Abstract
Wnt signaling involves multiple pathways that contribute to organ development, cell fate, inflammation, and normal stem cell renewal and maintenance. Although the homeostasis of stem cells in the gastrointestinal (GI) tract highly depends on the Wnt signaling pathway, this regulation is impaired in cancers and in aging. Overactive (uncontrolled) Wnt signaling can induce GI epithelial cancers such as colon and gastric cancer. Overactive Wnt signaling can also contribute to the initiation and progression of gastrointestinal stromal tumor, which is the most common human sarcoma occurring in the walls of the digestive organs, mainly the stomach and small intestine. Wnt expression is positively associated not only with the progression of oncogenesis but also with resistance to chemotherapy and radiotherapy. Of note, recent reports show that decreased Wnt signaling is related to intestinal stem cell aging and that overactivated Wnt signaling leads to gastric pacemaker stem cell aging in tunica muscularis. These findings indicate that Wnt signaling has different crucial aspects of cell fate determination with age in GI tunica mucosa and muscularis. In this review, we summarize the most recent advances in our understanding of Wnt signaling pathways and their role in regulating key aspects during development, carcinogenesis, inflammation, and aging, with the ultimate goal of identifying novel therapies.
1. Introduction
Wnt (wingless-type MMTV integration site) signaling is an evolutionarily conserved signaling mechanism that involves multiple pathways contributing to organ development and formation, stem cell renewal, and cell maintenance. Wnts are secreted, cysteine-rich glycoproteins that activate intracellular signaling cascades [1] by binding to many receptors and coreceptors. Wnt signaling has roles in both cancer and embryonic development. Uncontrolled (overactivated or underactivated) Wnt signaling can induce cellular senescence, a state of permanent cell-cycle arrest, or can lead to uncontrolled hypergrowth of cells (i.e., cancer) in the gastrointestinal (GI) tract. This implies that the Wnt pathway has relevance regarding the regulation of cell growth and proliferation. The regulation of important Wnt signaling targets has been shown to affect the differentiation of stem cells during embryonic development [2]. This suggests not only the importance of Wnt regarding early development but also the importance of Wnt in relation to stem cell function and proliferation. Due to the pathway’s cellular importance and potential to inflict diseases, there is ample reason to research the Wnt signaling pathway for novel treatments especially in regard to cancer treatments, as GI cancers’ inclination toward the resistance of chemotherapeutic drugs spurs efforts toward alternative therapies [3]. Understanding Wnt signaling in GI diseases is key to developing treatments to prevent or reverse them. In this review, we summarize the role of Wnt signaling in the GI tract in health and disease.
2. Pathway Types
Wnt signaling is divided into three pathways: canonical pathway, noncanonical planar cell polarity (PCP) pathway, and noncanonical Wnt/calcium pathway.
2.1. Canonical Wnt Signaling Pathway, β-Catenin Dependent
The canonical Wnt signaling pathway primarily involves β-catenin, a core component of cadherin proteins, which regulate cell adhesion and gene transcription. The absence of Wnt ligands leads to the phosphorylation of cytoplasmic β-catenin by glycogen synthase kinase-3β and proteasomal degradation of β-catenin (Figure 1A) [4]. Wnt signaling is active in response to normal development and the growth of various tissues. When Wnt signaling is turned “on,” Wnt proteins bind to Frizzled receptor and LRP5/6 (lipoprotein receptor-related protein 5/6) coreceptor, which inhibits the β-catenin destruction complex and increases the level of cytoplasmic unphosphorylated (stable) β-catenin (Figure 1B). β-catenin then enters the nucleus, where it acts as a cofactor for various proteins such as TCF/LEF (T-cell specific factor/lymphoid enhancer factor), p300, CREB-binding protein, Pygopus, and Bcl-9. These protein complexes, in turn, activate the transcription of Wnt target genes such as c-Myc, cyclin D1, and p21CIP1/WAF1 (or cyclin-dependent kinase [5]. The extracellular domain of α-klotho, an antiaging protein, can activate dickkopf (DKK; a secreted inhibitor of canonical Wnt signaling) to prevent Wnt ligands from binding to their receptors (Figure 1C) [6]. As a result, downstream elements such as the expression of β-catenin and the translocation of β-catenin to the nucleus are downregulated, which leads to the decreased expression of Wnt target genes [6].
