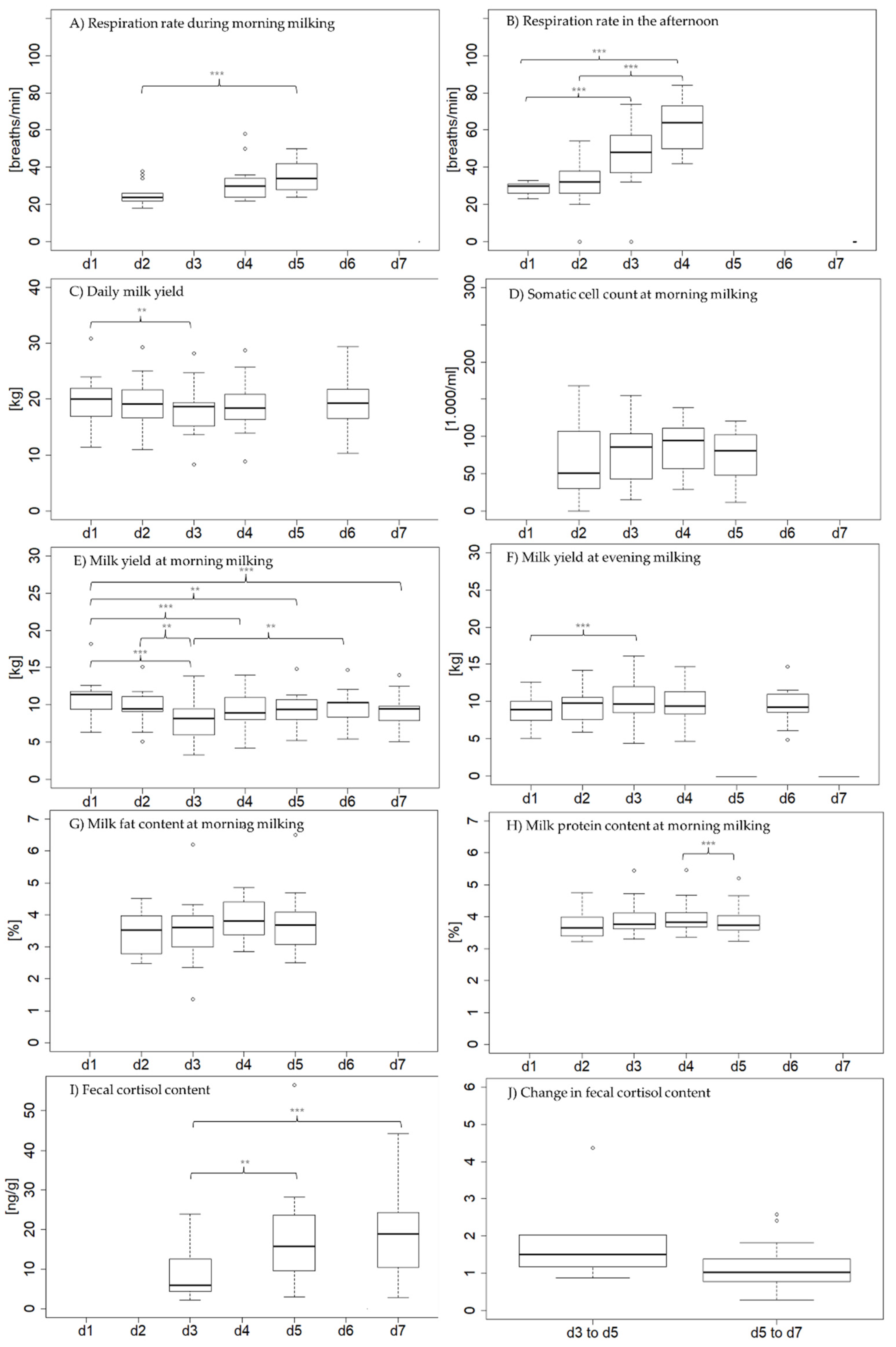
3.2. Physiological Parameters
3.3. Grazing Behavior
3.4. Ruminating Behavior
4. Discussion
4.1. Physiological Responses in Relation to Weather Conditions
4.2. Behavioral Changes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hammami, H.; Bormann, J.; M’hamdi, N.; Montaldo, H.H.; Gengler, N. Evaluation of heat stress effects on production traits and somatic cell score of Holsteins in a temperate environment. J. Dairy Sci. 2013, 96, 1844–1855. [Google Scholar] [CrossRef] [PubMed]
- Brügemann, K. Defining and evaluating heat stress thresholds in different dairy cow production systems. Arch. Tierz. 2012, 55, 13–24. [Google Scholar] [CrossRef]
- Carvalho, D.; Cardoso Pereira, S.; Rocha, A. Future surface temperatures over Europe according to CMIP6 climate projections: An analysis with original and bias-corrected data. Clim. Chang. 2021, 167, 10. [Google Scholar] [CrossRef]
- Wankar, A.K.; Rindhe, S.N.; Doijad, N.S. Heat stress in dairy animals and current milk production trends, economics, and future perspectives: The global scenario. Trop Anim. Health Prod. 2021, 53, 70. [Google Scholar] [CrossRef]
- Polsky, L.; von Keyserlingk, M.A.G. Invited review: Effects of heat stress on dairy cattle welfare. J. Dairy Sci. 2017, 100, 8645–8657. [Google Scholar] [CrossRef]
- Gantner, V.; Bobić, T.; Potočnik, K.; Gregić, M.; Kučević, D. Persistence of heat stress effect in dairy cows. Mljekarstvo/Dairy 2019, 69, 30–41. [Google Scholar] [CrossRef]
- Schüller, L.K.; Burfeind, O.; Heuwieser, W. Impact of heat stress on conception rate of dairy cows in the moderate climate considering different temperature-humidity index thresholds, periods relative to breeding, and heat load indices. Theriogenology 2014, 81, 1050–1057. [Google Scholar] [CrossRef]
- Gantner, V.; Kuterovac, K.; Potočnik, K. Effect of Heat Stress on Metabolic Disorders Prevalence Risk and Milk Production in Holstein Cows in Croatia. Ann. Anim. Sci. 2016, 16, 451–461. [Google Scholar] [CrossRef]
- Vitali, A.; Felici, A.; Lees, A.M.; Giacinti, G.; Maresca, C.; Bernabucci, U.; Gaughan, J.B.; Nardone, A.; Lacetera, N. Heat load increases the risk of clinical mastitis in dairy cattle. J. Dairy Sci. 2020, 103, 8378–8387. [Google Scholar] [CrossRef]
- Sanders, A.H.; Shearer, J.K.; de Vries, A. Seasonal incidence of lameness and risk factors associated with thin soles, white line disease, ulcers, and sole punctures in dairy cattle. J. Dairy Sci. 2009, 92, 3165–3174. [Google Scholar] [CrossRef]
- Thom, E.C. The Discomfort Index. Weatherwise 1959, 12, 57–61. [Google Scholar] [CrossRef]
- Buffington, D.E.; Collazo-Arocho, A.; Canton, G.H.; Pitt, D.; Thatcher, W.W.; Collier, R.J. Black Globe-Humidity Index (BGHI) as Comfort Equation for Dairy Cows. Trans. ASAE 1981, 24, 711–714. [Google Scholar] [CrossRef]
