Remodeling in Persistent Atrial Fibrillation: Pathophysiology and Therapeutic Targets—A Systematic Review
Abstract
1. Introduction
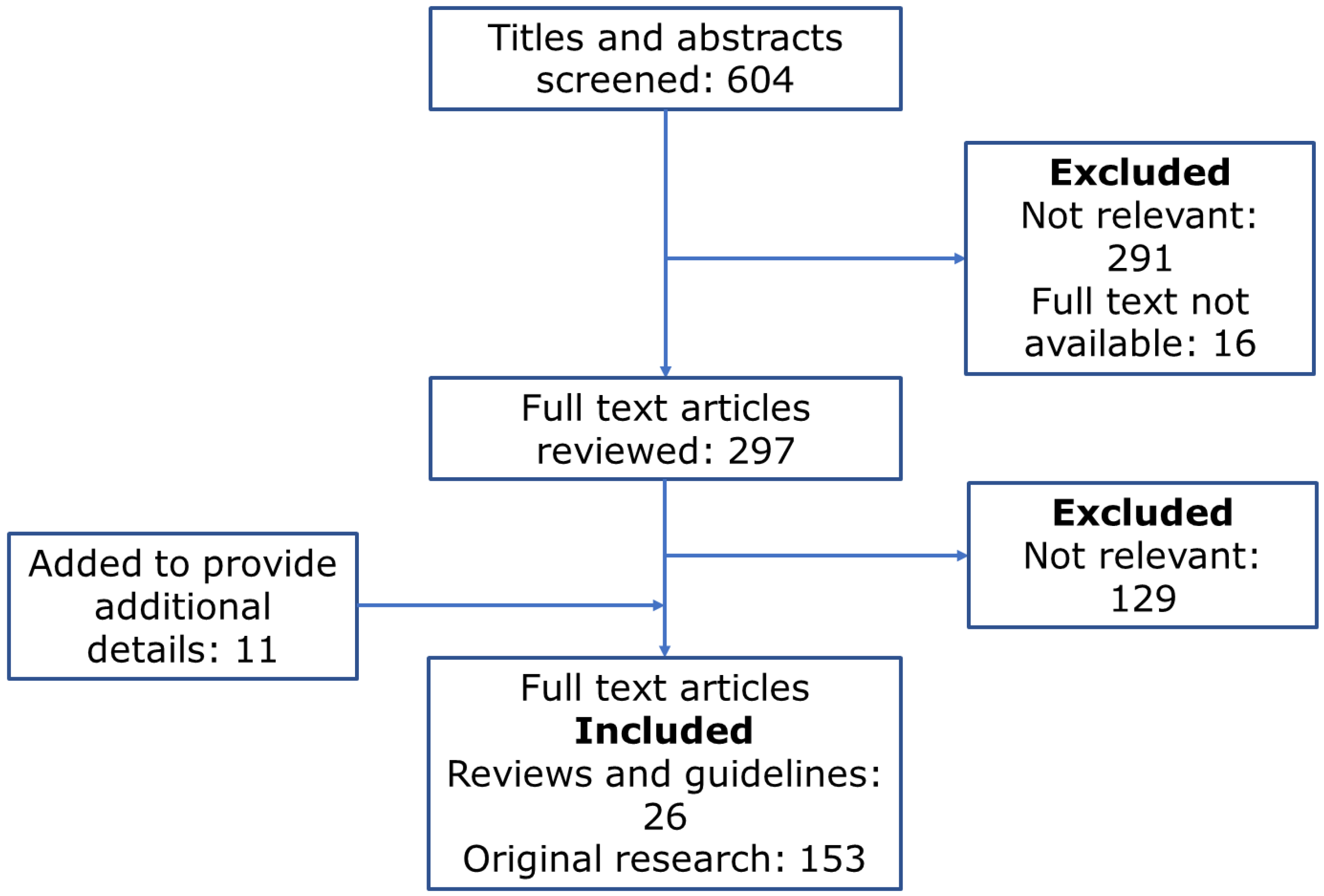
2. Methods
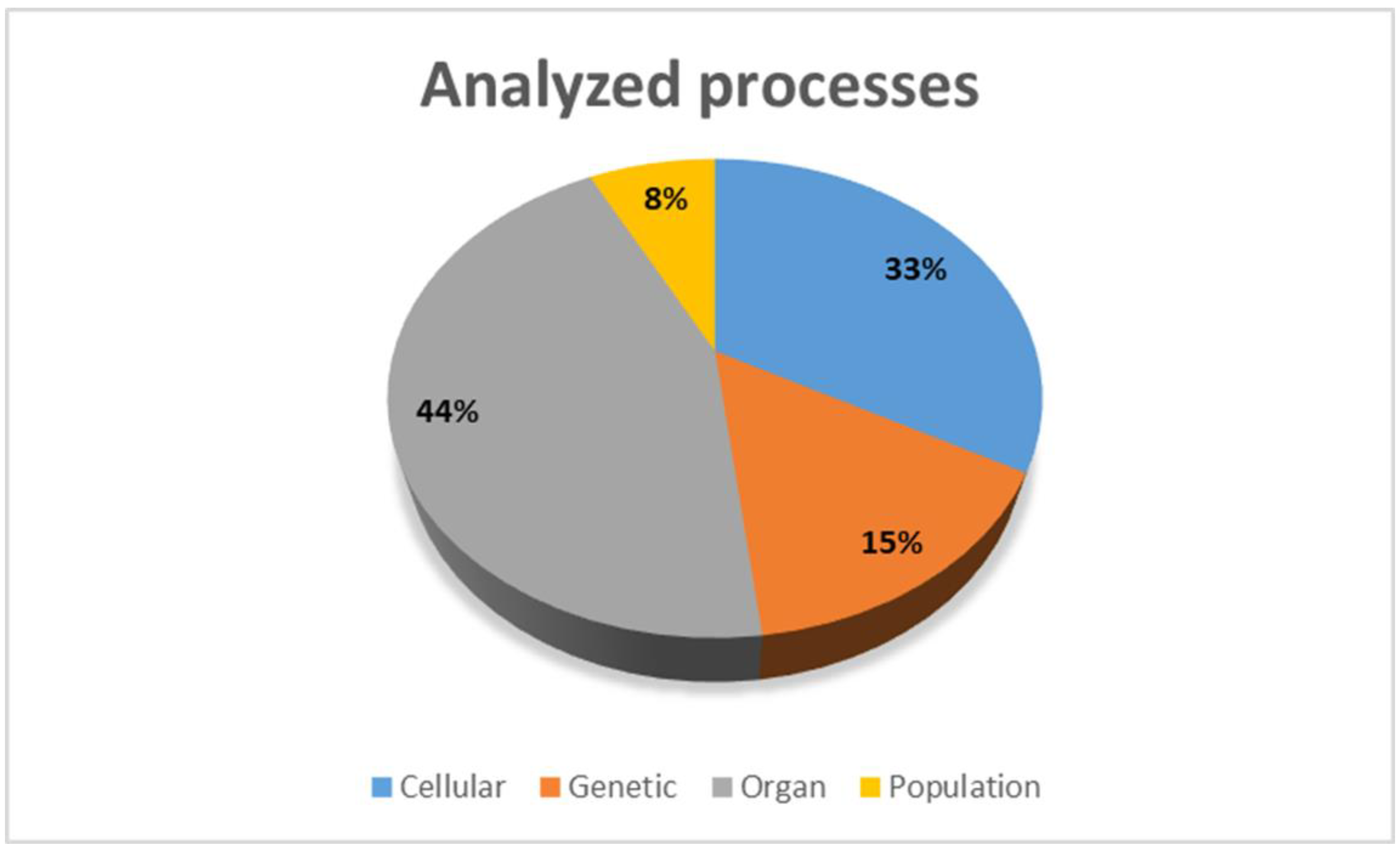
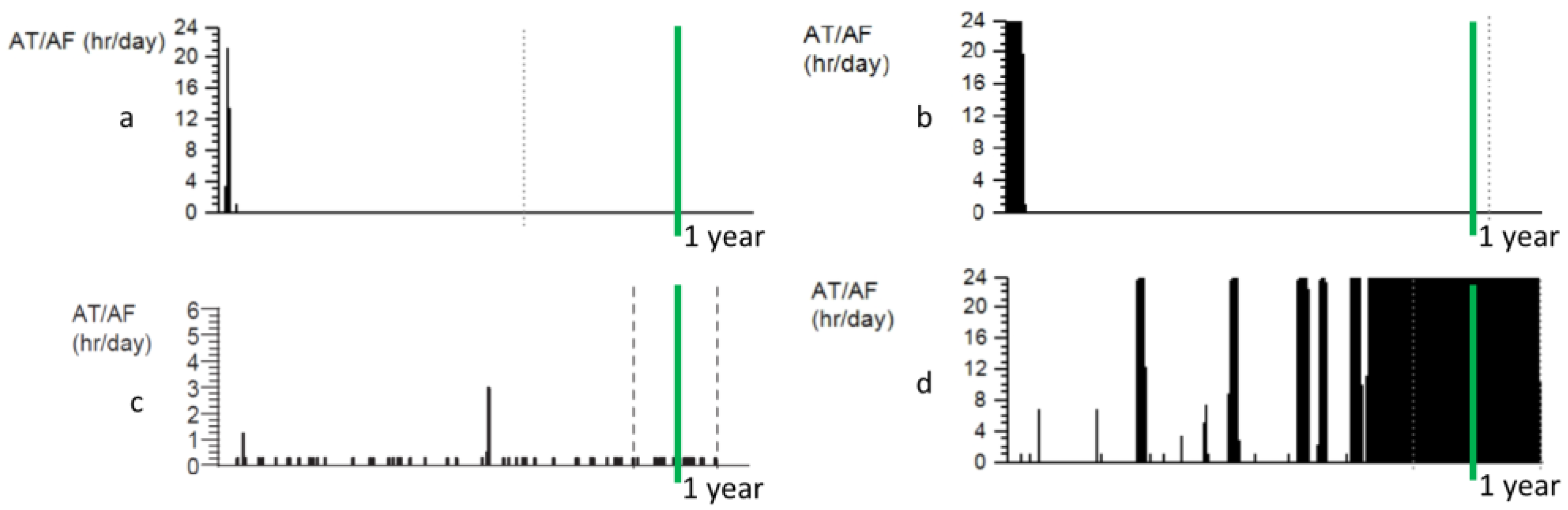
3. Progression of AF
4. Electrophysiological Remodeling
4.1. Arrhythmia Mechanisms—Initiation and Maintenance of AF
4.2. Electrical Remodeling
5. Genetic Processes in Atrial Remodeling
5.1. Genomic Risk of AF
5.2. Transcriptome Changes Affecting AF Risk
5.3. microRNA Abnormalities
6. Remodeling of Cellular Functions
6.1. Energy Homeostasis and Oxidative Stress
6.2. Intracellular Ca2+
6.3. Proteostasis
7. Organ Level Remodeling
7.1. Contractile Function
7.2. Atrial Geometry and Wall Thickness
7.3. Fibrosis
7.4. Epicardial Fat
7.5. Functional Mitral Regurgitation
8. Extracardiac Factors Affecting Remodeling
8.1. Vegetative Nervous System
8.2. Renin-Angiotensin-Aldosterone System
9. Systemic Factors in Remodeling
9.1. Inflammation
9.2. Hypercoagulability
9.3. Galectin-3
9.4. Homocysteine
9.5. Uric Acid
9.6. Gastrointestinal Effects
10. Clinical Risk Factors for AF Progression
10.1. Age
10.2. Obesity
10.3. Metabolic Syndrome
10.4. Sleep Apnea
10.5. Gender
10.6. Race
10.7. Biomarkers
11. Reversibility of Remodeling
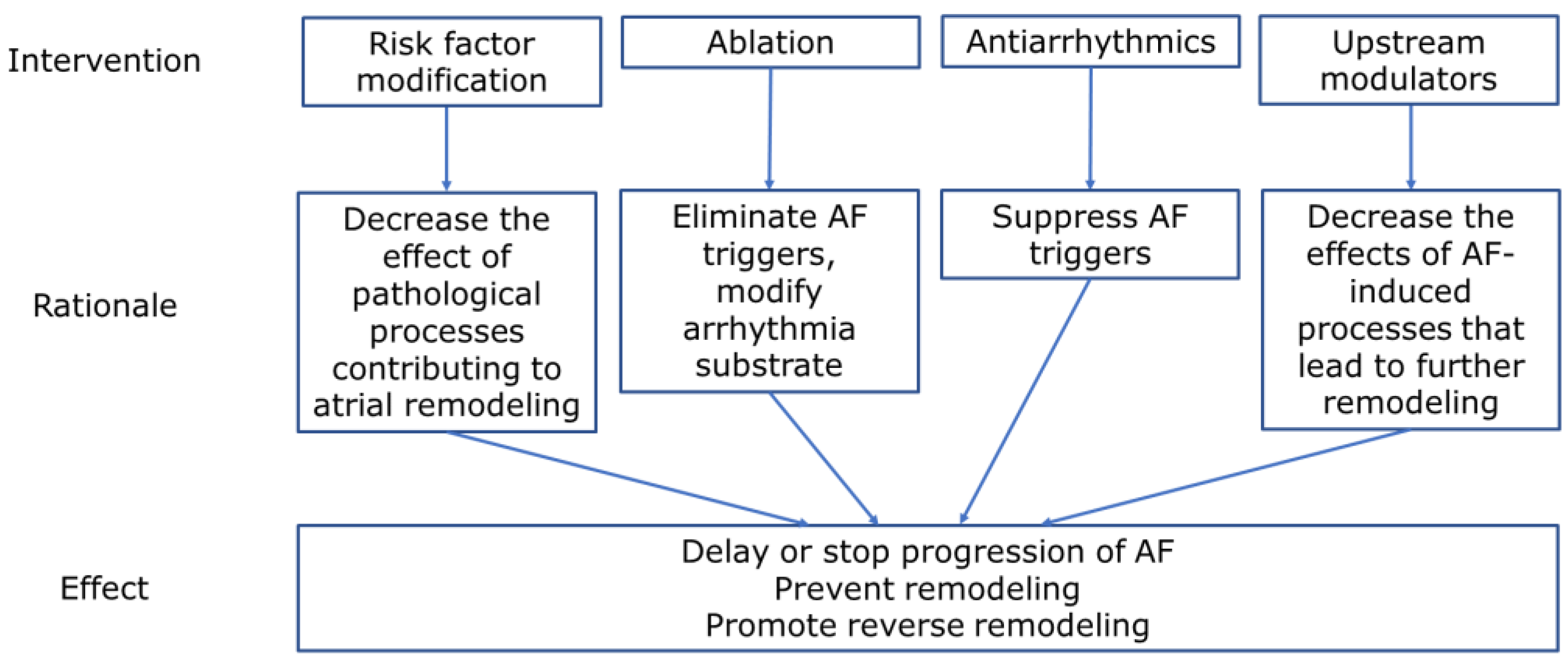
12. Potential Targets for the Treatment of Persistent Atrial Fibrillation
12.1. Simulation Models
12.2. Pharmacological Targets
12.3. Ablation of Persistent AF
12.4. Cardiac Resynchronization Therapy
13. Conclusions
14. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the european association for cardio-thoracic surgery (EACTS). Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Haïssaguerre, M.; Jaïs, P.; Shah, D.C.; Takahashi, A.; Hocini, M.; Quiniou, G.; Garrigue, S.; le Mouroux, A.; le Métayer, P.; Clémenty, J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N. Engl. J. Med. 1998, 339, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Wijffels, M.C.E.F.; Kirchhof, C.J.H.J.; Dorland, R.; Allessie, M.A. Atrial fibrillation begets atrial fibrillation. Circulation 1995, 92, 1954–1968. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 Statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.; McKenzie, J.E.; Sowden, A.; Katikireddi, S.V.; Brennan, S.E.; Ellis, S.; Hartmann-Boyce, J.; Ryan, R.; Shepperd, S.; Thomas, J.; et al. Synthesis without meta-analysis (SWiM) in systematic reviews: Reporting guideline. BMJ 2020, 368, l6890. [Google Scholar] [CrossRef]
- Vlachos, K.; Letsas, K.P.; Korantzopoulos, P.; Liu, T.; Georgopoulos, S.; Bakalakos, A.; Karamichalakis, N.; Xydonas, S.; Efremidis, M.; Sideris, A. Prediction of atrial fibrillation development and progression: Current perspectives. World J. Cardiol. 2016, 8, 267–276. [Google Scholar] [CrossRef]
- Nguyen, B.-O.; Weberndorfer, V.; Crijns, H.J.; Geelhoed, B.; ten Cate, H.; Spronk, H.; Kroon, A.; de With, R.; Al-Jazairi, M.; Maass, A.H.; et al. Prevalence and determinants of atrial fibrillation progression in paroxysmal atrial fibrillation. Heart 2022, in press. [Google Scholar] [CrossRef]
- Lillo-Castellano, J.M.; González-Ferrer, J.J.; Marina-Breysse, M.; Martínez-Ferrer, J.B.; Pérez-Álvarez, L.; Alzueta, J.; Martínez, J.G.; Rodríguez, A.; Rodríguez-Pérez, J.C.; Anguera, I.; et al. Personalized monitoring of electrical remodelling during atrial fibrillation progression via remote transmissions from implantable devices. EP Eur. 2020, 22, 704–715. [Google Scholar] [CrossRef]
- Jalife, J.; Kaur, K. Atrial remodeling, fibrosis, and atrial fibrillation. Trends Cardiovasc. Med. 2015, 25, 475–484. [Google Scholar] [CrossRef]
- Sánchez, J.; Gomez, J.F.; Martinez-Mateu, L.; Romero, L.; Saiz, J.; Trenor, B. Heterogeneous effects of fibroblast-myocyte coupling in different regions of the human atria under conditions of atrial fibrillation. Front. Physiol. 2019, 10, 847. [Google Scholar] [CrossRef]
- Cheniti, G.; Vlachos, K.; Pambrun, T.; Hooks, D.; Frontera, A.; Takigawa, M.; Bourier, F.; Kitamura, T.; Lam, A.; Martin, C.; et al. Atrial fibrillation mechanisms and implications for catheter ablation. Front. Physiol. 2018, 9, 1458. [Google Scholar] [CrossRef]
- Buhl, R.; Hesselkilde, E.M.; Carstensen, H.; Hopster-Iversen, C.; van Loon, G.; Decloedt, A.; van Steenkiste, G.; Marr, C.M.; Reef, V.B.; Schwarzwald, C.C.; et al. Atrial fibrillatory rate as predictor of recurrence of atrial fibrillation in horses treated medically or with electrical cardioversion. Equine Vet. J. 2022, 54, 1013–1022. [Google Scholar] [CrossRef]
- Luca, A.; Pittet, A.; Buttu, A.; McCann, A.; Vesin, J.-M.; Pascale, P.; le Bloa, M.; Herrera, C.; Park, C.-I.; Rollin, A.; et al. Severe and uniform bi-atrial remodeling measured by dominant frequency analysis in persistent atrial fibrillation unresponsive to ablation. J. Interv. Card. Electrophysiol. 2020, 59, 431–440. [Google Scholar] [CrossRef]
- Martins, R.P.; Kaur, K.; Hwang, E.; Ramirez, R.J.; Willis, B.C.; Filgueiras-Rama, D.; Ennis, S.R.; Takemoto, Y.; Ponce-Balbuena, D.; Zarzoso, M.; et al. Dominant frequency increase rate predicts transition from paroxysmal to long-term persistent atrial fibrillation. Circulation 2014, 129, 1472–1482. [Google Scholar] [CrossRef]
- Angel, N.; Kholmovski, E.G.; Ghafoori, E.; Dosdall, D.J.; MacLeod, R.S.; Ranjan, R. Regions of high dominant frequency in chronic atrial fibrillation anchored to areas of atrial fibrosis. Proc. Comput. Cardiol. 2019, 46, 10. [Google Scholar]
- Williams, S.E.; O’Neill, L.; Roney, C.H.; Julia, J.; Metzner, A.; Reißmann, B.; Mukherjee, R.K.; Sim, I.; Whitaker, J.; Wright, M.; et al. Left atrial effective conducting size predicts atrial fibrillation vulnerability in persistent but not paroxysmal atrial fibrillation. J. Cardiovasc. Electrophysiol. 2019, 30, 1416–1427. [Google Scholar] [CrossRef]
- Honarbakhsh, S.; Schilling, R.J.; Orini, M.; Providencia, R.; Keating, E.; Finlay, M.; Sporton, S.; Chow, A.; Earley, M.J.; Lambiase, P.D.; et al. Structural remodeling and conduction velocity dynamics in the human left atrium: Relationship with reentrant mechanisms sustaining atrial fibrillation. Heart Rhythm 2019, 16, 18–25. [Google Scholar] [CrossRef]
- Ohguchi, S.; Inden, Y.; Yanagisawa, S.; Shigematsu, T.; Yasuda, K.; Katagiri, K.; Oguri, M.; Murohara, T. Long P-wave duration immediately after pulmonary vein isolation on radiofrequency catheter ablation for atrial fibrillation predicts clinical recurrence: Correlation with atrial remodeling in persistent atrial fibrillation. Heart Vessel. 2022, 37, 476–488. [Google Scholar] [CrossRef]
- Walters, T.E.; Lee, G.; Lee, A.; Sievers, R.; Kalman, J.M.; Gerstenfeld, E.P. Site-specific epicardium-to-endocardium dissociation of electrical activation in a swine model of atrial fibrillation. JACC Clin. Electrophysiol. 2020, 6, 830–845. [Google Scholar] [CrossRef]