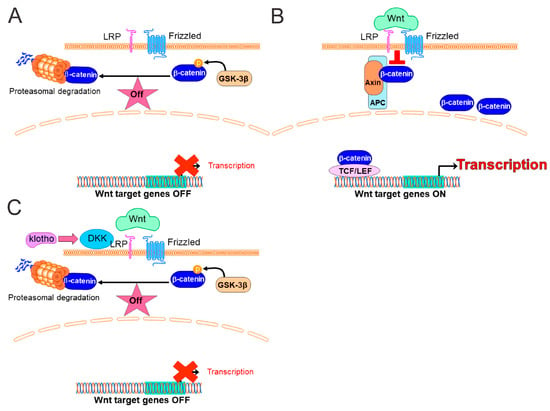
Figure 1.
The canonical Wnt (Wingless-Type MMTV Integration Site)/β-catenin Pathway. (A) Wnt in the “off” state. When Wnt receptor complexes are not bound by the ligands or if Wnt ligands are absent, the destruction of β-catenin, formed by Axin, APC (adenomatous polyposis coli), and GSK (glycogen synthase kinase)-3β, phosphorylate β-catenin. Phosphorylated β-catenin is targeted for rapid degradation by the proteasome. In the nucleus, the transcription of Wnt target genes is inhibited (red X). (B) Wnt in the “on” state. When Wnt proteins bind to Frizzled receptors and LRP (lipoprotein receptor-related protein) coreceptors in response to stimuli such as inflammation, cancer, and aging, the Frizzled/LRP coreceptor complex activates the canonical Wnt signaling pathway by suppressing the activity of GSK-3β and regulating the docking of Axin. The recruitment of Axin away from the destruction complex leads to the stabilization of β-catenin (unphosphorylated β-catenin). In the nucleus, the β-catenin and TCF/LEF complex leads to the transcriptional activation of Wnt target genes. (C) The antiaging protein Klotho is an endogenous Wnt antagonist. Soluble Klotho effectively binds to dickkopf (DKK), which prevents Wnt ligands from binding to their receptors, thereby negatively controlling Wnt action.
2.2. Noncanonical Wnt Pathways, β-Catenin Independent
β-catenin-independent pathways are the noncanonical PCP and Wnt/calcium pathways, which have some interactions with the canonical Wnt pathway. The noncanonical PCP pathway begins with Wnt proteins attaching to Frizzled receptors, along with participating coreceptors, receptor tyrosine kinases RYK and ROR, which control the activity of GTPases such as RhoA (Ras homolog gene family member A), RAC (Ras-related C3 botulinum toxin substrate), and CDC42 (cell division control protein 42). These GTPases have a role in cytoskeleton remodeling and affect the transcriptional activation of target genes, which leads to cell adhesion and migration [7].
The Wnt/calcium pathway is activated by Wnt5a and Frizzled 2, which cleaves G-proteins, inducing calcium (Ca2+) to be released into the cytoplasm. The noncanonical calcium-dependent pathway has an important role in the attachment of RYK or ROR (alternative receptors), which affects cell migration and inhibition of the Wnt canonical pathway. This is accomplished through the regulation of intracellular calcium flux and the activation of calmodulin kinase II, JNKs (c-Jun N-terminal kinases), and protein kinase C [8].