- Gebremedhin, K.G.; Lee, C.N.; Hillman, P.E.; Collier, R.J. Physiological Responses of Dairy Cows during Extended Solar Exposure. Trans. ASABE 2010, 53, 239–247. [Google Scholar] [CrossRef]
- Rashid, S.A.; Tomar, A.K.S.; Verma, M.R.; Mehrotra, S.; Bharti, P.K. Effect of skin and coat characteristics on growth and milk production traits in Tharparkar cattle. Indian J. Anim. Sci. 2019, 89, 1251–1254. [Google Scholar]
- Ji, B.; Banhazi, T.; Ghahramani, A.; Bowtell, L.; Wang, C.; Li, B. Modelling of heat stress in a robotic dairy farm. Part 2: Identifying the specific thresholds with production factors. Biosyst. Eng. 2020, 199, 43–57. [Google Scholar] [CrossRef]
- Ji, B.; Banhazi, T.; Ghahramani, A.; Bowtell, L.; Wang, C.; Li, B. Modelling of heat stress in a robotic dairy farm. Part 1: Thermal comfort indices as the indicators of production loss. Biosyst. Eng. 2020, 199, 27–42. [Google Scholar] [CrossRef]
- Abeni, F.; Galli, A. Monitoring cow activity and rumination time for an early detection of heat stress in dairy cow. Int. J. Biometeorol. 2017, 61, 417–425. [Google Scholar] [CrossRef]
- Navrátil, S.; Falta, D.; Chládek, G. Effect of Temperature Cumulation on Milk Yield of Czech Fleckvieh-Simmental Cattle. Acta Univ. Agric. Silvic. Mendel. Brun. 2017, 65, 1579–1584. [Google Scholar] [CrossRef]
- Heinicke, J.; Hoffmann, G.; Ammon, C.; Amon, B.; Amon, T. Effects of the daily heat load duration exceeding determined heat load thresholds on activity traits of lactating dairy cows. J. Therm. Biol. 2018, 77, 67–74. [Google Scholar] [CrossRef]
- Abdeltalif, A.M.; Alameen, A.O. Influence of Season and Pregnangy on Thermoregulation and Haematological Profile in Crossbred Dairy Cows in Tropical Environment. Glob. Vet. 2012, 9, 334–340. [Google Scholar] [CrossRef]
- Heinicke, J.; Ibscher, S.; Belik, V.; Amon, T. Cow individual activity response to the accumulation of heat load duration. J. Therm. Biol. 2019, 82, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Van Laer, E.; Tuyttens, F.A.M.; Ampe, B.; Sonck, B.; Moons, C.P.H.; Vandaele, L. Effect of summer conditions and shade on the production and metabolism of Holstein dairy cows on pasture in temperate climate. Animal 2015, 9, 1547–1558. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, J.; Peng, D.; Li, G.; Chen, J.; Gu, X. Exposure to heat-stress environment affects the physiology, circulation levels of cytokines, and microbiome in dairy cows. Sci. Rep. 2018, 8, 14606. [Google Scholar] [CrossRef] [PubMed]
- McManus, C.M.; Louvandini, H.; Paim, T.P.; Silva, F.C.P.; Bernal, F.E.M. Factors affecting heat tolerance in crossbred cattle in central Brazil. Ciênc. Anim. Bras. 2014, 15, 152–158. [Google Scholar] [CrossRef][Green Version]
