Abstract
This study aimed to evaluate the impact of a vapor freezing protocol on antioxidant enzyme activity (superoxide dismutase (SOD) and glutathione reductase (GR)), sperm with active mitochondria, DNA damage, and spermatic parameters. Twenty-six semen samples from men undergoing infertility investigation were cryopreserved in liquid nitrogen (LN) vapors and plunged into LN, with (method A) and without (method B) a commercial sperm freezing medium (SFM) and inherent removal with a sperm preparation medium (SPM). Most parameters were assessed before and after freezing, except for SOD and GR activity, which were only assessed after freezing. Although method A promoted better results than method B, the percentage of spermatozoa with active mitochondria, motility, vitality, and normal morphology decreased significantly. DNA damage (determined by comet assay) increased similarly with both methods, but the percentage of spermatozoa with fragmented DNA (by TUNEL assay) remained similar to fresh values when method A was applied. GR activity was higher and SOD activity lower with method A. The addition of SFM coupled with the sperm wash with SPM seems essential to preserve the quality of most of the analyzed spermatic parameters and active mitochondria. The detrimental effects promoted by freezing were shown to depend on the quality of the fresh semen, according to correlation coefficients. Interestingly, thawed samples of both methods shared similar DNA damage. These results highlight the need to find more effective protocols, especially for the freezing of low-quality semen samples.
1. Introduction
Freezing with permeable cryoprotectants prevails, and several cryopreservation methods have emerged over the years. Although cryoprotectant’s mode of action is known, few studies have analyzed its effects in detail (reviewed in [1]). Additionally, an optimal and standardized protocol does not yet exist [2]. One of the most frequently applied protocols is the vapor freezing protocol (VFP), based on placing the samples with cryoprotectant in liquid nitrogen (LN) vapors (≈−80 °C) and then immersing the samples in LN (≈−196 °C), using cryovials or straws [3,4]. Some fertility centers also perform cooling at slower and gradual rates. The major problem with these techniques is the formation of ice crystals if freezing is too fast or cell shrinkage if freezing is too slow [5,6]. Given the disadvantages of those techniques, one of the most widely used protocols for human semen cryopreservation remains the VFP, particularly in academic research laboratories and small andrology laboratories with funding and equipment availability constraints.
However, this freezing procedure is not innocuous for sperm cells and may increase oxidative stress [7]. Oxidative stress per se can promote DNA damage and lead to low overall sperm quality. In previous work, we have already verified that correlations between antioxidant enzymatic activity, the effect of ROS on proteins and lipids, and the classic sperm analysis indicators seem to exist [8]. Therefore, the use of cryoprotectants prior to freezing can be helpful to assure a better semen quality after thawing, but the extent of this effect is not so well defined.
Therefore, the purpose of this pilot study was to perform a complete evaluation of the impact of one of the most applied VFPs using a commercial sperm freezing medium (SFM) and washing step with a sperm preparation medium (SPM) on antioxidant enzyme activity, spermatic parameters, DNA damage, and mitochondrial activity. We expect to obtain a better understanding of the impact of cryopreservation on sperm parameters associated with infertility and how they correlate by correlating all these variables for the first time.
2. Materials and Methods
2.1. Study Design and Sample Collection
This pilot study was performed on anonymous semen samples provided by adult men living in Trás-os-Montes and Alto Douro (Portugal) who attended fertility consultations at the Hospital Centre of Trás-os-Montes and Alto Douro (CHTMAD). From 100 participants, samples with a sperm concentration less than 2 × 106 spermatozoa/mL (SPZ/mL) and/or a volume less than 1.5 mL were not considered, resulting in 26 semen samples (from 35 ± 5-year-old men).
Immediately after liquefaction, part of the semen samples was used to assess the spermatic quality of the fresh samples. The other part of the samples was used to continue the experiment, which consisted of cryopreserving the sperm samples and re-evaluating the sperm quality after thawing. All 26 samples were cryopreserved using a VFP with an SFM and washed after thawing with an SPM to remove the SFM (method A (MA)). The first 13 samples were also cryopreserved using the same VFP without the SFM and the inherent washing step after thawing (method B (MB)). This allowed us to observe which sperm parameters benefit the most from the use of SFM and the washing step after thawing with an SPM.
The percentage of spermatozoa with active mitochondria, DNA integrity, and the spermatic parameters (motility, vitality, morphology, and concentration) were assessed before and after cryopreservation by MA and MB. Superoxide dismutase (SOD) and glutathione reductase (GR) activities were only evaluated after thawing the cryopreserved sperm samples from both studied protocols. The experimental design is shown in Figure 1.
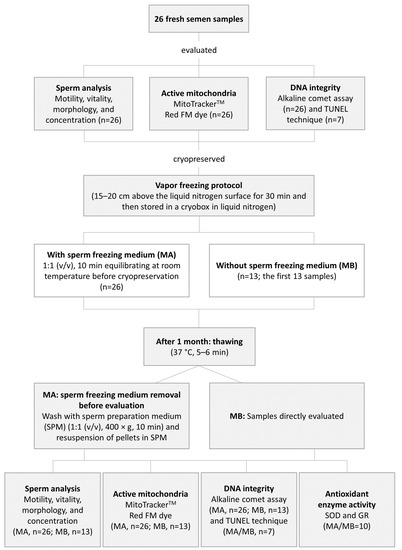
Figure 1.
Schematic diagram of the experimental design.
This study was approved by the ethics committee of the CHTMAD (434/2017—P.C.A). Participants were informed of the study, and informed consent and a statement of responsibility were obtained, following the Declaration of Helsinki.