2.3. Crosstalk between Wnt and Notch Signaling Pathway
The Notch pathway—another highly conserved signaling pathway that critically regulates cell fates, cell proliferation, and cell death during development [9]—interacts with the Wnt/β-catenin pathways for developmental processes [9]. The Notch receptor negatively regulates the stability of β-catenin. In contrast, HES1 (the gene encoding a Notch ligand) is a target of Wnt/β-catenin signaling and is regulated by the Wnt signaling pathway. In addition, glycogen synthase kinase-3β can phosphorylate the Notch intracellular domain, which increases its level in the nucleus [10].
3. Wnt Signaling in GI Stem Cells
Wnt signaling has been implicated in the control over adult mammalian tissue stem cells, including stem cells in the GI tract. Intestinal stem cells (ISCs) can self-renew by removing dead cells and renewing the epithelium with new cells every 3 to 4 days throughout life [11]. ISCs differentiate into a population of nonspecific transit-amplifying cells that rapidly proliferate and migrate vertically along an intestinal crypt to produce multiple cell lineages. The homeostasis of ISCs highly depends on the Wnt/β-catenin signaling pathway [12]. The gradient of Wnt levels along the crypt–villus axis is produced by Wnt3a, a canonical Wnt agonist, from Paneth cells (specialized secretory cells located at the bottom of intestinal crypts), not epithelial or subepithelial cells [13]. The ISC-specific expression of R-spondin 1 controls the upward movement of progeny cells from the crypt bottom to the villus and induces them to differentiate into various cell types [14]. Several key regulators of Wnt signaling in ISCs include knockdown of transcription factor 4, knockdown of Myc (mostly referred to as c-Myc), and upregulation of DKK. The manipulation of these key regulators can cause an aberrant gradient of Wnt activity, which disrupts the intestinal crypt–villus structure [15,16]. The conditional deletion of Myc from the intestinal epithelium causes crypt atrophy, which occurs by the clearing of Myc-deficient cells from the epithelium. Together, these findings emphasize the critical roles of canonical Wnt signaling in the maintenance of ISCs. Compared to canonical Wnt signaling, noncanonical signaling is involved in cell polarity and motility [17]. Of note, noncanonical Wnt signaling is required for the migration of ISCs to regenerate in response to injury [18,19]. Thus, the interrelationship between the Wnt signaling pathways might be a potential pathological mechanism of several diseases.
The enteric nervous system is an extensive network of enteric neurons and glial cells located along the GI tract which controls digestive functions [20]. Enteric neural progenitor/stem cells (ENSCs) for the enteric nervous system might serve as a possible source for cell-based treatment of neurogastrointestinal disorders such as Hirschsprung disease [21]. Although the maintenance of ENSCs also depends on Wnt signaling, the detailed mechanism of Wnt signaling in ENSCs and their roles in GI diseases remain unclear and will require further studies [22,23]. Recently, single nucleus RNA-seq for the isolation of intact nuclei and ribosome-bound RNA and mining rare cells sequencing (MIRACL-seq) for label-free profiling displayed diverse enteric neurons, epithelial, stromal, and immune cells and also revealed the putative interactions of these cells. Future works will dissect these interaction and clarify in the context of several GI diseases and disorders [24,25].