- Vizzotto, E.F.; Fischer, V.; Thaler Neto, A.; Abreu, A.S.; Stumpf, M.T.; Werncke, D.; Schmidt, F.A.; McManus, C.M. Access to shade changes behavioral and physiological attributes of dairy cows during the hot season in the subtropics. Animal 2015, 9, 1559–1566. [Google Scholar] [CrossRef] [PubMed]
- Herbut, P.; Angrecka, S. The effect of heat stress on time spent lying by cows in a housing system. Ann. Anim. Sci. 2018, 18, 825–833. [Google Scholar] [CrossRef]
- Tucker, C.B.; Rogers, A.R.; Schütz, K.E. Effect of solar radiation on dairy cattle behaviour, use of shade and body temperature in a pasture-based system. Appl. Anim. Behav. Sci. 2008, 109, 141–154. [Google Scholar] [CrossRef]
- Eslamizad, M.; Lamp, O.; Derno, M.; Kuhla, B. The control of short-term feed intake by metabolic oxidation in late-pregnant and early lactating dairy cows exposed to high ambient temperatures. Physiol. Behav. 2015, 145, 64–70. [Google Scholar] [CrossRef]
- Fraser, D.; Weary, D.M.; Pajor, E.; Milligan, B.N. A Scientific Conception of Animal Welfare that Reflects Ethical Concerns. Anim. Welf. 1997, 6, 187–205. [Google Scholar]
- Von Keyserlingk, M.A.G.; Rushen, J.; de Passillé, A.M.; Weary, D.M. Invited review: The welfare of dairy cattle-key concepts and the role of science. J. Dairy Sci. 2009, 92, 4101–4111. [Google Scholar] [CrossRef]
- Berckmans, D. Precision livestock farming technologies for welfare management in intensive livestock systems. Rev. Sci. Tech. OIE 2014, 33, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.D.; Hall, L.W.; Collier, R.J.; Smith, J.F. Effect of core body temperature, time of day, and climate conditions on behavioral patterns of lactating dairy cows experiencing mild to moderate heat stress. J. Dairy Sci. 2015, 98, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Arias, R.A.; Delgado, C.; Keim, J.P.; Gandarillas, M. Use of the Comprehensive Climate Index to estimate heat stress response of grazing dairy cows in a temperate climate region. J. Dairy Res. 2021, 88, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.A.; Aghalari, A.; Marufuzzaman, M.; Stone, A.E. Predicting dairy cattle heat stress using machine learning techniques. J. Dairy Sci. 2021, 104, 501–524. [Google Scholar] [CrossRef]
- Karimi, M.T.; Ghorbani, G.R.; Kargar, S.; Drackley, J.K. Late-gestation heat stress abatement on performance and behavior of Holstein dairy cows. J. Dairy Sci. 2015, 98, 6865–6875. [Google Scholar] [CrossRef]
- Boonsanit, D.; Chanpongsang, S.; Chaiyabutr, N. Effects of supplemental recombinant bovine somatotropin and mist-fan cooling on the renal tubular handling of sodium in different stages of lactation in crossbred Holstein cattle. Res. Vet. Sci. 2012, 93, 417–426. [Google Scholar] [CrossRef]
- Ortiz, X.A.; Smith, J.F.; Rojano, F.; Choi, C.Y.; Bruer, J.; Steele, T.; Schuring, N.; Allen, J.; Collier, R.J. Evaluation of conductive cooling of lactating dairy cows under controlled environmental conditions. J. Dairy Sci. 2015, 98, 1759–1771. [Google Scholar] [CrossRef]