2.2. Vapor Freezing and Thawing
Immediately after liquefaction, approximately 1.2–1.8 mL of the semen samples was used to test a VFP with (MA) and without (MB) SFM and sperm wash with SPM. For the first 13 semen samples, the semen was divided into 8 aliquots of 100–150 µL (4 for MA and 4 for MB) and for the other 13 samples, the semen was only divided into 4 aliquots of 100–150 µL for MA. For MA, the SFM (Sperm Freezing Medium; Origio, CooperSurgical®, Måløv, Denmark) was added dropwise to the cryovials (1:1 (v/v)) and carefully mixed. Mixtures were left at room temperature (RT) for 10 min to equilibrate. For method B, samples were left for 10 min at RT without the addition of any freezing medium. Then, all cryovials were frozen with a VFP. The VFP consisted of suspending the cryovials horizontally 15–20 cm above the LN surface for 30 min, transferring them to a cryobox, and then storing the box in the LN container (MVE CryoSystem 4000). After 1 month of storage, samples were thawed (37 °C, 5–6 min) and immediately processed. Samples with SFM (MA) underwent an additional step, compared to the aliquots cryopreserved by MB, for SFM removal. The SFM was removed by washing the samples with SPM (Sperm Preparation Medium, Origio, CooperSurgical®; 1:1 (v/v), 400× g, 10 min) and the pellets were resuspended in SPM to obtain the initial cryopreserved volume.
2.3. Semen Analysis
The semen analysis, supervised by internal and external quality controls (United Kingdom National External Quality Assessment Service for Reproductive Science, UK NEQAS, GameteExpert), was performed according to the World Health Organization guidelines (2010) [9] before and after cryopreservation by MA and MB for all samples. After 2–5 days of sexual abstinence, the semen samples were obtained by masturbation and collected into the provided sterile plastic container. The samples were analyzed after complete liquefaction at 37 °C within 1 h of ejaculation. Semen volume (mL) and pH (pH indicator strips MQuant®, Supelco®, Bellefonte, PA, USA) were measured. Manual evaluation of sperm motility (%) was performed and motility was classified into progressive (PR), non-progressive (NP), immotile, and total motility (PR + NP). Vitality (%) was evaluated by counting the percentage of viable spermatozoa (green) under a bright field microscope (400× magnification) after incubating 10 μL of the semen sample for 5 min with 10 μL of Eosin Y 0.5%. Sperm morphology (%) was analyzed after the Papanicolaou–Shörr staining procedure. For the staining procedure, one smear per sample was left to dry for at least 4 h. Then, the slides were immersed in methanol for 10 s, dried for 5 min, immersed in Papanicolaou stain (Merck) for 15 min, rinsed under running water, immersed in Shorr’s stain (Merck) for 5 min, rinsed under running water, immersed again in methanol for 10 sec, and then allowed to dry. After the definitive preparation with Entelan (Merck), spermatozoa were classified morphologically under a bright field microscope (1000× magnification). The percentage of morphologically normal spermatozoa and the percentage of spermatozoa with abnormal head/midpiece/tail and/or excess residual cytoplasm were registered. Each sample was tested at least in duplicate for all mentioned analyses and a total of 200 spermatozoa were evaluated. For the determination of sperm concentration (×106 SPZ/mL), samples were diluted with a fixative solution and, after incubation, at least 200 complete spermatozoa were counted in the central grid of a Neubauer chamber.
2.4. Percentage of Spermatozoa with Active Mitochondria
The percentage of spermatozoa with active mitochondria was assessed using the MitoTrackerTM Red FM (MTRFM) dye (ThermoFisher Scientific, Waltham, MA, USA) on 26 samples for MA and 13 samples for MB, as previously described (Figure S1) [10,11]. In brief, 10 × 106 SPZ/mL/sample were incubated (37 °C, 20 min, dark) with fresh secondary stock solution (10 µM) (primary stock solution (1 mM, in DMSO) mixed with PBS (pH 7.2)) at a final concentration of 300 nM. Then, two drops (10 μL) were slightly smeared, stained (5 μL, DAPI 125 ng/mL), and covered with a coverslip. After fluorescence stabilization (10 min, RT, dark), at least 200 spermatozoa/replicate were classified and the percentage of spermatozoa with active mitochondria/sample (midpiece with red fluorescence) was determined.
2.5. DNA Integrity
The DNA damage was assessed by the alkaline comet assay (ACA; Figure S2) and the percentage of spermatozoa with fragmented DNA from application of the terminal deoxynucleotidyl transferase dUTP nick-end labeling assay (TUNEL; Figure S3). Due to the scarcity of the sample, whenever we did not have enough volume to perform the two complementary techniques, we chose to perform the ACA since it is quantitative, provides more information than the TUNEL assay, and has shown to be better at predicting male infertility [12]. Therefore, the ACA was performed on 26 samples for MA and 13 samples for MB, and the TUNEL assay was performed on 7 samples for both methods.