4. Wnt Signaling in GI Inflammation
Inflammatory bowel disease (IBD) is an immune-mediated disorder characterized by long-lasting inflammation of the GI tract. Types of IBD include ulcerative colitis characterized by chronic inflammation and ulcer of the large intestine and rectum and Crohn’s disease characterized by inflammation of the whole GI tract, mainly the small intestine. The hallmark of IBD is chronic recurring inflammation of the intestinal mucosa, which cause diarrhea, rectal bleeding, abdominal pain, and weight loss. While the main causes of IBD are not fully understood despite decades of intensive investigation, the interaction between the immune systems, environmental factors and (epi)genetics may underlie the pathogenesis. As mentioned above, Wnt signaling is generally considered as a key player in cell fate determination, cell polarity and organogenesis, and it is not an inflammatory pathway. However, there is accumulating evidence that Wnt signaling is linked to an inflammatory pathway in IBD and might be a potential therapeutic target. During colitis, tumor necrosis factor-alpha (TNFα), a central regulator of inflammation, upregulates Wnt/β-catenin target genes, and TNFα-induced Wnt/β-catenin activation is protective against apoptosis in ISCs [26]. These findings indicate that TNFα-induced Wnt/β-catenin signaling plays crucial roles in wound healing in IBD. In contrast to TNFα, transforming growth factor-beta (TGFβ), an inhibitory cytokine, induces DKK leading to reduced Wnt signaling in intestinal epithelial cells [27]. These experimental findings are confirmed by a clinical study indicating that DKK expression was higher and β-catenin expression was lower in IBD patients [28]. Importantly, DKK expression was reduced and β-catenin expression was increased after TNFα blockade by infliximab, which is a chimeric monoclonal antibody against TNFα [28], suggesting DKK and β-catenin expression as potential biomarkers of inflammation in IBD patients. In contrast, non-canonical Wnt/β-catenin agonist Wnt5a plays a critical role in the regulation of inflammatory cytokines during colitis, and Wnt5a expression is inversely correlated with inflammation and TNF expression in IBD patients [29]. Interestingly, Wnt5a peptide suppresses experimental colitis in mice, and Wnt5a might be a potential therapeutic option for IBD [29].
Wnt signaling’s relationship with inflammation is not concentrated solely in the intestines, as the development of esophagitis has shown a clear correlation with the regulation of the Wnt pathway. Esophagitis is a disorder that is heavily characterized by an inflamed esophagus caused by either an overabundance of eosinophils or chronic acid reflux [30], and a dysregulation of Wnt signaling was reported in esophagitis [31]. A recent study demonstrates that the Wnt pathway regulates the cell lineages of the esophagus [32] and dysregulation of Wnt signaling and upregulation inflammatory cytokines were reported in esophagitis [31,33]. These findings suggest that tissue remodeling caused by the inflammation of esophagitis, and the subsequent diversion from the Wnt pathway implies Wnt’s role as a regulator of esophageal tissue maintenance during inflammation.
Helicobacter pylori (H. pylori) is a bacteria strain known to infect the gastric mucosa of humans and is associated with increased inflammation in the upper GI tract [34]. The bacterial infection increases in risk especially in the elderly, often bringing peptic ulcer diseases, chronic gastritis, and gastric cancer development, which is the fourth most common cause of cancer worldwide. Regarding cellular senescence (a state of irreversible cell cycle arrest associated with chronic inflammation), H. pylori often causes oxidative DNA damage to mucosal tissue [35]. Not only that, H. pylori increases the inflammatory environment via mucosal lesions and nitric oxidation. Given how oxidative DNA damage often leads to the accumulation of senescent cells, this implies that H. pylori increases senescence in the gastric mucosa. H. pylori infections have even been shown to upregulate Wnt/β-catenin target genes, which can lead to either cell proliferation or onset gastritis [36,37,38]. This suggests a possible connection between the Wnt/β-catenin pathway and H. pylori. Although inhibitors of Wnt/β-catenin have been studied in gastric cancer [39], these inhibitors have not been tested in H. pylori-induced gastritis, and further investigation is warranted.
Most reagents targeting Wnt signaling interact with both canonical and noncanonical Wnt signaling pathways and lack of cell specificity. Improvement of both the selectivity of signaling and cell specificity might increase the beneficial effects in various GI inflammatory disorders.