- Prabhu, S.; Voskoboinik, A.; McLellan, A.J.A.; Peck, K.Y.; Pathik, B.; Nalliah, C.J.; Wong, G.R.; Azzopardi, S.M.; Lee, G.; Mariani, J.; et al. A Comparison of the electrophysiologic and electroanatomic characteristics between the right and left atrium in persistent atrial fibrillation: Is the right atrium a window into the left? J. Cardiovasc. Electrophysiol. 2017, 28, 1109–1116. [Google Scholar] [CrossRef]
- Parikh, R.R.; Norby, F.L.; Wang, W.; Thenappan, T.; Prins, K.W.; Van’t Hof, J.R.; Lutsey, P.L.; Solomon, S.D.; Shah, A.M.; Chen, L.Y. Association of right ventricular afterload with atrial fibrillation risk in older adults. Chest 2022, 162, 884–893. [Google Scholar] [CrossRef] [PubMed]
- Sairaku, A.; Nakano, Y.; Suenari, K.; Tokuyama, T.; Kawazoe, H.; Matsumura, H.; Tomomori, S.; Amioka, M.; Kihara, Y. Electrical remodeling of the atrioventricular node caused by persistent atrial fibrillation in humans. J. Cardiovasc. Electrophysiol. 2016, 27, 918–922. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Shim, J.; Uhm, J.-S.; Joung, B.; Lee, M.-H.; Pak, H.-N. Post-shock sinus node recovery time is an independent predictor of recurrence after catheter ablation of longstanding persistent atrial fibrillation. Int. J. Cardiol. 2013, 168, 1937–1942. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Nagata, Y.; Nitta, G.; Okata, S.; Nagase, M.; Miyazaki, R.; Nagamine, S.; Kaneko, M.; Lee, T.; Nozato, T.; et al. Impact of the sinus node recovery time after termination of atrial fibrillation during catheter ablation on clinical outcomes in patients with persistent atrial fibrillation. PLoS ONE 2021, 16, e0259750. [Google Scholar] [CrossRef]
- Steenman, M. Insight into atrial fibrillation through analysis of the coding transcriptome in humans. Biophys. Rev. 2020, 12, 817–826. [Google Scholar] [CrossRef]
- Wong, G.R.; Nalliah, C.J.; Lee, G.; Voskoboinik, A.; Prabhu, S.; Parameswaran, R.; Sugumar, H.; Anderson, R.D.; Ling, L.-H.; McLellan, A.; et al. Genetic Susceptibility to atrial fibrillation is associated with atrial electrical remodeling and adverse post-ablation outcome. JACC Clin. Electrophysiol. 2020, 6, 1509–1521. [Google Scholar] [CrossRef]
- Husser, D.; Büttner, P.; Ueberham, L.; Dinov, B.; Sommer, P.; Arya, A.; Hindricks, G.; Bollmann, A. Association of atrial fibrillation susceptibility genes, atrial fibrillation phenotypes and response to catheter ablation: A Gene-based analysis of GWAS data. J. Transl. Med. 2017, 15, 71. [Google Scholar] [CrossRef]
- Husser, D.; Ueberham, L.; Dinov, B.; Kosiuk, J.; Kornej, J.; Hindricks, G.; Shoemaker, M.B.; Roden, D.M.; Bollmann, A.; Büttner, P. Genomic contributors to atrial electroanatomical remodeling and atrial fibrillation progression: Pathway enrichment analysis of GWAS data. Sci. Rep. 2016, 6, 36630. [Google Scholar] [CrossRef]
- Ezeani, M.; Prabhu, S. PI3K(P110α) as a determinant and gene therapy for atrial enlargement in atrial fibrillation. Mol. Cell. Biochem. 2022, in press. [Google Scholar] [CrossRef]
- Deshmukh, A.; Barnard, J.; Sun, H.; Newton, D.; Castel, L.; Pettersson, G.; Johnston, D.; Roselli, E.; Gillinov, A.M.; McCurry, K.; et al. Left atrial transcriptional changes associated with atrial fibrillation susceptibility and persistence. Circ. Arrhythm. Electrophysiol. 2015, 8, 32–41. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Chuang, J.-H.; Wang, H.-T.; Chen, H.-C.; Liu, W.-H.; Yang, M.-Y. Altered expression of circadian clock genes in patients with atrial fibrillation is associated with atrial high-rate episodes and left atrial remodeling. Diagnostics 2021, 11, 90. [Google Scholar] [CrossRef]
- Huang, L.; Yu, H.; Fan, X.; Li, X.; Mao, L.; Cheng, J.; Zeng, X.; Dang, X. A potential role of esophageal cancer related Gene-4 for atrial fibrillation. Sci. Rep. 2017, 7, 2717. [Google Scholar] [CrossRef]
- Oh, Y.; Yang, S.; Liu, X.; Jana, S.; Izaddoustdar, F.; Gao, X.; Debi, R.; Kim, D.-K.; Kim, K.-H.; Yang, P.; et al. Transcriptomic bioinformatic analyses of atria uncover involvement of pathways related to strain and post-translational modification of collagen in increased atrial fibrillation vulnerability in intensely exercised mice. Front. Physiol. 2020, 11, 605671. [Google Scholar] [CrossRef]
- Jiang, Y.-Y.; Hou, H.-T.; Yang, Q.; Liu, X.-C.; He, G.-W. Chloride channels are involved in the development of atrial fibrillation—A transcriptomic and proteomic study. Sci. Rep. 2017, 7, 10215. [Google Scholar] [CrossRef]
- Wang, R.; Bektik, E.; Sakon, P.; Wang, X.; Huang, S.; Meng, X.; Chen, M.; Han, W.; Chen, J.; Wang, Y.; et al. Integrated analysis of the MicroRNA–MRNA network predicts potential regulators of atrial fibrillation in humans. Cells 2022, 11, 2629. [Google Scholar] [CrossRef]
- Çubukçuoğlu Deniz, G.; Durdu, S.; Doğan, Y.; Erdemli, E.; Özdağ, H.; Akar, A.R. Molecular signatures of human chronic atrial fibrillation in primary mitral regurgitation. Cardiovasc. Ther. 2021, 2021, 5516185. [Google Scholar] [CrossRef]
- Alvarez-Franco, A.; Rouco, R.; Ramirez, R.J.; Guerrero-Serna, G.; Tiana, M.; Cogliati, S.; Kaur, K.; Saeed, M.; Magni, R.; Enriquez, J.A.; et al. Transcriptome and proteome mapping in the sheep atria reveal molecular featurets of atrial fibrillation progression. Cardiovasc. Res. 2021, 117, 1760–1775. [Google Scholar] [CrossRef]
- Liu, B.; Li, X.; Zhao, C.; Wang, Y.; Lv, M.; Shi, X.; Han, C.; Pandey, P.; Qian, C.; Guo, C.; et al. Proteomic analysis of atrial appendages revealed the pathophysiological changes of atrial fibrillation. Front. Physiol. 2020, 11, 573433. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, Y.; Fu, Y.; Wang, Q.; Liu, Z.; Hu, R.; Yang, X.; Chen, M. The potential regulatory role of hsa_circ_0004104 in the persistency of atrial fibrillation by promoting cardiac fibrosis via TGF-β pathway. BMC Cardiovasc. Disord. 2021, 21, 25. [Google Scholar] [CrossRef]
- Liu, L.; Chen, Y.; Shu, J.; Tang, C.-E.; Jiang, Y.; Luo, F. Identification of MicroRNAs enriched in exosomes in human pericardial fluid of patients with atrial fibrillation based on bioinformatic analysis. J. Thorac. Dis. 2020, 12, 5617–5627. [Google Scholar] [CrossRef]
- Galenko, O.; Jacobs, V.; Knight, S.; Taylor, M.; Cutler, M.J.; Muhlestein, J.B.; Carlquist, J.L.; Knowlton, K.U.; Jared Bunch, T. The role of MicroRNAs in the development, regulation, and treatment of atrial fibrillation. J. Int. Card. Electrophysiol. 2019, 55, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, F.; Zhang, Y.-L.; Yang, X.-C. Relationship between circulating MiRNA-21, atrial fibrosis, and atrial fibrillation in patients with atrial enlargement. Ann. Palliat. Med. 2021, 10, 12742–12749. [Google Scholar] [CrossRef] [PubMed]