2.5.1. ACA
The semen samples were processed based on Sipinen et al. (2010) [13]. In brief, two slides per sample were pre-coated with 1% normal melting point agarose (MPA). A total of 12 × 104 fresh SPZ/sample were washed with 1× PBS (1500× g, 10 min). Then, the pellet was mixed with 280 μL of low MPA (37 °C) and 70 μL was pipetted in duplicate onto each slide. Each gel was immediately covered by a coverslip. After 5 min at 4 °C, coverslips were removed and cells were lysed in two consecutive lysis solutions (4 °C, 1 h each). Lysis solution I consisted of 40 mL of a base lysis solution (2.5 M sodium chloride (NaCl); 0.1 M ethylenediamine tetraacetic acid (EDTA); 10 mM Tris Base; and 8 M sodium hydroxide (NaOH) to adjust the pH to 10.0) supplemented with 400 μL of Triton X and 1 mL of DTT (0.01 g/mL). After the first lysis, slides were placed in lysis solution II. This solution consisted of 40 mL of the base lysis solution, supplemented with 400 μL of Triton X and 100 μL of proteinase K (20 mg/mL). Then, slides remained in electrophoresis buffer for 30 min (1 L: 30 mL of 10 M NaOH, 10 mL of 0.1 M EDTA, and 960 mL of distilled water; pH 13.2) at 4 °C to unwind DNA and were subjected to electrophoresis at 17 V and 300 mA for 20 min (CSL-COM20, Cleaver Scientific Ltd., Rugby, UK; 31 × 34 × 9 cm, filled with 20 slides and 1.2 L of buffer). The slides were neutralized with 1× PBS for 10 min and then with distilled water for a further 10 min. After drying at RT, slides were stained (DAPI 125 ng/mL). A total of 50 comets/gel were analyzed by visual scoring on a scale of 0–400 arbitrary units (AU) according to Collins (2004) and represented in Figure S2 [14].
2.5.2. TUNEL
The TUNEL assay detects free 3′-OH ends of the DNA and was performed using the In Situ Cell Death Detection Kit (Roche Diagnostics, Basel, Switzerland), based on Muratori et al. (2000) [15]. For each sample, 150 μL of semen was washed three times in 1× PBS (pH 7.2), fixed in 4% paraformaldehyde, and frozen (−20 °C) until use. After thawing, the samples were centrifuged (500× g, 10 min) and two consecutive washes were carried out in 1× PBS supplemented with 1% BSA. The pellet was resuspended in permeabilizing solution for 2 min (2–8 °C) and two more washes were performed. Then, the samples were divided into 3 aliquots (sample, negative control, and positive control) and incubated with different solutions at 37 °C for 1 h in the dark. Both the sample and the negative control were incubated with 10 µL of TUNEL reaction (TR) solution, with and without the enzyme terminal deoxynucleotidyl transferase (TdT), respectively. For the positive control, the same protocol was used but with an additional treatment, where the samples were incubated (37 °C, 20 min) with 50 μL of recombinant DNase I before the reaction with the TR/TdT solution. After the 1h incubation, all aliquots underwent two consecutive washes (500× g, 10 min) to reduce nonspecific fluorescence and the pellet was resuspended in 25 µL of 1× PBS. For each sample, several drops were placed on a slide (overlapping) and dried. After staining with DAPI 125 ng/mL (4 °C, 15 min, dark), the percentage of spermatozoa with fragmented DNA (green fluorescence) was determined by observation of 400 SPZ/sample.
2.6. Antioxidant Enzymes Activity
SOD activity and glutathione reductase (GR) activity were assessed in 10 thawed samples cryopreserved by both methods. For samples cryopreserved using MA, the SFM was removed and the pellet was resuspended in ultra-pure deionized water. Samples cryopreserved using MB were directly assessed. All samples were sonicated (pulse 15 s, amplitude 70%, time 1 min), centrifuged (12,000× g, 15 min, 4 °C), and the supernatants were used in the following protocols. When needed, the supernatants were stored (−20 °C) for later assessment (Figure S4).
2.6.1. Superoxide Dismutase (SOD)
The activity of the superoxide dismutase enzyme was determined spectrophotometrically, according to the method described by Payá et al. (1992) [16], with some alterations. The molecule used for detection was nitrotetrazolium blue chloride (NBT). The reaction medium was composed of phosphate buffer, pH 7.4, NBT (10 mM), and hypoxanthine (10 mM). The sample supernatant (30 µL) was incubated at 25 °C (2 min) and the reaction was initiated by the addition of xanthine oxidase. SOD activity is expressed as U min-1 mg protein−1, which corresponds to the amount of SOD that inhibits 50% of the reduction of NBT.
2.6.2. Glutathione Reductase (GR)
The activity of the glutathione reductase enzyme was determined spectrophotometrically using an adapted method described by Carlberg and Mannervik (1985) [17]. The reaction medium was composed of phosphate buffer (pH 7.4) and sample supernatant (50 μL) to a final volume of 2 mL. The mixture was incubated at 25 °C (2 min) and then NADPH was added. The reaction started 1 min after with the addition of GSSG and was monitored (25 °C, 3 min, 340 nm). GR activity is expressed as µM NADPH/NADP+ min−1 mg protein−1.
2.6.3. Protein Quantification Assay
The total protein content of the samples was determined using a modified Bradford method [18], on a 96-well plate using BSA (2 mg/mL) as a standard.
2.7. Statistical Analysis
The statistical analysis was performed using IBM SPSS Statistics v25 software and a p-value ≤ 0.05 was considered statistically significant. Data were tested for normal distributions with the Shapiro–Wilk test. Considering that most of the parameters did not follow a normal distribution, only non-parametric tests were applied. For comparisons between the fresh raw samples and the post-thaw samples (groups A and B), the Wilcoxon signed-rank test was used. The strength of the correlation between the spermatic characteristics on fresh samples and the impact of MA on them was expressed using Spearman’s rank correlation coefficients (ρ).