5. Wnt Signaling in GI Cancer
The overactivation of Wnt signaling is mainly caused by sequence variations in adenomatous polyposis coli (APC) or β-catenin, as observed in GI cancers [40]. APC variations do not directly affect Wnt signaling, but regulation of APC is essential for this pathway. Tumors with APC variations have variable levels of nuclear β-catenin [41,42]. Interestingly, recent reports demonstrated that Apc mutant ISCs selectively replace normal (Apc wild type) ISCs and facilitate intestinal tumor formation [43]. This replacement is promoted by Notum, which is an Wnt deacylase that disrupts Wnt ligand binding to Frizzle receptors [44] secreted by Apc mutant ISCs. Importantly, the “supercompetitor” phenotype is also observed in KRAS (KRAS proto-oncogene, GTPase) and PI3K (phosphoinositide 3-kinase) mutant ISCs [45], suggesting these phenotypes are common to ISCs with different oncogenic mutations. The Wnt pathway also has a critical role in the epithelial–mesenchymal transition of tumor cells, which is a developmental program and key driver of cancer metastasis [46]. Another important aspect of Wnt signaling is that Wnt/β-catenin is positively associated with cancer cell resistance to chemotherapy and radiation [47]. The upregulation of this signaling pathway results in cancer cell protection from apoptosis and cell-cycle arrest [48]. Moreover, high levels of Wnt signaling increase DNA damage repair, which can increase resistance to the PARP (poly-ribose polymerase) inhibitor in gynecologic cancers [49]. In contrast, the promoter of the multidrug resistance gene ABCB1/multidrug resistance mutation 1 (MDR1) consists of several TCF binding parts that affect its transcription in colorectal cancer [50]. Oxaliplatin inhibits the Wnt/β-catenin signaling pathway by suppressing the expression of MDR1/P-glycoprotein, which suggests that β-catenin reduction could decrease the transcription and expression of the MDR1 gene [51,52].
Akt-regulated pathways are frequently dysregulated due to sequence variations of p100α subunit of PI3K in many cancers, including colorectal cancers [53]. Akt is phosphorylated by protein kinase CK2, which is formerly known as casein kinase 2. Sequentially, Akt also phosphorylates β-catenin at Ser-552 to stabilize and activate canonical β-catenin [54]. The integration of these two critical signaling pathways enhances cancerous growth and confers resistance against current therapies [55,56]. The molecular mechanism responsible for the crosstalk between the Wnt/β-catenin and CK2/PI3K/Akt pathways in cancer remains unclear and requires further investigations.
p53 is a key cell-cycle inhibitor that regulates cell death [57]. This pathway and other mechanisms mediated by p53 cause downregulation of the β-catenin level. Thus, p53 has both negative and positive roles in the regulation of Wnt/β-catenin signaling. The translation, stability, and transcriptional activity of p53 are increased by 5-fluorouracil (5-FU), which is one of the most commonly used drugs to treat cancers [52]. 5-FU promotes various anticancer processes such as apoptosis and cell-cycle arrest [52]. More particularly, 5-FU activates p53-induced Wnt3 transcription in colonic stem cells, which is followed by the activation of Wnt/β-catenin signaling in tumor cells [52]. These findings suggest that the blockade of Wnt/β-catenin signaling or one of the downstream pathways can increase the therapeutic responsiveness of cancers to chemotherapy.
Although Wnt/β-catenin signaling is important for the development of GI epithelial tumors, it also has a crucial role in gastrointestinal stromal tumor (GIST), which is a type of rare tumor that occurs in the GI tunica muscularis [58]. GIST is thought to arise from the interstitial cells of Cajal (ICC), electrical pacemakers and mediators of neuromuscular neurotransmission, or from the stem cells of ICC (ICC-SC) [58,59]. Imatinib mesylate, a KIT/platelet-derived growth factor α tyrosine kinase inhibitor, remains the first line of treatment for advanced GIST, but complete cure is rare [60]. Wnt/β-catenin signaling is critical for ICC/ICC-SC growth and maintenance, but its role in GIST is unclear [61]. A subtype of GIST expresses β-catenin and displays dephosphorylated (active) β-catenin during tumorigenesis [62]. The potent and clinically promising tankyrase inhibitor G007-LK, which inhibits the CREB-binding protein/β-catenin complex, has antitumor activity in KitV558Δ/+ mice (a mouse model of spontaneous GIST), and this effect is enhanced in the presence of imatinib mesylate [62]. Therefore, the inhibition of Wnt/β-catenin signaling seems to be a promising therapeutic approach for advanced GIST, especially imatinib-resistant GIST. Small interfering RNA (siRNA)-mediated knocking down β-catenin has also been found to prevent the proliferation of ICC-SC, a possible origin of GIST precursors [63], suggesting that inhibitors of Wnt/β-catenin signaling may prevent imatinib-resistant GIST via the targeting of their stem cells. Given the interaction of Wnt signaling with other key molecules and pathways in GI cancers as described above, future studies should focus on the crosstalk between Wnt and related signaling networks.