- Morishima, M.; Iwata, E.; Nakada, C.; Tsukamoto, Y.; Takanari, H.; Miyamoto, S.; Moriyama, M.; Ono, K. Atrial fibrillation-mediated upregulation of MiR-30d regulates myocardial electrical remodeling of the G-protein-gated K+ channel, IK.ACh. Circ. J. 2016, 80, 1346–1355. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Ren, W.; Zhang, X.; Wu, P.; Fan, J. MiR-425-5p is negatively associated with atrial fibrosis and promotes atrial remodeling by targeting CREB1 in atrial fibrillation. J. Cardiol. 2022, 79, 202–210. [Google Scholar] [CrossRef]
- Jie, Q.-Q.; Li, G.; Duan, J.-B.; Li, X.-B.; Yang, W.; Chu, Y.-P.; Yu, S.-D.; Liu, X.-Y.; Wang, C.-Y.; Liu, F.-F.; et al. Remodeling of myocardial energy and metabolic homeostasis in a sheep model of persistent atrial fibrillation. Biochem. Biophys. Res. Commun. 2019, 517, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Muszyński, P.; Bonda, T.A. Mitochondrial dysfunction in atrial fibrillation—Mechanisms and pharmacological interventions. J. Clin. Med. 2021, 10, 2385. [Google Scholar] [CrossRef] [PubMed]
- Watts, M.; Kolluru, G.K.; Dherange, P.; Pardue, S.; Si, M.; Shen, X.; Trosclair, K.; Glawe, J.; Al-Yafeai, Z.; Iqbal, M.; et al. Decreased bioavailability of hydrogen sulfide links vascular endothelium and atrial remodeling in atrial fibrillation. Redox Biol. 2021, 38, 101817. [Google Scholar] [CrossRef]
- Xue, X.; Ling, X.; Xi, W.; Wang, P.; Sun, J.; Yang, Q.; Xiao, J. Exogenous hydrogen sulfide reduces atrial remodeling and atrial fibrillation induced by diabetes mellitus via activation of the PI3K/Akt/ENOS pathway. Mol. Med. Rep. 2020, 22, 1759–1766. [Google Scholar] [CrossRef]
- Avula, U.M.R.; Dridi, H.; Chen, B.; Yuan, Q.; Katchman, A.N.; Reiken, S.R.; Desai, A.D.; Parsons, S.; Baksh, H.; Ma, E.; et al. Attenuating persistent sodium current-induced atrial myopathy and fibrillation by preventing mitochondrial oxidative stress. JCI Insight 2021, 6, e147371. [Google Scholar] [CrossRef]
- Dolce, B.; Christ, T.; Grammatika Pavlidou, N.; Yildirim, Y.; Reichenspurner, H.; Eschenhagen, T.; Nikolaev, V.O.; Kaumann, A.J.; Molina, C.E. Impact of phosphodiesterases PDE3 and PDE4 on 5-hydroxytryptamine receptor4-mediated increase of CAMP in human atrial fibrillation. Naunyn Schmiedebergs Arch. Pharmacol. 2021, 394, 291–298. [Google Scholar] [CrossRef]
- Chang, K.C.; Trayanova, N.A. Mechanisms of arrhythmogenesis related to calcium-driven alternans in a model of human atrial fibrillation. Sci. Rep. 2016, 6, 36395. [Google Scholar] [CrossRef]
- Onal, B.; Gratz, D.; Hund, T.J. Ca2+/Calmodulin-dependent Kinase II-dependent regulation of atrial myocyte late Na+ current, Ca2+ cycling, and excitability: A mathematical modeling study. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H1227–H1239. [Google Scholar] [CrossRef]
- Liu, Z.; Finet, J.E.; Wolfram, J.A.; Anderson, M.E.; Ai, X.; Donahue, J.K. Calcium/Calmodulin-dependent protein Kinase II causes atrial structural remodeling associated with atrial fibrillation and heart failure. Heart Rhythm 2019, 16, 1080–1088. [Google Scholar] [CrossRef]
- Munro, M.L.; van Hout, I.; Aitken-Buck, H.M.; Sugunesegran, R.; Bhagwat, K.; Davis, P.J.; Lamberts, R.R.; Coffey, S.; Soeller, C.; Jones, P.P. Human atrial fibrillation is not associated with remodeling of ryanodine receptor clusters. Front. Cell Dev. Biol. 2021, 9, 633704. [Google Scholar] [CrossRef]
- Hu, X.; van Marion, D.M.S.; Wiersma, M.; Zhang, D.; Brundel, B.J.J.M. The protective role of small heat shock proteins in cardiac diseases: Key role in atrial fibrillation. Cell Stress Chaperones 2017, 22, 665–674. [Google Scholar] [CrossRef]
- Wiersma, M.; Meijering, R.A.M.; Qi, X.; Zhang, D.; Liu, T.; Hoogstra-Berends, F.; Sibon, O.C.M.; Henning, R.H.; Nattel, S.; Brundel, B.J.J.M. Endoplasmic reticulum stress is associated with autophagy and cardiomyocyte remodeling in experimental and human atrial fibrillation. J. Am. Heart Assoc. 2017, 6, e006458. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhao, J.; Gong, Y.; Wang, D.; Wang, X.; Yun, F.; Liu, Z.; Zhang, S.; Li, W.; Zhao, X.; et al. Autophagy exacerbates electrical remodeling in atrial fibrillation by Ubiquitin-dependent degradation of L-type calcium channel. Cell Death Dis. 2018, 9, 873. [Google Scholar] [CrossRef]
- Adeniran, I.; MacIver, D.H.; Garratt, C.J.; Ye, J.; Hancox, J.C.; Zhang, H. Effects of persistent atrial fibrillation-induced electrical remodeling on atrial electro-mechanics—Insights from a 3D model of the human atria. PLoS ONE 2015, 10, e0142397. [Google Scholar] [CrossRef]
- Wałek, P.; Sielski, J.; Gorczyca, I.; Roskal-Wałek, J.; Starzyk, K.; Jaskulska-Niedziela, E.; Bartkowiak, R.; Wożakowska-Kapłon, B. Left atrial mechanical remodelling assessed as the velocity of left atrium appendage wall motion during atrial fibrillation is associated with maintenance of sinus rhythm after electrical cardioversion in patients with persistent atrial fibrillation. PLoS ONE 2020, 15, e0228239. [Google Scholar] [CrossRef]
- Kaufmann, R.; Rezar, R.; Strohmer, B.; Wernly, B.; Lichtenauer, M.; Hitzl, W.; Meissnitzer, M.; Hergan, K.; Granitz, M. Left atrial ejection fraction assessed by prior cardiac CT predicts recurrence of atrial fibrillation after pulmonary vein isolation. J. Clin. Med. 2021, 10, 752. [Google Scholar] [CrossRef]
- Zuo, K.; Li, K.; Liu, M.; Li, J.; Liu, X.; Liu, X.; Zhong, J.; Yang, X. Correlation of left atrial wall thickness and atrial remodeling in atrial fibrillation. Medicine 2019, 98, e15170. [Google Scholar] [CrossRef] [PubMed]
- Verheule, S.; Eckstein, J.; Linz, D.; Maesen, B.; Bidar, E.; Gharaviri, A.; Schotten, U. Role of endo-epicardial dissociation of electrical activity and transmural conduction in the development of persistent atrial fibrillation. Prog. Biophys. Mol. Biol. 2014, 115, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Maesen, B.; Zeemering, S.; Afonso, C.; Eckstein, J.; Burton, R.A.B.; van Hunnik, A.; Stuckey, D.J.; Tyler, D.; Maessen, J.; Grau, V.; et al. Rearrangement of atrial bundle architecture and consequent changes in anisotropy of conduction constitute the 3-dimensional substrate for atrial fibrillation. Circ. Arrhythm. Electrophysiol. 2013, 6, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Mouws, E.M.J.P.; Lanters, E.A.H.; Teuwen, C.P.; van der Does, L.J.M.E.; Kik, C.; Knops, P.; Bekkers, J.A.; Bogers, A.J.J.C.; de Groot, N.M.S. Epicardial breakthrough waves during sinus rhythm. Circ. Arrhythm. Electrophysiol. 2017, 10, e005145. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.E.; Na, J.O.; Im, S.I.; Choi, C.U.; Kim, S.H.; Kim, J.W.; Kim, E.J.; Han, S.W.; Rha, S.-W.; Park, C.G.; et al. Interatrial septal thickness as a marker of structural and functional remodeling of the left atrium in patients with atrial fibrillation. Korean J. Intern. Med. 2015, 30, 808–820. [Google Scholar] [CrossRef]
