Can Associative Effects Affect In Vitro Digestibility Estimates Using Artificial Fermenters?
Abstract
1. Introduction
2. Materials and Methods
2.1. Incubations and Measurements
2.2. Statistical Analysis
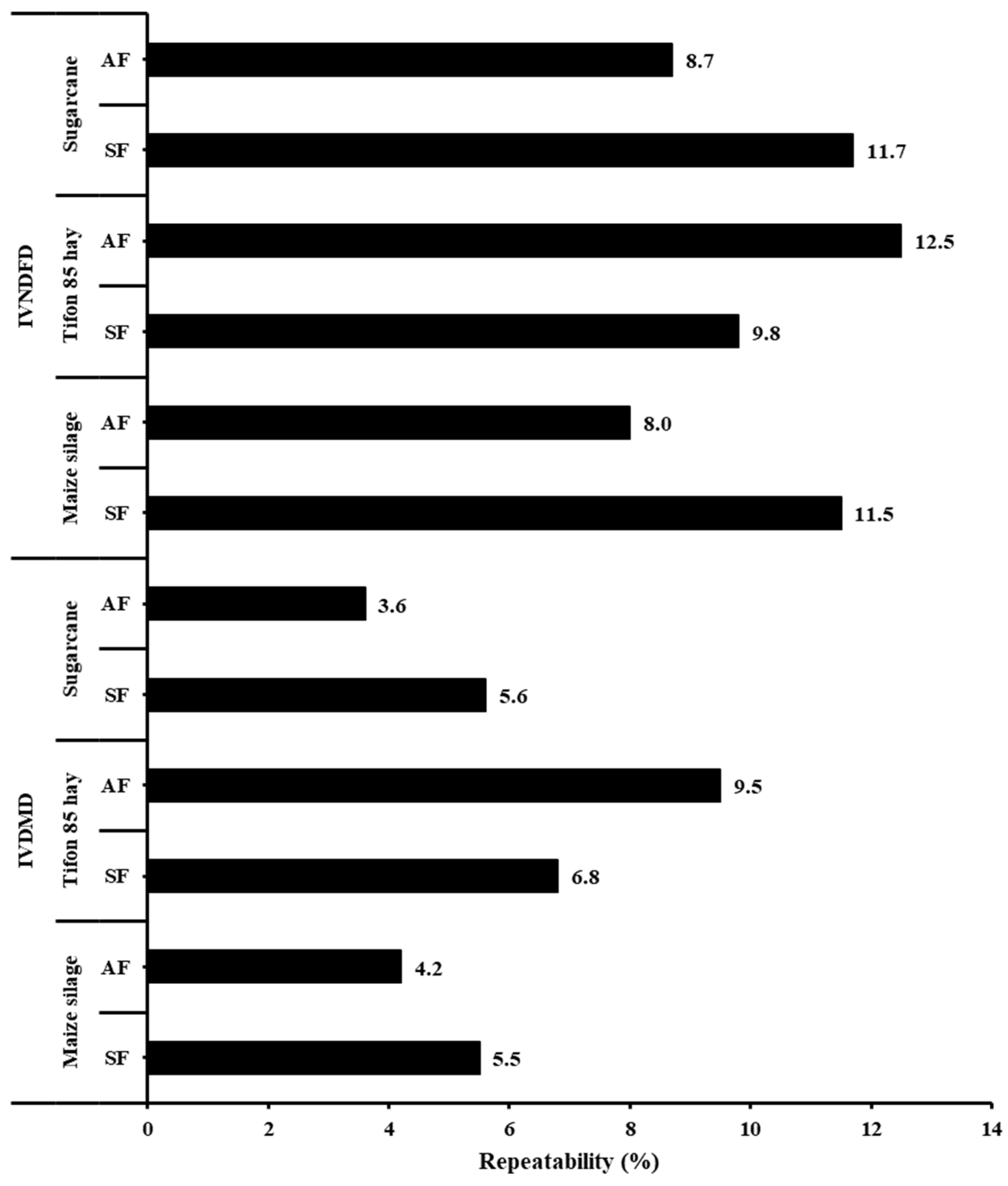
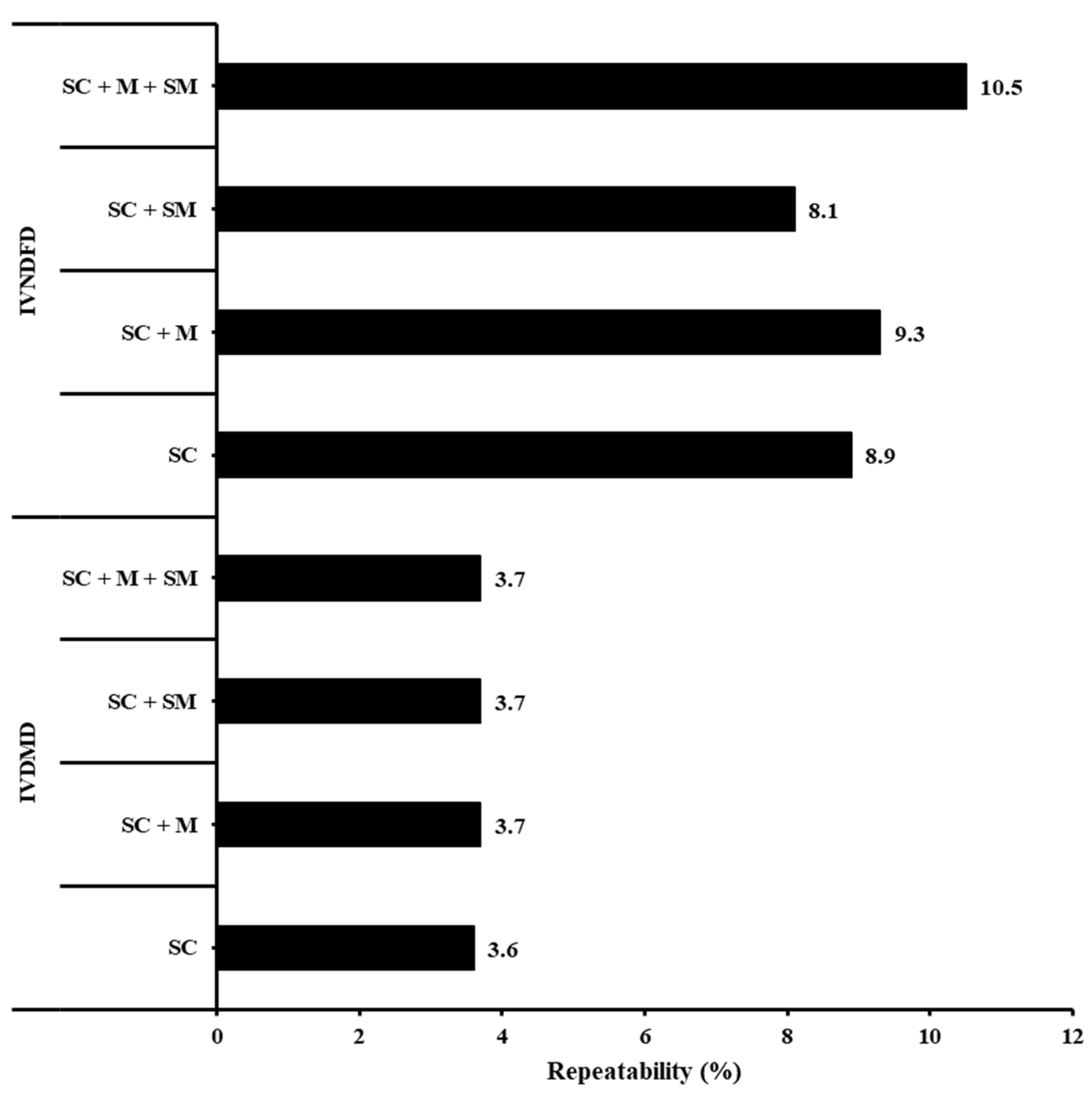
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tilley, J.M.A.; Terry, R.A. A two-stage technique for the in vitro digestion of forage crops. J. Br. Grassl. Soc. 1963, 18, 104–111. [Google Scholar] [CrossRef]
- Adesogan, A.T. What are feeds worth? A critical evaluation of selected nutritive values methods. In Proceedings of the 13th Annual Florida Ruminant Nutrition Symposium, Gainesville, University of Florida, Gainesville, FL, USA, 10–22 January 2002; pp. 33–47. [Google Scholar]
- Tassone, S.; Fortina, R.; Peiretti, P.G. In vitro techniques using the DaisyII incubator for the assessment of digestibility: A review. Animals 2020, 10, 775. [Google Scholar] [CrossRef] [PubMed]
- Camacho, L.F.; Silva, T.E.; Rodrigues, J.P.P.; Franco, M.O.; Detmann, E. A standard procedure for in vitro digestion using rumen fermenters: A collaborative study. Animals 2022, 12, 2842. [Google Scholar] [CrossRef] [PubMed]
- Lopes, F.C.F.; Dornelas, R.A.C.; Portugal, J.A.B.; Carneiro, J.C.; Verneque, R.S.; Oliveira, J.S.; Arcuri, P.B.; Duque, A.C.A. Dry matter digestibility of corn silages and concentrates determined by in vitro procedures. Arq. Bras. Med. Vet. Zootec. 2010, 62, 1167–1173. [Google Scholar] [CrossRef]
- Holden, L.A. Comparison of methods of in vitro dry matter digestibility for ten feeds. J. Dairy Sci. 1999, 82, 1791–1794. [Google Scholar] [CrossRef]
- Wilman, D.; Adesogan, A. A comparison of filter bags method with conventional tube methods of determining the in vitro digestibility of forages. Anim. Feed Sci. Technol. 2000, 84, 33–47. [Google Scholar] [CrossRef]
- Adesogan, A.T. Effect of bag type on the apparent digestibility of feeds in Ankom DaisyII incubators. Anim. Feed Sci. Technol. 2005, 119, 333–344. [Google Scholar] [CrossRef]