3. Results
3.1. Spermatic Parameters
Cryopreservation by MA and MB resulted in a significant reduction in total sperm motility (A: decreased 65.2%, p < 0.001; B: decreased 98.5%, p = 0.001) and progressive motility (A: decreased 78.0%, p < 0.001; B: decreased 99.0%, p = 0.001). Only MB promoted a significant decrease in non-progressive motility (A: decreased 7.3%, p > 0.05; B: decreased 95.9%, p = 0.001) (Table 1). Motility was more affected by MB (p = 0.001, n = 13; Figure 2).

Table 1.
Spermatic parameters (motility, vitality, morphology, and sperm concentration) before and after cryopreservation with (method A) and without (method B) sperm freezing medium and inherent wash with sperm preparation medium 1.
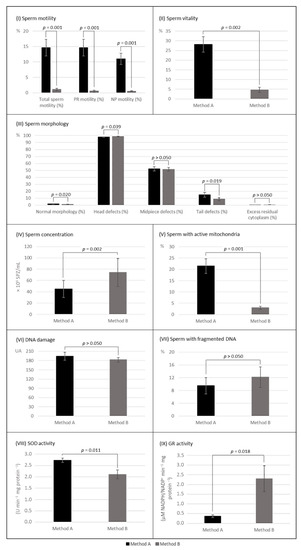
Figure 2.
Post-thaw parameters of sperm samples cryopreserved by both methods with (method A) and without (method B) Sperm Freezing Medium (mean values ± SEM). (I) Sperm motility, n = 13; (II) sperm vitality, n = 13; (III) sperm morphology, n = 13; (IV) sperm concentration, n = 13; (V) sperm with active mitochondria, n = 13; (VI) DNA damage assessed by comet assay, n = 13; (VII) sperm with fragmented DNA assessed by TUNEL assay, n = 7; (VIII) SOD activity, n = 10; and (IX) GR activity, n = 10. p ≤ 0.05 indicates a statistically significant difference between methods A and B. as determined by Wilcoxon signed-rank test. PR—progressive; NP—non-progressive.
Sperm vitality decreased significantly with cryopreservation (A: decreased 65.4%, p < 0.001; B: decreased 94.2%, p = 0.001) (Table 1) and was more affected by MB (p = 0.002) (Figure 2).
The percentage of morphologically normal spermatozoa decreased significantly with cryopreservation, regardless of the cryopreservation method (A: decreased 59.6%, p < 0.001; B: decreased 73.6%, p = 0.002) (Table 1) and was significantly more affected by MB (p = 0.020) (Figure 2).
Concerning morphological abnormalities, the excess residual cytoplasm decreased significantly after both cryopreservation methods (A: decreased 85.5%, p < 0.001; B: decreased 82.8%, p = 0.011) (Table 1). All other head, midpiece, and tail abnormalities increased in frequency significantly after the application of MA, while when applying MB, only the frequency of abnormal heads increased (p = 0.006) (Table 1). After thawing, samples of MA and MB differed significantly in the rate of abnormal heads (A: 98.5% vs. B: 98.8%, p = 0.039) and abnormal tails (A: 16.2% vs. B: 8.6%, p = 0.019), with a notable increase in coiled tails when applied MA (Figure 2). A representative figure can be found in the supplemental data file (Figure S5).
3.2. Percentage of Spermatozoa with Active Mitochondria
The percentage of spermatozoa with active mitochondria decreased with cryopreservation (A: decreased 67.6%, p < 0.001; B: decreased 95.8%, p = 0.001) (Table 2) and was significantly more affected when applied the MB (p = 0.002) (Figure 2).

Table 2.
Percentage of sperm with active mitochondria before and after cryopreservation with (method A) and without (method B) sperm freezing medium and inherent wash with sperm preparation medium 1.
3.3. DNA Integrity
The DNA damage, as assessed with the ACA, increased significantly after both cryopreservation methods (A: increased 95.3%, p < 0.001; B: increased 88.9%, p = 0.001) (Table 3). MA and MB promoted similar DNA damage on the thawed samples (p > 0.05) (Figure 2). Regarding the assessment of the percentage of spermatozoa with fragmented DNA using the TUNEL assay, due to limitations in the volume of semen samples only seven were evaluated. Although the percentage of spermatozoa with fragmented DNA only increased significantly when MB was applied (A: increased 71.7%, p > 0.05; B: increased 119.9%, p = 0.043) (Table 3), thawed samples of both MA and MB shared similar results (p > 0.05) (Figure 2).

Table 3.
DNA damage, assessed by alkaline comet assay and TUNEL assay, before and after cryopreservation with (method A) and without (method B) sperm freezing medium and inherent wash with sperm preparation medium 1.
3.4. Antioxidant Enzymes Activity
SOD activity was significantly higher in the samples cryopreserved by MA (A: 2.7 vs. B: 2.1 U min−1 mg protein−1, p = 0.011). Interestingly, the GR activity was significantly higher on the samples cryopreserved by MB (A: 0.4 vs. B: 2.3 µM NADPH/NADP+ min−1 mg protein−1, p = 0.018). GR activity varied more between samples cryopreserved by MA and MB than SOD activity (Figure 2).