- Park, Y.M.; Park, H.C.; Ban, J.-E.; Choi, J.-I.; Lim, H.E.; Park, S.W.; Kim, Y.-H. Interatrial septal thickness is associated with the extent of left atrial complex fractionated atrial electrograms and acute procedural outcome in patients with persistent atrial fibrillation. Europace 2015, 17, 1700–1707. [Google Scholar] [CrossRef]
- Xie, E.; Yu, R.; Ambale-Venkatesh, B.; Bakhshi, H.; Heckbert, S.R.; Soliman, E.Z.; Bluemke, D.A.; Kawut, S.M.; Wu, C.O.; Nazarian, S.; et al. Association of right atrial structure with incident atrial fibrillation: A longitudinal cohort cardiovascular magnetic resonance study from the multi-ethnic study of atherosclerosis (MESA). J. Cardiovasc. Magn. Reson. 2020, 22, 36. [Google Scholar] [CrossRef]
- Kishima, H.; Mine, T.; Takahashi, S.; Ashida, K.; Ishihara, M.; Masuyama, T. Morphologic remodeling of left atrial appendage in patients with atrial fibrillation. Heart Rhythm 2016, 13, 1823–1828. [Google Scholar] [CrossRef]
- Platonov, P.G. Atrial fibrosis: An obligatory component of arrhythmia mechanisms in atrial fibrillation? J. Geriatr. Cardiol. 2017, 14, 233–237. [Google Scholar] [CrossRef]
- Hopman, L.H.G.A.; Mulder, M.J.; van der Laan, A.M.; Demirkiran, A.; Bhagirath, P.; van Rossum, A.C.; Allaart, C.P.; Götte, M.J.W. Impaired left atrial reservoir and conduit strain in patients with atrial fibrillation and extensive left atrial fibrosis. J. Cardiovasc. Magn. Reson. 2021, 23, 131. [Google Scholar] [CrossRef]
- Kato, T.; Sekiguchi, A.; Sagara, K.; Tanabe, H.; Takamura, M.; Kaneko, S.; Aizawa, T.; Fu, L.-T.; Yamashita, T. Endothelial–mesenchymal transition in human atrial fibrillation. J. Cardiol. 2017, 69, 706–711. [Google Scholar] [CrossRef]
- Van den Berg, N.W.E.; Kawasaki, M.; Fabrizi, B.; Nariswari, F.A.; Verduijn, A.C.; Neefs, J.; Wesselink, R.; Al-Shama, R.F.M.; van der Wal, A.C.; de Boer, O.J.; et al. Epicardial and endothelial cell activation concurs with extracellular matrix remodeling in atrial fibrillation. Clin. Transl. Med. 2021, 11, e558. [Google Scholar] [CrossRef]
- Benito, E.M.; Cabanelas, N.; Nuñez-Garcia, M.; Alarcón, F.; Figueras, I.; Ventura, R.M.; Soto-Iglesias, D.; Guasch, E.; Prat-Gonzalez, S.; Perea, R.J.; et al. Preferential regional distribution of atrial fibrosis in posterior wall around left inferior pulmonary vein as identified by late gadolinium enhancement cardiac magnetic resonance in patients with atrial fibrillation. EP Eur. 2018, 20, 1959–1965. [Google Scholar] [CrossRef]
- Kogawa, R.; Okumura, Y.; Watanabe, I.; Nagashima, K.; Takahashi, K.; Iso, K.; Watanabe, R.; Arai, M.; Kurokawa, S.; Ohkubo, K.; et al. Left atrial remodeling: Regional differences between paroxysmal and persistent atrial fibrillation. J. Arrhythm. 2017, 33, 483–487. [Google Scholar] [CrossRef]
- Callegari, S.; Macchi, E.; Monaco, R.; Magnani, L.; Tafuni, A.; Croci, S.; Nicastro, M.; Garrapa, V.; Banchini, A.; Becchi, G.; et al. Clinicopathological bird’s-eye view of left atrial myocardial fibrosis in 121 patients with persistent atrial fibrillation. Circ. Arrhythm. Electrophysiol. 2020, 13, e007588. [Google Scholar] [CrossRef]
- Maesen, B.; Verheule, S.; Zeemering, S.; la Meir, M.; Nijs, J.; Lumeij, S.; Lau, D.H.; Granier, M.; Crijns, H.J.; Maessen, J.G.; et al. Endomysial fibrosis, rather than overall connective tissue content, is the main determinant of conduction disturbances in human atrial fibrillation. EP Eur. 2022, 24, 1015–1024. [Google Scholar] [CrossRef]
- Sánchez, J.; Trenor, B.; Saiz, J.; Dössel, O.; Loewe, A. Fibrotic remodeling during persistent atrial fibrillation: In silico investigation of the role of calcium for human atrial myofibroblast electrophysiology. Cells 2021, 10, 2852. [Google Scholar] [CrossRef]
- Szilágyi, J.; Sághy, L. Atrial remodeling in atrial fibrillation. Comorbidities and markers of disease progression predict catheter ablation outcome. Curr. Cardiol. Rev. 2021, 17, 217–229. [Google Scholar] [CrossRef]
- Musa, H.; Kaur, K.; O’Connell, R.; Klos, M.; Guerrero-Serna, G.; Avula, U.M.R.; Herron, T.J.; Kalifa, J.; Anumonwo, J.M.B.; Jalife, J. Inhibition of platelet-derived growth factor-AB signaling prevents electromechanical remodeling of adult atrial myocytes that contact myofibroblasts. Heart Rhythm 2013, 10, 1044–1051. [Google Scholar] [CrossRef]
- Chen, J.; Guo, Y.; Chen, Q.; Cheng, X.; Xiang, G.; Chen, M.; Wu, H.; Huang, Q.; Zhu, P.; Zhang, J. TGFβ1 and HGF regulate CTGF expression in human atrial fibroblasts and are involved in atrial remodelling in patients with rheumatic heart disease. J. Cell. Mol. Med. 2019, 23, 3032–3039. [Google Scholar] [CrossRef]
- Sai, C.; Yunhan, J.; Zhao, J.; Yu, Z.; Yun, Z.; Zhezhe, C.; Fuqin, T.; Yingbin, X.; Ruiyan, M. Cyclin dependent kinase 1 (CDK1) activates cardiac fibroblasts via directly phosphorylating paxillin at Ser244. Int. Heart J. 2019, 60, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Ciuffo, L.A.; Lima, J.; Vasconcellos, H.D.; de Balouch, M.; Tao, S.; Nazarian, S.; Spragg, D.D.; Marine, J.E.; Berger, R.D.; Calkins, H.; et al. Intra-atrial dyssynchrony using cardiac magnetic resonance to quantify tissue remodeling in patients with atrial fibrillation. Arq. Bras. Cardiol. 2019, 4, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Holzwirth, E.; Kornej, J.; Erbs, S.; Obradovic, D.; Bollmann, A.; Hindricks, G.; Thiele, H.; Büttner, P. Myeloperoxidase in atrial fibrillation: Association with progression, origin and influence of renin-angiotensin system antagonists. Clin. Res. Cardiol. 2020, 109, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Kornej, J.; Zeynalova, S.; Büttner, P.; Burkhardt, R.; Bae, Y.J.; Willenberg, A.; Baber, R.; Thaler, A.; Hindricks, G.; Loeffler, M.; et al. Differentiation of atrial fibrillation progression phenotypes using troponin, T. Int. J. Cardiol. 2019, 297, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, H.; Kou, W.; Tang, K.; Zhao, D.; Zhang, J.; Zhuang, J.; Zhao, Y.; Ji, S.; Peng, W.; et al. Increased plasma microfibrillar-associated protein 4 is associated with atrial fibrillation and more advanced left atrial remodelling. Arch. Med. Sci. 2019, 15, 632–640. [Google Scholar] [CrossRef]
- Lau, D.H.; Schotten, U.; Mahajan, R.; Antic, N.A.; Hatem, S.N.; Pathak, R.K.; Hendriks, J.M.L.; Kalman, J.M.; Sanders, P. Novel mechanisms in the pathogenesis of atrial fibrillation: Practical applications. Eur. Heart J. 2016, 37, 1573–1581. [Google Scholar] [CrossRef]
- Oba, K.; Maeda, M.; Maimaituxun, G.; Yamaguchi, S.; Arasaki, O.; Fukuda, D.; Yagi, S.; Hirata, Y.; Nishio, S.; Iwase, T.; et al. Effect of the epicardial adipose tissue volume on the prevalence of paroxysmal and persistent atrial fibrillation. Circ. J. 2018, 82, 1778–1787. [Google Scholar] [CrossRef]