- Schütz, K.E.; Rogers, A.R.; Poulouin, Y.A.; Cox, N.R.; Tucker, C.B. The amount of shade influences the behavior and physiology of dairy cattle. J. Dairy Sci. 2010, 93, 125–133. [Google Scholar] [CrossRef]
- Calamari, L.; Petrera, F.; Stefanini, L.; Abeni, F. Effects of different feeding time and frequency on metabolic conditions and milk production in heat-stressed dairy cows. Int. J. Biometeorol. 2013, 57, 785–796. [Google Scholar] [CrossRef]
- Steinwidder, A.; Gasteiner, J. 8 Tipps Gegen Hitzestress bei Milchkühen. Available online: https://landwirt-media.com/8-tipps-gegen-hitzestress-bei-milchkuehen/ (accessed on 16 September 2021).
- Galán, E.; Llonch, P.; Villagrá, A.; Levit, H.; Pinto, S.; Del Prado, A. A systematic review of non-productivity-related animal-based indicators of heat stress resilience in dairy cattle. PLoS ONE 2018, 13, e0206520. [Google Scholar] [CrossRef]
- National Research Council (U.S.). A Guide to Environmental Research on Animals; National Academy of Sciences: Washington, DC, USA, 1971; ISBN 0309018692 9780309018692. [Google Scholar]
- Hajizadeh, R.; Farhang Dehghan, S.; Golbabaei, F.; Jafari, S.M.; Karajizadeh, M. Offering a model for estimating black globe temperature according to meteorological measurements. Met. Apps 2017, 24, 303–307. [Google Scholar] [CrossRef]
- Lawrence, M.G. The Relationship between Relative Humidity and the Dewpoint Temperature in Moist Air: A Simple Conversion and Applications. Bull. Am. Meteorol. Soc. 2005, 86, 225–234. [Google Scholar] [CrossRef]
- Schmeling, L.; Elmamooz, G.; Nicklas, D.; Rauch, E.; Thurner, S. Evaluation of a sensor-based system for monitoring rumination in dairy cows with access to pasture. In Proceedings of the EurAgEng 2021 Conference, New Challaneges for Agricultural Engineering towards a Digital World, Online, 5–8 July 2021; University of Évora: Évora, Portugal, 2021; pp. 154–155. [Google Scholar]
- Schmeling, L.; Thurner, S.; Nicklas, D.; Erhard, M.; Rauch, E. Automated detection of grazing behaviour with a collar-based monitoring system. In Sensing-New Insights into Grassland and Science and Practice, Proceedings of the 21st Symposium of the European Grassland Federation, 21st Symposium of the European Grassland Federation, Online, 17–19 May 2021; Astor, T., Dzene, I., Eds.; EGF: Witzenhausen, Germany, 2021; pp. 193–195. ISBN 978-3-00-068789-1. [Google Scholar]
- German Federal Ministry of Food and Agriculture. Verordnung zur Fortenwicklung des Rohmilchgüterechts: RohmilchGütV. Available online: https://www.bgbl.de/xaver/bgbl/text.xav?SID=&tf=xaver.component.Text_0&tocf=&qmf=&hlf=xaver.component.Hitlist_0&bk=bgbl&start=%2F%2F*%5B%40node_id%3D%27817545%27%5D&skin=pdf&tlevel=-2&nohist=1 (accessed on 8 September 2021).