3.5. The Influence of Spermatic Quality on Cryopreservation
The impact of cryopreservation by MA on the spermatic parameters was shown to be influenced by the quality of the fresh sperm samples. Based on correlations, it was observed that the higher the progressive motility (ρ = −0.659, p < 0.01) or the percentage of spermatozoa with active mitochondria (ρ = −0.428, p < 0.05) in fresh samples, the less non-progressive motility decreases with cryopreservation. Furthermore, the higher the percentage of morphologically normal spermatozoa in fresh samples, the less progressive motility (ρ = −0.474), total motility (ρ = −0.431), and vitality (ρ = −0.466) are affected by cryopreservation (p < 0.05). On the other hand, the higher the non-progressive motility in fresh samples (p < 0.01), the more total motility (ρ = 0.557), vitality (ρ = 0.564), and percentage of spermatozoa with active mitochondria (ρ = 0.652) are affected by cryopreservation. In addition, the higher the level of DNA fragmentation in fresh samples, the more progressive motility (ρ = 0.599, p < 0.01), total motility (ρ = 0.539, p < 0.01), and vitality (ρ = 0.495, p < 0.05) decrease with cryopreservation.
4. Discussion
Human semen cryopreservation is an everyday practice in assisted reproduction and VFPs with commercial cryoprotectants and a washing step after cryopreservation with sperm medium are commonly applied. The SFM used in this protocol contains glycerol and sucrose as cryoprotectant agents, but it also contains numerous constituents that promote better sperm protection and recovery common to the composition of SPM. One of these constituents is human albumin solution, which operates as an antioxidant, a scavenger of overabundant ions, and a reservoir for fatty acids, vitamins, and cholesterols. Sodium bicarbonate is also present to simulate in vivo conditions, which promotes better motility and supports sperm capacitation, as is synthetic serum replacement, which is a pro-survival factor for spermatozoa [19,20]. Similar media are used to cryopreserve and wash sperm samples; therefore, we expected better results when using MA. However, to understand the extent of the usefulness of both procedures, we also performed MB, where none of the media and respective procedures were applied. This study differs from previous studies since it allows us to observe the impact and importance of adding cryoprotectants and washing the samples with an SPM after cryopreservation based on a global analysis of the sperm quality. Simultaneously considering key factors in semen samples allowed for a better understanding of how the factors are related and how they influence the impact that cryopreservation has on sperm samples.
Overall, regardless of the freezing protocol applied in our study, the analyzed spermatic parameters worsened with freezing, especially with MB. The drastic results obtained with MB were similar to those of Lusignan et al. (2018) [21]. The only parameter that improved was the residual cytoplasm, which decreased, probably as a consequence of their release from spermatozoa due to ice crystals formed during freezing. As expected, sperm motility, vitality, normal morphology, and head defects were less affected when SFM and sperm wash was applied (MA). These results are consistent with other works, such as the study of Raad et al. (2018) [22], where the impact on progressive motility and head defects was comparable to that observed in the present study. However, two parameters were more affected by MA. Compared to MB, we observed (1) more coiled tails, which has also been reported in two other studies that evaluated sperm morphology [23] and ultrastructural morphology [24] after a rapid freezing protocol in nitrogen vapors. and (2) a major sperm concentration decrease. The latter may be due to the SFM removal process. Especially in viscous samples, the spermatozoa did not always form a compact cell pellet, which hampered the washing of the sample and may have contributed to a loss in spermatozoa when aspirating the supernatant.
Therefore, the SFM and/or the washing step with SPM help, but they are not a perfect solution. It has been proposed that the main reason for cell changes during the freezing of extracellular fluids results from the formation of ice crystals [24]. Additionally, solute exchanges, promoted by the addition and removal of the cryoprotectant, cause swelling and cellular shrinkage, which can be intolerable for most organelles [7] and cause the destruction of the plasma membrane in the spermatozoa tail [24].
Mitochondria are an important energy source for sperm, necessary for good motility and fertilization capacity, and their integrity is essential for proper cell function [25]. Using the MTRFM probe, we observed that the percentage of spermatozoa with active mitochondria decreased significantly after both VFPs, with worse results in MB. Still, MA promoted less damage to mitochondria (decreased by 68%) than the VFP presented by Pabón et al. (2019) (decreased by 80%) [26], where semen with cryoprotectant was frozen on the surface of dry ice and pellets stored in cryotubes immersed in LN. We noticed that both methods led to a greater decrease in progressive motility than in the percentage of spermatozoa with active mitochondria. This probably occurred not only as a consequence of mitochondrial damage and changes in the membrane integrity and sperm chromatin but also due to the increase in morphological defects, namely in the tail.
The influence of cryopreservation on sperm DNA is still a topic of discussion [4]. Some authors argue that damage generally increases [27], while others argue that it only increases in infertile men [28] or that it has no impact on DNA damage [29]. In our research, we employed two tools to assess DNA integrity: TUNEL and ACA. They detect distinct types of DNA damage and hence complement one another. TUNEL is a qualitative assay that only detects DNA fragmentation and, therefore, it is mostly used to assess apoptosis (reviewed by [27]). On the other hand, the comet assay is a quantitative method for classifying DNA damage in AU. In addition to determination of the presence of DNA single and double-strand breaks, it is more sensitive than TUNEL, as it identifies lower levels of damage which can be repaired after fertilization. Additionally, since we performed the ACA instead of the neutral comet assay (NCA), we were able to detect other forms of damage, such as alkali-labile sites (e.g., apurinic and apyrimidinic sites) not detected by TUNEL [14,30,31]. The ideal scenario would be to perform both techniques on all samples to obtain an overview of what would be occurring during the cryopreservation process and to indirectly validate one technique with the other. However, because only seven samples could be analyzed by the TUNEL assay, the results must be interpreted carefully. Our results showed that MB caused a substantial increase in both TUNEL and ACA-measured DNA damage, but MA only promoted a significant increase in ACA-measured DNA damage. Nevertheless, the DNA damage assessed on thawed samples A and B did not differ substantially. This suggests that while MA appears to be more protective than MB, the overall DNA damage following cryopreservation seems to be identical whether SFM is applied or not. Furthermore, the increase in DNA damage was overall more substantial when assessed via ACA, rather than the TUNEL assay. As a result, we hypothesized that this VFP causes mostly DNA damage that can be repaired (as quantified by ACA) and not necessarily apoptosis (as detected by the TUNEL assay).