- Gaeta, M.; Bandera, F.; Tassinari, F.; Capasso, L.; Cargnelutti, M.; Pelissero, G.; Malavazos, A.E.; Ricci, C. Is epicardial fat depot associated with atrial fibrillation? A systematic review and meta-analysis. EP Eur. 2017, 19, 747–752. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, X.; Wang, J.; Zhong, J.; Zhang, H.; Wu, B.; Zheng, Z.; Xie, X.; Zhu, J.; Tang, X.; et al. Redistribution of adipose tissue is associated with left atrial remodeling and dysfunction in patients with atrial fibrillation. Front. Cardiovasc. Med. 2022, 9, 969513. [Google Scholar] [CrossRef]
- Zhao, L.; Ma, Z.; Guo, Z.; Zheng, M.; Li, K.; Yang, X. Analysis of long non-coding RNA and MRNA profiles in epicardial adipose tissue of patients with atrial fibrillation. Biomed. Pharmacother. 2020, 121, 109634. [Google Scholar] [CrossRef]
- Tsao, H.-M.; Hu, W.-C.; Tsai, P.-H.; Lee, C.-L.; Liu, F.-C.; Wang, H.-H.; Lo, L.-W.; Chang, S.-L.; Chao, T.-F.; Chen, S.-A. The abundance of epicardial adipose tissue surrounding left atrium is associated with the occurrence of stroke in patients with atrial fibrillation. Medicine 2016, 95, e3260. [Google Scholar] [CrossRef]
- Haemers, P.; Hamdi, H.; Guedj, K.; Suffee, N.; Farahmand, P.; Popovic, N.; Claus, P.; LePrince, P.; Nicoletti, A.; Jalife, J.; et al. Atrial fibrillation is associated with the fibrotic remodelling of adipose tissue in the subepicardium of human and sheep atria. Eur. Heart J. 2017, 38, 53–61. [Google Scholar] [CrossRef]
- Uno, G.; Omori, T.; Shimada, S.; Rader, F.; Siegel, R.J.; Shiota, T. Differences in mitral valve geometry between atrial and ventricular functional mitral regurgitation in patients with atrial fibrillation: A 3D transoesophageal echocardiography study. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 1106–1116. [Google Scholar] [CrossRef]
- Kim, D.-H.; Heo, R.; Handschumacher, M.D.; Lee, S.; Choi, Y.-S.; Kim, K.-R.; Shin, Y.; Park, H.-K.; Bischoff, J.; Aikawa, E.; et al. Mitral valve adaptation to isolated annular dilation. JACC Cardiovasc. Imaging 2019, 12, 665–677. [Google Scholar] [CrossRef]
- Machino-Ohtsuka, T.; Seo, Y.; Ishizu, T.; Sato, K.; Sugano, A.; Yamamoto, M.; Hamada-Harimura, Y.; Aonuma, K. Novel mechanistic insights into atrial functional mitral regurgitation—3-dimensional echocardiographic study. Circ. J. 2016, 80, 2240–2248. [Google Scholar] [CrossRef]
- Guta, A.C.; Badano, L.P.; Tomaselli, M.; Mihalcea, D.; Bartos, D.; Parati, G.; Muraru, D. The pathophysiological link between right atrial remodeling and functional tricuspid regurgitation in patients with atrial fibrillation: A three-dimensional echocardiography study. J. Am. Soc. Echocardiogr. 2021, 34, 585–594.e1. [Google Scholar] [CrossRef]
- Tang, B. Atrial fibrillation electrical remodelling via ablation of the epicardial neural networks and suprathreshold stimulation of vagosympathetic nerve. Med. Sci. Monit. 2015, 21, 82–89. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, X.; Li, Z.; Wang, W.; Zhang, Y.; Liu, J.; Hou, Y. Atrial heterogeneous autonomic neural remodeling in rabbits with experimental atrial fibrillation and the effect of intervention by rosuvastatin. Pacing Clin. Electrophysiol. 2016, 39, 598–606. [Google Scholar] [CrossRef]
- De Oliveira, Í.M.; Silva, E.L.d.; Martins, Y.d.O.; Rocha, H.A.L.; Scanavacca, M.I.; Gutierrez, P.S. Cardiac autonomic nervous system remodeling may play a role in atrial fibrillation: A study of the autonomic nervous system and myocardial receptors. Arq. Bras. Cardiol. 2021, 117, 999–1007. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, M.; Zhang, Y.; Xie, X.; Wang, W.; Li, Z.; Gao, M.; Wang, Z.; Hou, Y. Long-term effects of ganglionated plexi ablation on electrophysiological characteristics and neuron remodeling in target atrial tissues in a canine model. Circ. Arrhythm. Electrophysiol. 2015, 8, 1276–1283. [Google Scholar] [CrossRef]
- Moss, E.; Cardinal, R.; Yin, Y.; Pagé, P. Biatrial neuroablation attenuates atrial remodeling and vulnerability to atrial fibrillation in canine chronic rapid atrial pacing. Auton. Neurosci. 2015, 189, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.-J.; Yao, F.-J.; Lu, G.-H.; Xu, C.-G.; Xu, Z.; Tang, K.; Cheng, Y.-J.; Gao, X.-R.; Wu, S.-H. The role of the Rho/ROCK pathway in Ang II and TGF-Β1-induced atrial remodeling. PLoS ONE 2016, 11, e0161625. [Google Scholar] [CrossRef] [PubMed]
- Walters, T.E.; Kalman, J.M.; Patel, S.K.; Mearns, M.; Velkoska, E.; Burrell, L.M. Angiotensin converting enzyme 2 activity and human atrial fibrillation: Increased plasma angiotensin converting enzyme 2 activity is associated with atrial fibrillation and more advanced left atrial structural remodelling. Europace 2016, 19, 1280–1287. [Google Scholar] [CrossRef]
- Zhou, T.; Han, Z.; Gu, J.; Chen, S.; Fan, Y.; Zhang, H.; Yin, Y.; Zhang, J.; Wang, C. Angiotensin-converting enzyme-2 overexpression improves atrial electrical remodeling through TRPM7 signaling pathway. Oncotarget 2017, 8, 78726–78733. [Google Scholar] [CrossRef] [PubMed]
- Ihara, K.; Sasano, T. Role of inflammation in the pathogenesis of atrial fibrillation. Front. Physiol. 2022, 13, 862164. [Google Scholar] [CrossRef]
- Pauklin, P.; Zilmer, M.; Eha, J.; Tootsi, K.; Kals, M.; Kampus, P. Markers of inflammation, oxidative stress, and fibrosis in patients with atrial fibrillation. Oxid. Med. Cell. Longev. 2022, 2022, 4556671. [Google Scholar] [CrossRef]
- Smorodinova, N.; Bláha, M.; Melenovský, V.; Rozsívalová, K.; Přidal, J.; Ďurišová, M.; Pirk, J.; Kautzner, J.; Kučera, T. Analysis of immune cell populations in atrial myocardium of patients with atrial fibrillation or sinus rhythm. PLoS ONE 2017, 12, e0172691. [Google Scholar] [CrossRef]
- Yao, C.; Veleva, T.; Scott, L.; Cao, S.; Li, L.; Chen, G.; Jeyabal, P.; Pan, X.; Alsina, K.M.; Abu-Taha, I.; et al. Enhanced cardiomyocyte NLRP3 inflammasome signaling promotes atrial fibrillation. Circulation 2018, 138, 2227–2242. [Google Scholar] [CrossRef]
- Hiram, R.; Xiong, F.; Naud, P.; Xiao, J.; Sirois, M.; Tanguay, J.-F.; Tardif, J.-C.; Nattel, S. The inflammation-resolution promoting molecule resolvin-D1 prevents atrial proarrhythmic remodelling in experimental right heart disease. Cardiovasc. Res. 2021, 117, 1776–1789. [Google Scholar] [CrossRef]
- Kornej, J.; Büttner, P.; Hammer, E.; Engelmann, B.; Dinov, B.; Sommer, P.; Husser, D.; Hindricks, G.; Völker, U.; Bollmann, A. Circulating proteomic patterns in AF related left atrial remodeling indicate involvement of coagulation and complement cascade. PLoS ONE 2018, 13, e0198461. [Google Scholar] [CrossRef]
- Kondo, H.; Abe, I.; Fukui, A.; Saito, S.; Miyoshi, M.; Aoki, K.; Shinohara, T.; Teshima, Y.; Yufu, K.; Takahashi, N. Possible role of rivaroxaban in attenuating pressure-overload-induced atrial fibrosis and fibrillation. J. Cardiol. 2018, 71, 310–319. [Google Scholar] [CrossRef]
- Wałek, P.; Grabowska, U.; Cieśla, E.; Sielski, J.; Roskal-Wałek, J.; Wożakowska-Kapłon, B. Analysis of the correlation of galectin-3 concentration with the measurements of echocardiographic parameters assessing left atrial remodeling and function in patients with persistent atrial fibrillation. Biomolecules 2021, 11, 1108. [Google Scholar] [CrossRef]