- Mould, F.L.; Kliem, K.E.; Morgan, R.; Mauricio, R.M. In vitro microbial inoculums: A review of its function and properties. Anim. Feed Sci. Technol. 2005, 123–124, 31–50. [Google Scholar] [CrossRef]
- Huhtanen, P.; Rinne, M.; Nousiainen, J. A meta-analysis of feed digestion in dairy cows. 2. The effects of feeding level and diet composition on digestibility. J. Dairy Sci. 2009, 92, 5031–5042. [Google Scholar] [CrossRef]
- Silva, T.E.; Detmann, E.; Camacho, L.F.; Saliba, E.O.S.; Palma, M.N.N.; Valadares Filho, S.C. Comparison of in vitro methods to quantify the dry matter and neutral detergent fibre digestibility of forages and concentrates. Arq. Bras. Med. Vet. Zootec. 2017, 69, 1635–1644. [Google Scholar] [CrossRef]
- Detmann, E.; Costa e Silva, L.F.; Rocha, G.C.; Palma, M.N.N.; Rodrigues, J.P.P. Métodos Para Análise de Alimentos, 2nd ed.; Suprema: Visconde do Rio Branco, Brazil, 2021; 350p. [Google Scholar]
- Valente, T.N.P.; Detmann, E.; Valadares Filho, S.C.; Da Cunha, M.; Queiroz, A.C.; Sampaio, C.B. In situ estimation of indigestible compounds contents in cattle feed and feces using bags made from different textiles. Rev. Bras. Zootecn. 2011, 40, 666–675. [Google Scholar] [CrossRef]
- McDougall, E.I. Studies on ruminant saliva. 1. The composition and output of sheep’s saliva. Bioch. J. 1948, 43, 99–109. [Google Scholar] [CrossRef]
- Camacho, L.F.; Silva, T.E.; Palma, M.N.N.; Assunção, A.S.; Rodrigues, J.P.; Costa e Silva, L.F.; Detmann, E. Evaluation of buffer solutions and urea addition for estimating the in vitro digestibility of feeds. J. Anim. Sci. 2019, 97, 922–931. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.G.; Detmann, E.; Mantovani, H.C.; Valadares Filho, S.C.; Bento, C.B.P.; Marcondes, M.I.; Assunção, A.S. Evaluation of the length of adaptation period for changeover and crossover nutritional experiments with cattle fed tropical forage-based diets. Anim. Feed Sci. Technol. 2016, 222, 133–148. [Google Scholar] [CrossRef]