- Abeni, F.; Calamari, L.; Stefanini, L. Metabolic conditions of lactating Friesian cows during the hot season in the Po valley. 1. Blood indicators of heat stress. Int. J. Biometeorol. 2007, 52, 87–96. [Google Scholar] [CrossRef]
- Pesenhofer, G.; Palme, R.; Pesenhofer, R.M.; Kofler, J. Comparison of two methods of fixation during functional claw trimming-walk-in crush versus tilt table-in dairy cows using faecal cortisol metabolite concentrations and daily milk yield as parameters. Wien. Tierärztliche Mitschrift 2006, 93, 288–294. [Google Scholar]
- Palme, R.; Robia, C.; Messmann, S.; Hofer, J.; Möstl, E. Measurement of faecal cortisol metabolites in ruminants: A non-invasive parameter of adrenocortical function. Wien. Tierärztliche Mitschrift 1999, 86, 237–241. [Google Scholar]
- Morrow, C.J.; Kolver, E.S.; Verkerk, G.A.; Matthews, L.R. Fecal glucocorticoid metabolites as a measure of adrenal activity in dairy cattle. Gen. Comp. Endocrinol. 2002, 126, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Geischeder, S. Auswirkungen von Hitzestress auf Milchkühe der Rasse Fleckvieh unter Bayerischen Klimabedingungen und Einfluss Einer Unterstützungslüftung Durch Ventilatoren. Ph.D. Thesis, Ludwig-Maximilians-Universität München, München, Germany, 2017. [Google Scholar]
- Palme, R.; Möstl, E. Measurement of cortisol metabolites in faeces of sheep as a parameter of cortisol concentration in blood. In Proceedings of the 1st International Symposium on Physiology and Ethology of Wild and Zoo Animals, 1st International Symposium on Physiology and Ethology of Wild and Zoo Animals, Berlin, Germany, 18–21 September 1996; Deutsche Gesellschaft Für Säugetierkunde EV Berlin, European Association of Zoos and Aquaria, Ed.; Gustav Fischer Verlag: Berlin, Germany, 1997; pp. 192–197. [Google Scholar]
- Elischer, M.F.; Arceo, M.E.; Karcher, E.L.; Siegford, J.M. Validating the accuracy of activity and rumination monitor data from dairy cows housed in a pasture-based automatic milking system. J. Dairy Sci. 2013, 96, 6412–6422. [Google Scholar] [CrossRef]
- Giraudoux, P. Pgirmess: Spatial Analysis and Data Mining for Field Ecologists: R Package Version 1.7.0. 2021. Available online: https://CRAN.R-project.org/package=pgirmess (accessed on 8 September 2021).
- Signorell, A.; Aho, K.; Alfons, A.; Anderegg, N.; Aragon, T.; Arachchige, C.; Arppe, A.; Baddeley, A.; Barton, K.; Bolker, B.; et al. DescTools: Tools for Descriptive Statistics: R Package Version 0.99.42. 2021. Available online: https://cran.r-project.org/package=DescTools (accessed on 8 September 2021).
- Crump, A.; Jenkins, K.; Bethell, E.J.; Ferris, C.P.; Arnott, G. Pasture Access Affects Behavioral Indicators of Wellbeing in Dairy Cows. Animals 2019, 9, 902. [Google Scholar] [CrossRef]
- Fleiss, J.L. Measuring nominal scale agreement among many raters. Psychol. Bull. 1971, 76, 378–382. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159. [Google Scholar] [CrossRef]
- Schmeling, L.; Elmamooz, G.; Hoang, P.T.; Kozar, A.; Nicklas, D.; Sünkel, M.; Thurner, S.; Rauch, E. Training and Validating a Machine Learning Model for the Sensor-based Monitoring of Lying Behavior in Dairy Cows on Pasture and in the Barn. Animals 2021, 11, 2660. [Google Scholar] [CrossRef] [PubMed]
- Zimbelman, R.B.; Rhoads, R.; Rhoads, M.L.; Duff, G.C.; Baumgard, L.H.; Collier, R.J. A Re-Evaluation of the Impact of Temperature Humidity Index (THI) and Black Globe Humidity Index (BGHI) on Milk Production in High Producing Dairy Cows. In Proceedings of the Southwest Nutrition and Management Conference, Southwest Nutrition and Management Conference, American Registry of Professional Animal Scientists, Tempe, AZ, USA, 26–27 February 2009; Southwest Nutrition Conference: Phoenix, AZ, USA, 2009; pp. 158–168. [Google Scholar]
- Dalcin, V.C.; Fischer, V.; Daltro, D.D.S.; Alfonzo, E.P.M.; Stumpf, M.T.; Kolling, G.J.; da Silva, M.V.G.B.; McManus, C. Physiological parameters for thermal stress in dairy cattle. R. Bras. Zootec. 2016, 45, 458–465. [Google Scholar] [CrossRef]
- Kibler, H.H. Environmental Physiology and Shelter Engineering: LXVII. Thermal Effects of Various Temperature-Humidity Combinations on Holstein Cattle as Measured by Eight Physiological Responses; Research Bulletin No. 862: Columbia, MO, USA, 1964; Available online: https://mospace.umsystem.edu/xmlui/handle/10355/58200 (accessed on 8 September 2021).