Levels of reactive oxygen species, oxidized and reduced glutathione, and antioxidant enzymes such as glutathione reductase determine the most suitable conditions for redox control within a cell. As we were interested in comparing the antioxidant activity of the semen samples after both protocols and found no other studies that analyzed this, GR and SOD activities were also assessed. GR catalyzes the reduction of oxidized glutathione (GSSG) to glutathione (GSH), which is the main endogenous antioxidant in most cells [32]. The excessive production of reactive oxygen species during sperm freezing and thawing [33] will lead to the oxidation of endogenous GSH. The SFM contains glycine, known for scavenging radicals [34] and those that form during cryopreservation [35]. Samples cryopreserved by MA were subjected to the SFM removal without resuspending the spermatozoa in the SPM for this analysis and showed lower GR activity and higher SOD activity than samples cryopreserved by MB. Seminal fluid was not eliminated for this analysis. GR is responsible for keeping cell glutathione reduced. Since the SFM provides better antioxidant protection, we were expecting no excessive oxidation of GSH. Samples treated with MA also had a greater number of functional mitochondria. The mitochondrial electron transport chain is the major source of superoxide anion (O2−•), during cellular metabolism [36]. Thus, it is admissible that during thawing and manipulation of the sample, there is an increase in superoxide ions that may subsequently induce SOD activity.
The impact of cryopreservation on the different sperm parameters remains controversial and different results are obtained by researchers. This may be due to the different cryoprotectants and sperm evaluation techniques used, as well as variations in the freezing and thawing protocols. The diverse groups (fertile vs. infertile) and the sample size may also promote these dissimilarities. We controlled the dissimilarities in our work since we used the same VFP conditions, but the impact on sperm characteristics varied between the samples. Thus, in our study, the obtained Spearman’s correlation coefficients demonstrated that not only the freezing protocols, but also the quality of the fresh sperm samples influence post-thaw sperm quality. Overall, the better the percentage of sperm with active mitochondria, the DNA integrity, and the spermatic parameters of fresh samples, the less they were affected by cryopreservation. Several works support that variations in the capacity of resistance to cryopreservation may be due to differences in the phospholipids, glycolipids, and sterol content of the sperm membrane [37], chromatin stability [38], and seminal plasma antioxidant capacity [39].
We are aware that the sample size is sometimes too small to draw definite conclusions about some variables. With our pilot study, we aimed to verify the tendencies for each sperm parameter and its correlations with cryopreservation, with and without SFM and SPM, hoping to draw cohesive internal conclusions. Although our results should be considered exploratory due to the small n in some groups, we believe it remains a proof of concept in nature.
5. Conclusions
Regardless of the SFM use and inherent wash and resuspension of the spermatozoa in SPM, freezing showed a detrimental effect on the sperm quality which we demonstrated to be related to the quality of the fresh semen samples. The use of SFM and SPM on the VFP seems to be essential for conserving the quality of most of the analyzed spermatic parameters and active mitochondria, but not so important when it comes to DNA integrity, since both protocols promoted a relatively similar negative impact. The two VFPs and subsequent sample treatments performed in this analysis induced differences in the antioxidant response. The increase in SOD observed in samples A could mean a greater production of superoxide radicals and a consequent cellular response for its neutralization. As for samples B, the observed increase in GR activity could indicate a greater involvement of GSH in response to cryopreservation. This aspect justifies clarification, even though the cellular regulation of the glutathione system is highly complex and involves a sophisticated signaling network that is not entirely understood. Our results highlight the need to find more effective cryopreservation methods. This is particularly important when cryopreserving sperm samples from infertile patients, where the reduced initial quality makes it more difficult to obtain viable and intact spermatozoa after the freeze–thaw cycle.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/physiologia2030008/s1, Figure S1: representative scheme of the protocol applied to assess the percentage of spermatozoa with active mitochondria, using the MitoTrackerTM Red FM (MTRFM) dye; Figure S2: representative scheme of the protocol applied to assess the sperm DNA damage by alkaline comet assay; Figure S3: representative scheme of the protocol applied to assess the percentage of spermatozoa with fragmented DNA with the terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay; Figure S4: representative scheme of the protocol applied to assess the activity of the antioxidant enzymes superoxide dismutase (SOD) and glutathione reductase (GR); and Figure S5: sperm morphology assessment after Papanicolaou–Shörr staining of a sperm sample cryopreserved by method A (MA) (1000× magnification). A large number of spermatozoa with coiled tails (circled in blue) are evident when MA is applied.