- Barbosa, M.M.; Detmann, E.; Rocha, G.C.; Franco, M.O.; Valadares Filho, S.C. Evaluation of laboratory procedures to quantify the neutral detergent fiber content in forage, concentrate, and ruminant feces. J. AOAC Internat. 2015, 98, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Huhtanen, P. Associative effects of feeds in ruminants. Norw. J. Agric. Sci. 1991, 5, 37–57. [Google Scholar]
- Moss, A.R.; Givens, D.I.; Phipps, R.H. Digestibility and energy value of combinations of forage mixture. Anim. Feed Sci. Technol. 1992, 39, 151–172. [Google Scholar] [CrossRef]
- Ramin, M.; Vaga, M.; Cabezas-Garcia, E.H.; Detmann, E. Comparison of methane production from individual feeds and total diets—An in vitro evaluation. Agraarteadus 2016, 27, 42–47. [Google Scholar]
- Detmann, E.; Paulino, M.F.; Valadares Filho, S.C. Avaliação nutricional de alimentos ou dietas? In Uma Abordagem Conceitual, Proceedings of the 6th Simpósio de Produção de Gado de Corte, Viçosa, Brazil; Universidade Federal de Viçosa: Viçosa, Brazil, 22–24 May 2008; pp. 21–52. [Google Scholar]
- Michalet-Doreau, B.; Ould-Bah, M.Y. In vitro and in sacco methods for the estimation of dietary nitrogen degradability in the rumen: A review. Anim. Feed Sci. Technol. 1992, 40, 57–86. [Google Scholar] [CrossRef]
- Leng, R.A. Factors affecting the utilization of ‘poor-quality’ forages by ruminants particularly under tropical conditions. Nutr. Res. Rev. 1990, 3, 277–303. [Google Scholar] [CrossRef]
- Detmann, E.; Paulino, M.F.; Mantovani, H.C.; Valadares Filho, S.C.; Sampaio, C.B.; Souza, M.A.; Lazzarini, I.; Detmann, K.S.C. Parameterization of ruminal fibre degradation in low-quality tropical forage using Michaelis-Menten kinetics. Livest. Sci. 2009, 126, 136–146. [Google Scholar] [CrossRef]