- Gorniak, T.; Meyer, U.; Südekum, K.-H.; Dänicke, S. Impact of mild heat stress on dry matter intake, milk yield and milk composition in mid-lactation Holstein dairy cows in a temperate climate. Arch. Anim. Nutr. 2014, 68, 358–369. [Google Scholar] [CrossRef]
- Gantner, V. The differences in heat stress resistance due to dairy cattle breed. Mljekarstvo/Dairy 2017, 62, 112–122. [Google Scholar] [CrossRef][Green Version]
- Collier, R.J.; Baumgard, L.H.; Zimbelman, R.B.; Xiao, Y. Heat stress: Physiology of acclimation and adaptation. Anim. Front. 2019, 9, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.D.; Roberts, N.; Bluett, S.J.; Verkerk, G.A.; Matthews, L.R. Effects of shade provision on the behaviour, body temperature and milk production of grazing dairy cows during a New Zealand summer. N. Z. J. Agric. Res. 2008, 51, 99–105. [Google Scholar] [CrossRef]
- Suzuki, T.; Kamiya, Y.; Tanaka, M.; Hattori, I.; Sakaigaichi, T.; Terauchi, T.; Nonaka, I.; Terada, F. Effect of fiber content of roughage on energy cost of eating and rumination in Holstein cows. Anim. Feed. Sci. Technol. 2014, 196, 42–49. [Google Scholar] [CrossRef]
- Ternman, E.; Pastell, M.; Hänninen, L.; Agenäs, S.; Nielsen, P.P. First-night effect on sleep time in dairy cows. PLoS ONE 2018, 13, e0195593. [Google Scholar] [CrossRef]
- Gregorini, P.; DelaRue, B.; McLeod, K.; Clark, C.; Glassey, C.B.; Jago, J. Rumination behavior of grazing dairy cows in response to restricted time at pasture. Livest. Sci. 2012, 146, 95–98. [Google Scholar] [CrossRef]
- Flury, R.; Gygax, L. Daily patterns of synchrony in lying and feeding of cows: Quasi-natural state and (anti-) synchrony factors. Behav. Processes 2016, 133, 56–61. [Google Scholar] [CrossRef]

| Day | Time of Day | DBT [°C] | RH [%] | RAD [W/m2] | WS [m/s] | THI | BGHI |
|---|---|---|---|---|---|---|---|
| 0 | 06:00–12:00 | 20 | 66 | 180 | 2.0 | 67 | 65 |
| 12:00–18:00 | 23 | 45 | 213 | 2.4 | 69 | 70 | |
| 18:00–24:00 | 18 | 66 | 39 | 1.5 | 63 | 60 | |
| 00:00–06:00 | 13 | 78 | 0 | 1.5 | 56 | 52 | |
| daily average | 19 | 64 | 105 | 1.8 | 64 | 62 | |
| 1 | 06:00–12:00 | 19 | 59 | 79 | 2.8 | 64 | 62 |
| 12:00–18:00 | 23 | 39 | 128 | 3.1 | 68 | 68 | |
| 18:00–24:00 | 19 | 52 | 48 | 3.3 | 64 | 62 | |
| 00:00–06:00 | 14 | 82 | 0 | 1.8 | 57 | 52 | |
| daily average | 19 | 58 | 64 | 2.8 | 63 | 61 | |
| 2 | 06:00–12:00 | 16 | 73 | 189 | 2.1 | 61 | 59 |
| 12:00–18:00 | 22 | 51 | 356 | 2.5 | 68 | 71 | |
| 18:00–24:00 | 16 | 78 | 73 | 3.0 | 61 | 57 | |
| 00:00–06:00 | 10 | 97 | 0 | 0.6 | 51 | 47 | |
| daily average | 16 | 75 | 155 | 2.0 | 60 | 58 | |
| 3 | 06:00–12:00 | 18 | 72 | 63 | 1.7 | 63 | 60 |