- Takemoto, Y.; Ramirez, R.J.; Yokokawa, M.; Kaur, K.; Ponce-Balbuena, D.; Sinno, M.C.; Willis, B.C.; Ghanbari, H.; Ennis, S.R.; Guerrero-Serna, G.; et al. Galectin-3 regulates atrial fibrillation remodeling and predicts catheter ablation outcomes. JACC Basic Transl. Sci. 2016, 1, 143–154. [Google Scholar] [CrossRef]
- Tang, Z.; Zeng, L.; Lin, Y.; Han, Z.; Gu, J.; Wang, C.; Zhang, H. Circulating galectin-3 is associated with left atrial appendage remodelling and thrombus formation in patients with atrial fibrillation. Heart Lung Circ. 2019, 28, 923–931. [Google Scholar] [CrossRef]
- Ivanov, V.; Smereka, Y.; Rasputin, V.; Dmytriiev, K. Homocysteine and atrial fibrillation: Novel evidences and insights. Monaldi Arch. Chest Dis. 2022, in press. [Google Scholar] [CrossRef]
- Taufiq, F.; Li, P.; Miake, J.; Hisatome, I. Hyperuricemia as a risk factor for atrial fibrillation due to soluble and crystalized uric acid. Circ. Rep. 2019, 1, 469–473. [Google Scholar] [CrossRef]
- Wang, X.-D.; Liu, J.; Zhang, Y.-C.; Wang, Y.; Wang, Y.; Ma, D. Correlation between the elevated uric acid levels and circulating renin-angiotensin-aldosterone system activation in patients with atrial fibrillation. Cardiovasc. Diagn. Ther. 2021, 11, 50–55. [Google Scholar] [CrossRef]
- Markus, M.R.P.; Meffert, P.J.; Baumeister, S.E.; Lieb, W.; Siewert, U.; Schipf, S.; Koch, M.; Kors, J.A.; Felix, S.B.; Dörr, M.; et al. Association between hepatic steatosis and serum liver enzyme levels with atrial fibrillation in the general population. Atherosclerosis 2016, 245, 123–131. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.; Zang, M.; Yao, T.; Mao, J.; Pu, J. Circulating primary bile acid is correlated with structural remodeling in atrial fibrillation. J. Interv. Card. Electrophysiol. 2020, 57, 371–377. [Google Scholar] [CrossRef]
- Zuo, K.; Fang, C.; Liu, Z.; Fu, Y.; Liu, Y.; Liu, L.; Wang, Y.; Yin, X.; Liu, X.; Li, J.; et al. Commensal microbe-derived SCFA alleviates atrial fibrillation via GPR43/NLRP3 signaling. Int. J. Biol. Sci. 2022, 18, 4219–4232. [Google Scholar] [CrossRef]
- Zuo, K.; Yin, X.; Li, K.; Zhang, J.; Wang, P.; Jiao, J.; Liu, Z.; Liu, X.; Liu, J.; Li, J.; et al. Different types of atrial fibrillation share patterns of gut microbiota dysbiosis. mSphere 2020, 5, e00071-20. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Chen, K.; Li, S.; Cai, M.; Yuan, M.; Wang, Y.; Zhang, X.; Wei, M.; Yan, M.-L.; Ma, X.-X.; et al. Impaired left atrial performance resulting from age-related arial fibrillation is associated with increased fibrosis burden: Insights from a clinical study combining with an in vivo experiment. Front. Cardiovasc. Med. 2021, 7, 615065. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.M.; Lee, D.I.; Park, H.C.; Shim, J.; Choi, J.; Park, S.W.; Kim, Y. The extent of complex fractionated atrial electrograms in the left atrium reflects age-related electrical remodeling in patients with persistent atrial fibrillation. J. Arrhythm. 2019, 35, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Javed, S.; Gupta, D.; Lip, G.Y.H. Obesity and atrial fibrillation: Making inroads through fat. Eur. Heart J. Cardiovasc. Pharmacother. 2021, 7, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Chirinos, J.A.; Sardana, M.; Satija, V.; Gillebert, T.C.; de Buyzere, M.L.; Chahwala, J.; de Bacquer, D.; Segers, P.; Rietzschel, E.R. Effect of obesity on left atrial strain in persons aged 35–55 years (The Asklepios study). Am. J. Cardiol. 2019, 123, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Mishima, R.S.; Ariyaratnam, J.P.; Pitman, B.M.; Malik, V.; Emami, M.; McNamee, O.; Stokes, M.B.; Lau, D.H.; Sanders, P.; Elliott, A.D. Cardiorespiratory fitness, obesity and left atrial function in patients with atrial fibrillation. IJC Heart Vasc. 2022, 42, 101083. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-C.; Lin, Y.-H. The pathogenic role of very low density lipoprotein on atrial remodeling in the metabolic syndrome. Int. J. Mol. Sci. 2020, 21, 891. [Google Scholar] [CrossRef] [PubMed]
- Rafaqat, S. Biomarkers of metabolic syndrome: Role in pathogenesis and pathophysiology of atrial fibrillation. J. Atr. Fibrillation 2021, 14, 20200495. [Google Scholar] [CrossRef]
- Nalliah, C.J.; Wong, G.R.; Lee, G.; Voskoboinik, A.; Kee, K.; Goldin, J.; Watts, T.; Linz, D.; Wirth, D.; Parameswaran, R.; et al. Sleep apnoea has a dose-dependent effect on atrial remodelling in paroxysmal but not persistent atrial fibrillation: A high-density mapping study. EP Eur. 2021, 23, 691–700. [Google Scholar] [CrossRef]
- Vizzardi, E.; Sciatti, E.; Bonadei, I.; D’Aloia, A.; Curnis, A.; Metra, M. Obstructive sleep apnoea—Hypopnoea and arrhythmias. J. Cardiovasc. Med. 2017, 18, 490–500. [Google Scholar] [CrossRef]
- Wong, G.R.; Nalliah, C.J.; Lee, G.; Voskoboinik, A.; Chieng, D.; Prabhu, S.; Parameswaran, R.; Sugumar, H.; Al-Kaisey, A.; McLellan, A.; et al. Sex-related differences in atrial remodeling in patients with atrial fibrillation: Relationship to ablation outcomes. Circ. Arrhythm. Electrophysiol. 2022, 15, e009925. [Google Scholar] [CrossRef]
- Akoum, N.; Mahnkopf, C.; Kholmovski, E.G.; Brachmann, J.; Marrouche, N.F. Age and sex differences in atrial fibrosis among patients with atrial fibrillation. EP Eur. 2018, 20, 1086–1092. [Google Scholar] [CrossRef]
- Paliwal, N.; Ali, R.L.; Salvador, M.; O’Hara, R.; Yu, R.; Daimee, U.A.; Akhtar, T.; Pandey, P.; Spragg, D.D.; Calkins, H.; et al. Presence of left atrial fibrosis may contribute to aberrant hemodynamics and increased risk of stroke in atrial fibrillation patients. Front. Physiol. 2021, 12, 657452. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Z.; Yin, Z.; Zhang, Y.; Xue, X.; Han, J.; Zhu, Y.; Zhang, J.; Emmert, M.Y.; Wang, H. Gender differences in fibrosis remodeling in patients with long-standing persistent atrial fibrillation. Oncotarget 2017, 8, 53714–53729. [Google Scholar] [CrossRef]
- Polejaeva, I.A.; Ranjan, R.; Davies, C.J.; Regouski, M.; Hall, J.; Olsen, A.L.; Meng, Q.; Rutigliano, H.M.; Dosdall, D.J.; Angel, N.A.; et al. Increased susceptibility to atrial fibrillation secondary to atrial fibrosis in transgenic goats expressing transforming growth factor-Β1. J. Cardiovasc. Electrophysiol. 2016, 27, 1220–1229. [Google Scholar] [CrossRef]
- Ammar-Busch, S.; Buiatti, A.; Tatzber, A.; Reents, T.; Bourier, F.; Semmler, V.; Telishevska, M.; Hessling, G.; Deisenhofer, I. Predictors of low voltage areas in persistent atrial fibrillation: Is it really a matter of time? J. Interv. Card. Electrophysiol. 2020, 57, 345–352. [Google Scholar] [CrossRef]
- Ríos-Muñoz, G.R.; Soto, N.; Ávila, P.; Carta, A.; Atienza, F.; Datino, T.; González-Torrecilla, E.; Fernández-Avilés, F.; Arenal, Á. Structural remodeling and rotational activity in persistent/long-lasting atrial fibrillation: Gender-effect differences and impact on post-ablation outcome. Front. Cardiovasc. Med. 2022, 9, 819429. [Google Scholar] [CrossRef]