- Russell, J.B.; Baldwin, R.L. Substrate preferences in rumen bacteria: Evidence of catabolite regulatory mechanisms. Appl. Environ. Microbiol. 1978, 36, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, I.P.C.; Detmann, E.; Mantovani, H.C.; Paulino, M.F.; Valadares Filho, S.C.; Costa, V.A.C.; Gomes, D.I. Growth and antimicrobial activity of lactic acid bacteria from rumen fluid according to energy or nitrogen source. Rev. Bras. Zootecn. 2011, 40, 1260–1265. [Google Scholar] [CrossRef]
- Mould, F.L.; Ørskov, E.R.; Manns, O. Associative effects of mixed feeds. I. Effects of type and level of supplementation and the influence of the rumen pH on cellulolysis in vivo and dry matter digestion of various roughages. Anim. Feed Sci. Technol. 1983, 10, 15–30. [Google Scholar] [CrossRef]
- Costa, V.A.C.; Detmann, E.; Valadares Filho, S.C.; Paulino, M.F.; Henriques, L.T.; Mantovani, H.C. In vitro degradation of neutral detergent fiber from high-quality tropical forage according to supplementation with protein and (or) carbohydrates. Rev. Bras. Zootecn. 2009, 38, 1803–1811. [Google Scholar] [CrossRef]
| CP | NDF | |
|---|---|---|
| Feeds | g/kg Dry Matter | |
| Sugarcane | 30.2 | 586 |
| Maize silage | 79.3 | 562 |
| Tifton 85 hay | 75.9 | 797 |
| Soybean meal | 515 | 233 |
| Maize grain | 84.0 | 198 |
| Incubation Condition 1 | |||
|---|---|---|---|
| Forage | Single Feed | All Feeds | p-Value |
| IVDMD (g/kg) | |||
| Sugarcane | 574 ± 20.2 (60) | 600 ± 21.3 (20) | 0.007 |
| Maize silage | 666 ± 20.2 (59) | 625 ± 21.3 (20) | <0.001 |
| Tifton 85 hay | 552 ± 20.3 (55) | 557 ± 21.3 (20) | 0.599 |
| IVNDFD (g/kg) | |||
| Sugarcane | 347 ± 21.4 (58) | 377 ± 23.1 (20) | 0.019 |
| Maize silage | 503 ± 21.3 (60) | 442 ± 23.1 (20) | <0.001 |
| Tifton 85 hay | 508 ± 21.4 (55) | 501 ± 23.1 (20) | 0.577 |
| Incubation Condition | IVDMD 1 | IVNDFD 1 |
|---|---|---|
| Sugarcane + maize + soybean meal | 660 ± 21.6 a (16) | 422 ± 31.5 a (18) |
| Sugarcane + soybean meal | 651 ± 21.2 a (29) | 418 ± 31.0 a (29) |
| Sugarcane + maize | 615 ± 21.2 b (29) | 370 ± 31.1 b (29) |
| Sugarcane | 641 ± 21.0 a (56) | 407 ± 30.6 a (57) |