| 12:00–18:00 | 25 | 53 | 190 | 3.3 | 72 | 73 | |
| 18:00–24:00 | 19 | 83 | 59 | 3.2 | 65 | 61 | |
| 00:00–06:00 | 16 | 95 | 0 | 1.0 | 61 | 56 | |
| daily average | 20 | 76 | 78 | 2.3 | 65 | 62 | |
| 4 | 06:00–12:00 | 22 | 73 | 83 | 1.9 | 69 | 66 |
| 12:00–18:00 | 27 | 50 | 224 | 3.8 | 75 | 76 | |
| 18:00–24:00 | 18 | 86 | 27 | 2.8 | 64 | 60 | |
| 00:00–06:00 | 16 | 96 | 0 | 0.6 | 62 | 57 | |
| daily average | 21 | 76 | 83 | 2.3 | 67 | 65 |
| Day | Time of Day | DBT [°C] | RH [%] | RAD [W/m2] | WS [m/s] | THI | BGHI |
|---|---|---|---|---|---|---|---|
| 0 | 18:00–24:00 | 20 | 68 | 82 | 0.7 | 66 | 64 |
| 00:00–06:00 | 17 | 83 | 0 | 0.3 | 61 | 56 | |
| daily average | - 7 | - 7 | - 7 | - 7 | - 7 | - 7 | |
| 1 | 06:00–12:00 | 17 | 78 | 128 | 0.9 | 62 | 60 |
| 12:00–18:00 | 19 | 74 | 315 | 0.7 | 64 | 64 | |
| 18:00–24:00 | 18 | 74 | 91 | 0.5 | 63 | 60 | |
| 00:00–06:00 | 15 | 85 | 0 | 0.6 | 58 | 54 | |
| daily average | 17 | 78 | 133 | 0.7 | 62 | 59 | |
| 2 | 06:00–12:00 | 15 | 79 | 258 | 0.7 | 59 | 58 |
| 12:00–18:00 | 22 | 49 | 632 | 0.9 | 68 | 75 | |
| 18:00–24:00 | 20 | 63 | 101 | 0.6 | 65 | 63 | |
| 00:00–06:00 | 15 | 78 | 0 | 0.9 | 58 | 54 | |
| daily average | 18 | 67 | 248 | 0.8 | 63 | 63 | |
| 3 | 06:00–12:00 | 16 | 77 | 216 | 1.0 | 60 | 59 |
| 12:00–18:00 | 27 | 42 | 721 | 1.1 | 73 | 84 | |
| 18:00–24:00 | 25 | 51 | 115 | 0.8 | 72 | 72 | |
| 00:00–06:00 | 19 | 67 | 0 | 1.6 | 65 | 61 | |
| daily average | 22 | 59 | 263 | 1.1 | 68 | 69 | |
| 4 | 06:00–12:00 | 19 | 70 | 247 | 1.3 | 65 | 65 |
| 12:00–18:00 | 30 | 39 | 738 | 1.1 | 77 | 89 | |
| 18:00–24:00 | 28 | 45 | 111 | 0.8 | 74 | 76 | |
| 00:00–06:00 | 20 | 77 | 0 | 0.6 | 67 | 62 | |
| daily average | 24 | 58 | 274 | 0.9 | 71 | 73 | |
| 5 | 06:00–12:00 | 21 | 72 | 46 | 2.3 | 68 | 65 |
| 12:00–18:00 | 19 | 89 | 26 | 2.7 | 66 | 61 | |
| 18:00–24:00 | 18 | 96 | 9 | 1.6 | 65 | 59 | |
| 00:00–06:00 | 16 | 99 | 0 | 0.7 | 60 | 55 | |
| daily average | 19 | 89 | 46 | 1.8 | 65 | 60 | |
| 6 | 06:00–12:00 | 19 | 80 | 126 | 2.6 | 64 | 62 |
| 12:00–18:00 | 21 | 59 | 155 | 4.9 | 68 | 66 | |
| 18:00–24:00 | 16 | 78 | 11 | 1.8 | 61 | 57 | |
| 00:00–06:00 | 12 | 91 | 0 | 1.0 | 54 | 50 | |
| daily average | 17 | 77 | 73 | 2.5 | 62 | 59 | |
| 7 | 06:00–12:00 | 16 | 76 | 167 | 0.9 | 60 | 58 |
| 12:00–18:00 | 20 | 50 | 487 | 2.6 | 66 | 70 | |
| 18:00–24:00 | 15 | 81 | 23 | 0.9 | 59 | 55 |
| d1 | d2 | d3 | d4 | |||||
|---|---|---|---|---|---|---|---|---|
| Ø | SEM | Ø | SEM | Ø | SEM | Ø | SEM | |
| round 1 | ||||||||
| Grazing time/24 h [h] | 8.9 a | 0.37 | 7.8 b | 0.39 | 7.9 a,b | 0.30 | - 1 | |