Author Contributions
Conceptualization, P.P.-P., B.C. and R.P.-L.; data curation, P.P.-P.; formal analysis, P.P.-P.; funding acquisition, I.G., F.P., O.M., B.C. and R.P.-L.; investigation, P.P.-P.; methodology, P.P.-P., B.C. and R.P.-L.; project administration, B.C. and R.P.-L.; resources, P.P.-P., I.G., F.P., Z.G., M.B., B.C. and R.P.-L.; supervision, B.C. and R.P.-L.; validation, R.A.-R., I.G., F.P., B.C. and R.P.-L.; writing—original draft, P.P.-P.; writing—review and editing, P.P.-P., R.A.-R., I.G., F.P., B.C. and R.P.-L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Centro Hospitalar de Trás-os-Montes e Alto Douro and by the Portuguese Foundation for Science and Technology (FCT) under the grants UIDB/CVT/00772/2020 (CECAV), UIDB/04033/2020 (CITAB), UIDB/00616/2020 (CQ-VR), UIDP/00616/2020 (CQ-VR), and PEst-OE/QUI/UI0616/2020 (CQ-VR). The authors also thank FCT for the Ph.D. grant SFRH/BD/146867/2019 and the grant EXPL/CVT-CVT/1112/2021.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the Hospital de Trás-os-Montes e Alto Douro (434/2017—P.C.A, 26 October 2017).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Due to the nature of this research, participants of this study did not agree for their data to be shared publicly, so supporting data is not available.
Acknowledgments
The authors thank Jorge Colaço for his assistance with the statistical analysis.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Best, B.P. Cryoprotectant Toxicity: Facts, Issues, and Questions. Rejuvenation Res. 2015, 18, 422–436. [Google Scholar] [CrossRef] [PubMed]
- Majzoub, A.; Argawal, A. Preface. In The Complete Guide to Male Fertility Preservation; Springer International: Cham, Switzerland, 2018. [Google Scholar]
- Creemers, E.; Nijs, M.; Vanheusden, E.; Ombelet, W. Cryopreservation of human sperm: Efficacy and use of a new nitrogen-free controlled rate freezer versus liquid nitrogen vapour freezing. Andrologia 2011, 43, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Talwar, P.; Ghosh, P. Sperm Freezing Injuries. In The Complete Guide to Male Fertility Preservation; Springer International: Cham, Switzerland, 2018; pp. 205–226. [Google Scholar]
- Gupta, S.; Sharma, R.; Agarwal, A. The Process of Sperm Cryopreservation, Thawing and Washing Techniques. In The Complete Guide to Male Fertility Preservation; Majzoub, A., Argawal, A., Eds.; Springer International Springer International: Cham, Switzerland, 2018; pp. 183–204. [Google Scholar]
- Nallella, K.P.; Sharma, R.K.; Allamaneni, S.S.; Aziz, N.; Agarwal, A. Cryopreservation of human spermatozoa: Comparison of two cryopreservation methods and three cryoprotectants. Fertil. Steril. 2004, 82, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, C.; Forell, F.; Oliveira, A.; Rodrigues, J. Current status of sperm cryopreservation: Why isn’t it better? Theriogenology 2002, 57, 327–344. [Google Scholar] [CrossRef]
- Lopes, F.; Pinto-Pinho, P.; Gaivão, I.; Martins-Bessa, A.; Gomes, Z.; Moutinho, O.; Oliveira, M.M.; Peixoto, F.; Pinto-Leite, R. Sperm DNA damage and seminal antioxidant activity in subfertile men. Andrologia 2021, 53, e14027. [Google Scholar] [CrossRef]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen; WHO: Geneva, Switzerland, 2010. [Google Scholar]
- Amaral, A.; Ramalho-Santos, J. Assessment of mitochondrial potential: Implications for the correct monitoring of human sperm function. Int. J. Androl. 2010, 33, e180–e186. [Google Scholar] [CrossRef]
- Invitrogen. MitoTracker® Mitochondrion—Selective Probes; Thermo Fisher Scientific: Waltham, MA, USA, 2008. [Google Scholar]
- Ribas-Maynou, J.; García-Peiró, A.; Fernández-Encinas, A.; Abad, C.; Amengual, M.J.; Prada, E.; Navarro, J.; Benet, J. Comprehensive analysis of sperm DNA fragmentation by five different assays: TUNEL assay, SCSA, SCD test and alkaline and neutral Comet assay. Andrology 2013, 1, 715–722. [Google Scholar] [CrossRef]
- Sipinen, V.; Laubenthal, J.; Baumgartner, A.; Cemeli, E.; Linschooten, J.O.; Godschalk, R.W.L.; Van Schooten, F.J.; Anderson, D.; Brunborg, G. In vitro evaluation of baseline and induced DNA damage in human sperm exposed to benzo[a]pyrene or its metabolite benzo[a]pyrene-7,8-diol-9,10-epoxide, using the comet assay. Mutagenesis 2010, 25, 417–425. [Google Scholar] [CrossRef]
- Collins, A.R. The Comet Assay for DNA Damage and Repair: Principles, Applications, and Limitations. Mol. Biotechnol. 2004, 26, 249–261. [Google Scholar] [CrossRef]
- Muratori, M.; Piomboni, P.; Baldi, E.; Filimberti, E.; Pecchioli, P.; Moretti, E.; Gambera, L.; Baccetti, B.; Biagiotti, R.; Forti, G.; et al. Functional and Ultrastructural Features of DNA-Fragmented Human Sperm. J. Androl. 2000, 21, 903–912. [Google Scholar]
- Payá, M.; Halliwell, B.; Hoult, J. Interactions of a series of coumarins with reactive oxygen species: Scavenging of superoxide, hypochlorous acid and hydroxyl radicals. Biochem. Pharmacol. 1992, 44, 205–214. [Google Scholar] [CrossRef]
- Carlberg, I.; Mannervik, B. [59] Glutathione reductase. Methods Enzymol. 1985, 113, 484–490. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- CooperSurgical. Sperm Freezing Medium. Available online: https://fertility.coopersurgical.com/art_media/sperm-freezing-medium/ (accessed on 30 May 2020).