| p-value | <0.001 | <0.001 |
| Ph 1 | NH3-N (mg/dL) 1 | |||
|---|---|---|---|---|
| Incubation Condition | Initial | Final | Initial | Final |
| Forages (Assay 1) | ||||
| Sugarcane | 7.01 | 6.76 | 0.47 | 5.73 |
| Maize silage | 6.99 | 6.77 | 0.62 | 6.27 |
| Tifton 85 hay | 7.00 | 6.72 | 0.29 | 4.03 |
| All forages together | 6.99 | 6.70 | 0.66 | 3.72 |
| p-value | ||||
| Incubation condition (IC) | 0.764 | 0.305 | ||
| Time (T) | <0.001 | <0.001 | ||
| IC × T | 0.809 | 0.350 | ||
| SEM | 0.040 | 0.762 | ||
| Forage plus concentrates (Assay 2) | ||||
| Sugarcane + soybean meal + maize | 6.91 | 6.69 ab | 1.90 | 8.12 ab |
| Sugarcane + soybean meal | 6.92 | 6.86 a | 2.04 | 13.59 a |
| Sugarcane + maize | 6.93 | 6.61 b | 2.54 | 4.05 b |
| Sugarcane | 6.90 | 6.63 b | 2.17 | 3.69 b |
| p-value | ||||
| Incubation condition (IC) | 0.009 | 0.039 | ||
| Time (T) | <0.001 | 0.001 | ||
| IC × T | 0.012 | 0.028 | ||
| SEM | 0.037 | 1.429 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camacho, L.F.; Silva, T.E.d.; Franco, M.d.O.; Detmann, E. Can Associative Effects Affect In Vitro Digestibility Estimates Using Artificial Fermenters? Ruminants 2023, 3, 100-110. https://doi.org/10.3390/ruminants3020009
Camacho LF, Silva TEd, Franco MdO, Detmann E. Can Associative Effects Affect In Vitro Digestibility Estimates Using Artificial Fermenters? Ruminants. 2023; 3(2):100-110. https://doi.org/10.3390/ruminants3020009
Chicago/Turabian StyleCamacho, Larissa Frota, Tadeu Eder da Silva, Marcia de Oliveira Franco, and Edenio Detmann. 2023. "Can Associative Effects Affect In Vitro Digestibility Estimates Using Artificial Fermenters?" Ruminants 3, no. 2: 100-110. https://doi.org/10.3390/ruminants3020009
APA StyleCamacho, L. F., Silva, T. E. d., Franco, M. d. O., & Detmann, E. (2023). Can Associative Effects Affect In Vitro Digestibility Estimates Using Artificial Fermenters? Ruminants, 3(2), 100-110. https://doi.org/10.3390/ruminants3020009