| Grazing time/12 h daytime [h] | 4.9 a | 0.22 | 3.9 b | 0.25 | 4.1 a,b | 0.19 | - 1 | |
| Grazing time/12 h nighttime [h] | 4.0 | 0.16 | 3.9 | 0.20 | 3.8 | 0.17 | - 1 | |
| Rumination time/24 h [h] | 7.1 | 0.25 | 6.8 | 0.33 | 7.1 | 0.17 | - 1 | |
| Rumination time/12 h daytime [h] | 2.7 | 0.34 | 2.6 | 0.26 | 3.0 | 0.13 | - 1 | |
| Rumination time/12 h nighttime [h] | 4.4 | 0.12 | 4.2 | 0.14 | 4.1 | 0.06 | - 1 | |
| round 2 | ||||||||
| Grazing time/24 h [h] | 7.0 | 0.34 | 6.8 | 0.48 | 7.1 | 0.38 | 6.4 | 0.38 |
| Grazing time/12 h daytime [h] | 3.7 a,b | 0.22 | 4.6 a | 0.21 | 4.3 a,b | 0.26 | 2.9 b | 0.28 |
| Grazing time/12 h nighttime [h] | 3.3 a,b | 0.14 | 2.2 a | 0.30 | 2.8 a,b | 0.19 | 3.5 b | 0.13 |
| Rumination time/24 h [h] | 7.0 | 0.31 | 7.5 | 0.19 | 7.4 | 0.26 | 8.2 | 0.14 |
| Rumination time/12 h daytime [h] | 2.9 a,b | 0.14 | 2.3 a | 0.14 | 2.6 a,b | 0.16 | 3.6 b | 0.22 |
| Rumination time/12 h nighttime [h] | 4.1 b | 0.19 | 5.2 a | 0.19 | 4.8 a,b | 0.26 | 4.6 a,b | 0.19 |
| Round 1 | Round 2 | ||||||
|---|---|---|---|---|---|---|---|
| d1 | d2 | d3 | d1 | d2 | d3 | d4 | |
| Synchrony of grazing behavior within 24 h | 0.63 | 0.73 | 0.62 | 0.71 | 0.59 | 0.59 | 0.73 |
| Synchrony of grazing behavior during the day | 0.59 | 0.67 | 0.63 | 0.60 | 0.82 | 0.56 | 0.50 |
| Synchrony of grazing behavior during the night | 0.67 | 0.79 | 0.61 | 0.80 | 0.41 | 0.62 | 0.92 |
| Synchrony of ruminating behavior within 24 h | 0.30 | 0.32 | 0.43 | 0.45 | 0.42 | 0.39 | 0.27 |
| Synchrony of ruminating behavior during the day | 0.04 | 0.26 | 0.35 | 0.28 | 0.11 | 0.41 | 0.34 |
| Synchrony of ruminating behavior during the night | 0.38 | 0.31 | 0.48 | 0.52 | 0.40 | 0.34 | 0.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmeling, L.; Thurner, S.; Erhard, M.; Rauch, E. Physiological and Behavioral Reactions of Simmental Dairy Cows to Increasing Heat Load on Pasture. Ruminants 2022, 2, 157-172. https://doi.org/10.3390/ruminants2020010
Schmeling L, Thurner S, Erhard M, Rauch E. Physiological and Behavioral Reactions of Simmental Dairy Cows to Increasing Heat Load on Pasture. Ruminants. 2022; 2(2):157-172. https://doi.org/10.3390/ruminants2020010
Chicago/Turabian StyleSchmeling, Lara, Stefan Thurner, Michael Erhard, and Elke Rauch. 2022. "Physiological and Behavioral Reactions of Simmental Dairy Cows to Increasing Heat Load on Pasture" Ruminants 2, no. 2: 157-172. https://doi.org/10.3390/ruminants2020010
APA StyleSchmeling, L., Thurner, S., Erhard, M., & Rauch, E. (2022). Physiological and Behavioral Reactions of Simmental Dairy Cows to Increasing Heat Load on Pasture. Ruminants, 2(2), 157-172. https://doi.org/10.3390/ruminants2020010