- Lau, C.-P.; Tse, H.-F.; Siu, C.-W.; Gbadebo, D. Atrial electrical and structural remodeling: Implications for racial differences in atrial fibrillation. J. Cardiovasc. Electrophysiol. 2012, 23, s36–s40. [Google Scholar] [CrossRef]
- Dmour, B.-A.; Miftode, R.-S.; Iliescu Halitchi, D.; Anton-Paduraru, D.T.; Iliescu Halitchi, C.-O.; Miftode, I.-L.; Mitu, O.; Costache, A.-D.; Stafie, C.-S.; Costache, I.I. Latest insights into mechanisms behind atrial cardiomyopathy: It is not always about ventricular function. Diagnostics 2021, 11, 449. [Google Scholar] [CrossRef]
- Wan, D.; Andrade, J.; Laksman, Z. Thromboembolic risk stratification in atrial fibrillation—Beyond clinical risk scores. Rev. Cardiovasc. Med. 2021, 22, 353. [Google Scholar] [CrossRef]
- Maille, B.; Das, M.; Hussein, A.; Shaw, M.; Chaturvedi, V.; Williams, E.; Morgan, M.; Ronayne, C.; Snowdon, R.L.; Gupta, D. Reverse electrical and structural remodeling of the left atrium occurs early after pulmonary vein isolation for persistent atrial fibrillation. J. Interv. Card. Electrophysiol. 2020, 58, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-H.; Li, H.-K.; Couri, D.M.; Araoz, P.A.; Lee, Y.-H.; Ma, C.-S.; Packer, D.L.; Cha, Y.-M. Reversal of pulmonary vein remodeling after catheter ablation of atrial fibrillation. J. Geriatr. Cardiol. 2016, 13, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Hori, Y.; Nakahara, S.; Fukuda, R.; Sato, H.; Ukaji, T.; Koshikawa, Y.; Nishiyama, N.; Ishikawa, T.; Kobayashi, S.; Taguchi, I. Atrial reverse remodeling represented by the atrial conduction time in persistent atrial fibrillation patients after catheter ablation: Its impact on predicting late atrial fibrillation recurrence. J. Cardiol. 2020, 75, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Kriatselis, C.; Unruh, T.; Kaufmann, J.; Gerds-Li, J.-H.; Kelle, S.; Gebker, R.; Jahnke, C.; Paetsch, I.; Pieske, B. Long-term left atrial remodeling after ablation of persistent atrial fibrillation: 7-year follow-up by cardiovascular magnetic resonance imaging. J. Interv. Card. Electrophysiol. 2020, 58, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Park, H.-S.; Han, S.; Jun, S.-W.; Kang, N.-Y.; Jeon, J.-H.; Choi, S.-W.; Lee, C.H.; Kim, I.-C.; Cho, Y.-K.; et al. The impact of catheter ablation of atrial fibrillation on the left atrial volume and function: Study using three-dimensional echocardiography. J. Interv. Card. Electrophysiol. 2020, 57, 87–95. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Q.; Yang, Y.; Zhang, C.; Yin, H.; Wu, J.; Yao, L.; Jin, L.; Yang, J.; Feng, L.; et al. Effect of radiofrequency catheter ablation on left atrial structure and function in patients with different types of atrial fibrillation. Sci. Rep. 2022, 12, 9511. [Google Scholar] [CrossRef]
- Liżewska-Springer, A.; Dąbrowska-Kugacka, A.; Lewicka, E.; Królak, T.; Drelich, Ł.; Kozłowski, D.; Raczak, G. Echocardiographic assessment in patients with atrial fibrillation (AF) and normal systolic left ventricular function before and after catheter ablation: If AF begets AF, does pulmonary vein isolation terminate the vicious circle? Cardiol. J. 2020, 27, 126–135. [Google Scholar] [CrossRef]
- Nagai, T.; Arakawa, J.; Hamabe, A.; Tabata, H. Improvement of left ventricular function after successful radiofrequency catheter ablation in persistent atrial fibrillation with preserved left ventricular ejection fraction: A comprehensive echocardiographic assessment using two-dimensional speckle tracking analysis. J. Echocardiogr. 2019, 17, 95–103. [Google Scholar] [CrossRef]
- Nishino, S.; Watanabe, N.; Ashikaga, K.; Morihisa, K.; Kuriyama, N.; Asada, Y.; Shibata, Y. Reverse remodeling of the mitral valve complex after radiofrequency catheter ablation for atrial fibrillation. Circ. Cardiovasc. Imaging 2019, 12, e009317. [Google Scholar] [CrossRef]
- Masuda, M.; Sekiya, K.; Asai, M.; Iida, O.; Okamoto, S.; Ishihara, T.; Nanto, K.; Kanda, T.; Tsujimura, T.; Matsuda, Y.; et al. Influence of catheter ablation for atrial fibrillation on atrial and ventricular functional mitral regurgitation. ESC Heart Fail. 2022, 9, 1901–1913. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Lv, M.; Yang, Z.; Zhu, S.; Wei, L.; Hong, T.; Ding, W.; Lin, Y.; Wang, C. Mitral Valve Repair and surgical ablation for atrial functional mitral regurgitation. Ann. Transl. Med. 2020, 8, 1420. [Google Scholar] [CrossRef]
- Kawakami, H.; Inoue, K.; Nagai, T.; Fujii, A.; Sasaki, Y.; Shikano, Y.; Sakuoka, N.; Miyazaki, M.; Takasuka, Y.; Ikeda, S.; et al. Persistence of left atrial abnormalities despite left atrial volume normalization after successful ablation of atrial fibrillation. J. Arrhythm. 2021, 37, 1318–1329. [Google Scholar] [CrossRef]
- Miwa, Y.; Mohri, T.; Katsume, Y.; Tashiro, M.; Momose, Y.; Nonoguchi, N.; Hoshida, K.; Togashi, I.; Hagiwara, Y.; Maeda, A.; et al. Left atrial reverse remodeling following the modified box isolation with centerline in patients with persistent atrial fibrillation. Int. Heart J. 2021, 62, 21–108. [Google Scholar] [CrossRef]
- Sasaki, N.; Okumura, Y.; Watanabe, I.; Mano, H.; Nagashima, K.; Sonoda, K.; Kogawa, R.; Ohkubo, K.; Nakai, T.; Hirayama, A. Increased levels of inflammatory and extracellular matrix turnover biomarkers persist despite reverse atrial structural remodeling during the first year after atrial fibrillation ablation. J. Interv. Card. Electrophysiol. 2014, 39, 241–249. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, M.; Hao, Z.; Wang, N.; Zhang, M. Sacubitril/valsartan attenuates atrial structural remodelling in atrial fibrillation patients. ESC Heart Fail. 2022, 9, 2428–2434. [Google Scholar] [CrossRef]
- Takemoto, Y.; Ramirez, R.J.; Kaur, K.; Salvador-Montañés, O.; Ponce-Balbuena, D.; Ramos-Mondragón, R.; Ennis, S.R.; Guerrero-Serna, G.; Berenfeld, O.; Jalife, J. Eplerenone reduces atrial fibrillation burden without preventing atrial electrical remodeling. J. Am. Coll. Cardiol. 2017, 70, 2893–2905. [Google Scholar] [CrossRef]
- Tong, M.; Wang, J.; Ji, Y.; Chen, X.; Wang, J.; Wang, S.; Ruan, L.; Cui, H.; Zhou, Y.; Zhang, Q.; et al. Effect of eicosapentaenoic acid and pitavastatin on electrophysiology and anticoagulant gene expression in mice with rapid atrial pacing. Exp. Ther. Med. 2017, 14, 2310–2316. [Google Scholar] [CrossRef]
- Heijman, J.; Sutanto, H.; Crijns, H.J.G.M.; Nattel, S.; Trayanova, N.A. Computational models of atrial fibrillation: Achievements, challenges, and perspectives for improving clinical care. Cardiovasc. Res. 2021, 117, 1682–1699. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Hwang, M.; Song, J.-S.; Li, C.; Joung, B.; Sobie, E.A.; Pak, H.-N. The contribution of ionic currents to rate-dependent action potential duration and pattern of reentry in a mathematical model of human atrial fibrillation. PLoS ONE 2016, 11, e0150779. [Google Scholar] [CrossRef]