- CooperSurgical. Sperm Preparation Medium. Available online: https://fertility.coopersurgical.com/art_media/sperm-preparation-medium/ (accessed on 30 May 2020).
- Lusignan, M.F.; Li, X.; Herrero, B.; Delbes, G.; Chan, P.T.K. Effects of different cryopreservation methods on DNA integrity and sperm chromatin quality in men. Andrology 2018, 6, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Raad, G.; Lteif, L.; Lahoud, R.; Azoury, J.; Azoury, J.; Tanios, J.; Hazzouri, M.; Azoury, J. Cryopreservation media differentially affect sperm motility, morphology and DNA integrity. Andrology 2018, 6, 836–845. [Google Scholar] [CrossRef] [PubMed]
- Agha-Rahimi, A.; Khalili, M.A.; Nabi, A.; Ashourzadeh, S. Vitrification is not superior to rapid freezing of normozoospermic spermatozoa: Effects on sperm parameters, DNA fragmentation and hyaluronan binding. Reprod. Biomed. Online 2014, 28, 352–358. [Google Scholar] [CrossRef][Green Version]
- Ozkavukcu, S.; Erdemli, E.; Isik, A.; Oztuna, D.; Karahuseyinoglu, S. Effects of cryopreservation on sperm parameters and ultrastructural morphology of human spermatozoa. J. Assist. Reprod. Genet. 2008, 25, 403–411. [Google Scholar] [CrossRef]
- O’Connell, M.; McClure, N.; Lewis, S.E.M. The effects of cryopreservation on sperm morphology, motility and mitochondrial function. Hum. Reprod. 2002, 17, 704–709. [Google Scholar] [CrossRef]
- Pabón, D.; Meseguer, M.; Sevillano, G.; Cobo, A.; Romero, J.L.; Remohi, J.; de Los Santos, M.J. A new system of sperm cryopreservation: Evaluation of survival, motility, DNA oxidation, and mitochondrial activity. Andrology 2019, 7, 293–301. [Google Scholar] [CrossRef]
- Thomson, L.K.; Fleming, S.D.; Aitken, R.J.; De Iuliis, G.N.; Zieschang, J.A.; Clark, A.M. Cryopreservation-induced human sperm DNA damage is predominantly mediated by oxidative stress rather than apoptosis. Hum. Reprod. 2009, 24, 2061–2070. [Google Scholar] [CrossRef]
- Kalthur, G.; Adiga, S.K.; Upadhya, D.; Rao, S.; Kumar, P. Effect of cryopreservation on sperm DNA integrity in patients with teratospermia. Fertil. Steril. 2008, 89, 1723–1727. [Google Scholar] [CrossRef] [PubMed]
- Isachenko, V.; Isachenko, E.; Katkov, I.I.; Montag, M.; Dessole, S.; Nawroth, F.; Van Der Ven, H. Cryoprotectant-Free Cryopreservation of Human Spermatozoa by Vitrification and Freezing in Vapor: Effect on Motility, DNA Integrity, and Fertilization Ability. Biol. Reprod. 2004, 71, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Majtnerová, P.; Roušar, T. An overview of apoptosis assays detecting DNA fragmentation. Mol. Biol. Rep. 2018, 45, 1469–1478. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.R.; Oscoz, A.A.; Brunborg, G.; Gaivao, I.; Giovannelli, L.; Kruszewski, M.; Smith, C.C.; Štětina, R. The comet assay: Topical issues. Mutagenesis 2008, 23, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Gregg, X.T.; Prchal, J.T. Red Blood Cell Enzymopathies. In Hematology; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Chatterjee, S.; Gagnon, C. Production of reactive oxygen species by spermatozoa undergoing cooling, freezing, and thawing. Mol. Reprod. Dev. 2001, 59, 451–458. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, J.; Chen, L.; Li, J.; Zhang, H.; Guo, X. Glycine Suppresses AGE/RAGE Signaling Pathway and Subsequent Oxidative Stress by Restoring Glo1 Function in the Aorta of Diabetic Rats and in HUVECs. Oxidative Med. Cell. Longev. 2019, 2019, 4628962. [Google Scholar] [CrossRef]
- Ghorbani, M.; Vatannejad, A.; Khodadadi, I.; Amiri, I.; Tavilani, H. Protective effects of glutathione supplementation against oxidative stress during cryopreservation of human spermatozoa. Cryoletters 2016, 37, 34–40. [Google Scholar]
- Boveris, A.; Chance, B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem. J. 1973, 134, 707–716. [Google Scholar] [CrossRef]
- Vutyavanich, T.; Piromlertamorn, W.; Nunta, S. Rapid freezing versus slow programmable freezing of human spermatozoa. Fertil. Steril. 2010, 93, 1921–1928. [Google Scholar] [CrossRef]
- Choucair, F.B.; Rachkidi, E.G.; Raad, G.C.; Saliba, E.M.; Zeidan, N.S.; Jounblat, R.A.; Abou Jaoude, I.F.; Hazzouri, M.M. High level of DNA fragmentation in sperm of Lebanese infertile men using Sperm Chromatin Dispersion test. Middle East Fertil. Soc. J. 2016, 21, 269–276. [Google Scholar] [CrossRef]
- Thomson, L.K.; Fleming, S.D.; Schulke, L.; Barone, K.; Zieschang, J.-A.; Clark, A.M. The DNA integrity of cryopreserved spermatozoa separated for use in assisted reproductive technology is unaffected by the type of cryoprotectant used but is related to the DNA integrity of the fresh separated preparation. Fertil. Steril. 2009, 92, 991–1001. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).