- Liberos, A.; Bueno-Orovio, A.; Rodrigo, M.; Ravens, U.; Hernandez-Romero, I.; Fernandez-Aviles, F.; Guillem, M.S.; Rodriguez, B.; Climent, A.M. Balance between sodium and calcium currents underlying chronic atrial fibrillation termination: An in silico intersubject variability study. Heart Rhythm 2016, 13, 2358–2365. [Google Scholar] [CrossRef]
- Palacio, L.C.; Ugarte, J.P.; Saiz, J.; Tobón, C. The effects of fibrotic cell type and its density on atrial fibrillation dynamics: An in silico study. Cells 2021, 10, 2769. [Google Scholar] [CrossRef] [PubMed]
- Jost, N.; Christ, T.; Magyar, J. New strategies for the treatment of atrial fibrillation. Pharmaceuticals 2021, 14, 926. [Google Scholar] [CrossRef] [PubMed]
- Calvo, D.; Filgueiras-Rama, D.; Jalife, J. Mechanisms and drug development in atrial fibrillation. Pharmacol. Rev. 2018, 70, 505–525. [Google Scholar] [CrossRef] [PubMed]
- Fenner, M.F.; Gatta, G.; Sattler, S.; Kuiper, M.; Hesselkilde, E.M.; Adler, D.M.T.; Smerup, M.; Schotten, U.; Sørensen, U.; Diness, J.G.; et al. Inhibition of small-conductance calcium-activated potassium current (IK,Ca) leads to differential atrial electrophysiological effects in a horse model of persistent atrial fibrillation. Front. Physiol. 2021, 12, 614483. [Google Scholar] [CrossRef] [PubMed]
- Yakabe, D.; Fukuyama, Y.; Araki, M.; Nakamura, T. Responsiveness to bepridil predicts atrial substrate in patients with persistent atrial fibrillation. J. Arrhythm. 2021, 37, 79–87. [Google Scholar] [CrossRef]
- Nakatani, Y.; Sakamoto, T.; Nishida, K.; Kataoka, N.; Yamaguchi, Y.; Sakabe, M.; Fujiki, A.; Mizumaki, K.; Inoue, H. Bepridil enhances aprindine-induced prolongation of atrial effective refractory period in a canine atrial rapid pacing model. J. Cardiol. 2015, 66, 445–450. [Google Scholar] [CrossRef]
- Zhao, Z.; Niu, X.; Dong, Z.; Qi, W.; Liu, E.; Liu, T.; Li, L.; Liang, Y.; Li, G. Upstream therapeutic strategies of valsartan and fluvastatin on hypertensive patients with non-permanent atrial fibrillation. Cardiovasc. Ther. 2018, 36, e12478. [Google Scholar] [CrossRef]
- Shao, Q.; Meng, L.; Lee, S.; Tse, G.; Gong, M.; Zhang, Z.; Zhao, J.; Zhao, Y.; Li, G.; Liu, T. Empagliflozin, a sodium glucose co-transporter-2 inhibitor, alleviates atrial remodeling and improves mitochondrial function in high-fat diet/streptozotocin-induced diabetic rats. Cardiovasc. Diabetol. 2019, 18, 165. [Google Scholar] [CrossRef]
- Nishinarita, R.; Niwano, S.; Niwano, H.; Nakamura, H.; Saito, D.; Sato, T.; Matsuura, G.; Arakawa, Y.; Kobayashi, S.; Shirakawa, Y.; et al. Canagliflozin suppresses atrial remodeling in a canine atrial fibrillation model. J. Am. Heart Assoc. 2021, 10, e017483. [Google Scholar] [CrossRef]
- Kiedrowicz, R.M.; Wielusiński, M.; Wojtarowicz, A.; Kaźmierczak, J. Atrial fibrillation risk scores to evaluate left atrial substrate based on voltage analysis in long-standing persistent type of arrhythmia. Kardiol. Pol. 2021, 79, 525–530. [Google Scholar] [CrossRef]
- Marchandise, S.; Garnir, Q.; Scavée, C.; Varnavas, V.; le Polain de Waroux, J.-B.; Wauters, A.; Beauloye, C.; Roelants, V.; Gerber, B.L. Prediction of left atrial fibrosis and success of catheter ablation by speckle tracking echocardiography in patients imaged in persistent atrial fibrillation. Front. Cardiovasc. Med. 2022, 9, 856796. [Google Scholar] [CrossRef]
- Sardu, C.; Santulli, G.; Guerra, G.; Trotta, M.C.; Santamaria, M.; Sacra, C.; Testa, N.; Ducceschi, V.; Gatta, G.; d’Amico, M.; et al. Modulation of SERCA in patients with persistent atrial fibrillation treated by epicardial thoracoscopic ablation: The CAMAF study. J. Clin. Med. 2020, 9, 544. [Google Scholar] [CrossRef]
- Roney, C.H.; Beach, M.L.; Mehta, A.M.; Sim, I.; Corrado, C.; Bendikas, R.; Solis-Lemus, J.A.; Razeghi, O.; Whitaker, J.; O’Neill, L.; et al. In silico comparison of left atrial ablation techniques that target the anatomical, structural, and electrical substrates of atrial fibrillation. Front. Physiol. 2020, 11, 1145. [Google Scholar] [CrossRef]
- Kim, I.-S.; Lim, B.; Shim, J.; Hwang, M.; Yu, H.T.; Kim, T.-H.; Uhm, J.-S.; Kim, S.-H.; Joung, B.; On, Y.K.; et al. Clinical usefulness of computational modeling-guided persistent atrial fibrillation ablation: Updated outcome of multicenter randomized study. Front. Physiol. 2019, 10, 1512. [Google Scholar] [CrossRef]
- Kanemaru, Y.; Arima, Y.; Kaikita, K.; Kiyama, T.; Kaneko, S.; Ito, M.; Yamabe, H.; Motozato, K.; Yamanaga, K.; Fujisue, K.; et al. Elongation of the high right atrium to coronary sinus conduction time predicts the recurrence of atrial fibrillation after radiofrequency catheter ablation. Int. J. Cardiol. 2020, 300, 147–153. [Google Scholar] [CrossRef]
- Higuchi, S.; Ejima, K.; Shoda, M.; Yamamoto, E.; Iwanami, Y.; Yagishita, D.; Hagiwara, N. Impact of a prolonged interatrial conduction time for predicting the recurrence of atrial fibrillation after circumferential pulmonary vein isolation of persistent atrial fibrillation. Heart Vessel. 2019, 34, 616–624. [Google Scholar] [CrossRef]
- Mathias, A.; Moss, A.J.; McNitt, S.; Zareba, W.; Goldenberg, I.; Solomon, S.D.; Kutyifa, V. Clinical implications of complete left-sided reverse remodeling with cardiac resynchronization therapy. J. Am. Coll. Cardiol. 2016, 68, 1268–1276. [Google Scholar] [CrossRef]
- Alexander, B.; Sadiq, F.; Azimi, K.; Glover, B.; Antiperovitch, P.; Hopman, W.M.; Jaff, Z.; Baranchuk, A. Reverse atrial electrical remodeling induced by cardiac resynchronization therapy. J. Electrocardiol. 2017, 50, 610–614. [Google Scholar] [CrossRef]
- Kugler, L.; Markendorf, S.; Bachmann, M.; Eriksson, U. Cardiac resynchronization therapy in the presence of total atrioventricular block reduces long-lasting atrial fibrillation episodes. J. Arrhythm. 2022, 38, 723–729. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roka, A.; Burright, I. Remodeling in Persistent Atrial Fibrillation: Pathophysiology and Therapeutic Targets—A Systematic Review. Physiologia 2023, 3, 43-72. https://doi.org/10.3390/physiologia3010004
Roka A, Burright I. Remodeling in Persistent Atrial Fibrillation: Pathophysiology and Therapeutic Targets—A Systematic Review. Physiologia. 2023; 3(1):43-72. https://doi.org/10.3390/physiologia3010004
Chicago/Turabian StyleRoka, Attila, and Isaac Burright. 2023. "Remodeling in Persistent Atrial Fibrillation: Pathophysiology and Therapeutic Targets—A Systematic Review" Physiologia 3, no. 1: 43-72. https://doi.org/10.3390/physiologia3010004
APA StyleRoka, A., & Burright, I. (2023). Remodeling in Persistent Atrial Fibrillation: Pathophysiology and Therapeutic Targets—A Systematic Review. Physiologia, 3(1), 43-72. https://doi.org/10.3390/physiologia3010004