There have been no FDA (Food and Drug Administration)-approved Wnt/β-catenin inhibitors despite great efforts. Clinical trials of several inhibitors for GI cancers are ongoing or/and completed (Table 1). As described above, the Wnt/β-catenin targeted interventions have been proved to represent promising candidates for GI cancers. Further investigations of clinical trials are warranted to confirm the safety, efficacy and patient stratification.

Table 1.
Wnt/β-catenin inhibitors in clinical trials for GI cancers.
6. Aging and Cellular Senescence
Aging is a complicated biological process regulated by various factors including genetic background and environmental stresses. Cellular senescence, a stress-responsive, irreversible cell-cycle arrest, seems to have a central role in aging and aging-related disorders and diseases. Interestingly, a connection exists between the Wnt signaling pathway and senescence via the interaction between the antiaging α-klotho protein and Wnt proteins [64,65]. α-Klotho is a transmembrane protein that is cleaved to form a circulating peptide [66,67]. The protein is primarily present in the kidney, and the α-klotho gene, KL, is located on chromosome 13 in humans and on chromosome 5 in mice [68,69]. α-Klotho has multiple functions for organ protection [70]. Transgenic mice engineered to overexpress α-klotho under the control of human elongation factor 1α promoter (EFmKL46 and EFmKL48 mice) have extended life spans [71]. In contrast to EFmKL46 and EFmKL48 mice, progeric mice hypomorphic for the α-klotho gene (klotho mice) have the development of multiple aging phenotypes after weaning and have premature death around age 60 days [69].
As described above and shown in Figure 1C, the cleaved (secreted) α-klotho protein inhibits canonical Wnt signaling via DKK [72]. Thus, Wnt signaling activity is augmented in progeric klotho mice, which is associated with a decreased stem cell population and more senescent cells in the skin and the small intestine [6]. These findings suggest that canonical Wnt signaling activation has an important causal role in the aging of some organs. Similar to the process of skeletal muscle aging, canonical Wnt signaling is activated with age, and this overactivation of Wnt signaling is associated with a decrease in ICC (Figure 2B) [61]. This decrease reflects reduced ICC-SC proliferation and self-renewal [73]. p53 is involved in this phenomenon owing to the upregulation of canonical Wnt signaling [61]. In sharp contrast to GI tunica muscularis, aged intestinal mucosa has decreased levels of canonical Wnts, which leads to a decreased number and function of ISCs, as shown in both mice and humans (Figure 2A) [74,75]. The decrease in Wnt with age is mediated by Notum, an extracellular Wnt inhibitor, which is produced by aged intestinal Paneth cells [76]. This ISC aging mechanism is consistent with the mechanism of hematopoietic stem cell aging, in which their aging is accompanied by a related decrease in Wnt signaling [77]. Importantly, the addition of exogenous Wnt3a or pharmacologic Notum inhibition can restore aged ISC function [74]. The cause of differential Wnt signaling with age between tunica mucosa and tunica muscularis in the GI tract remains unclear, but further investigations into this cause may lead to a better understanding of the (patho)physiology in the GI tract apparatus [61,74].
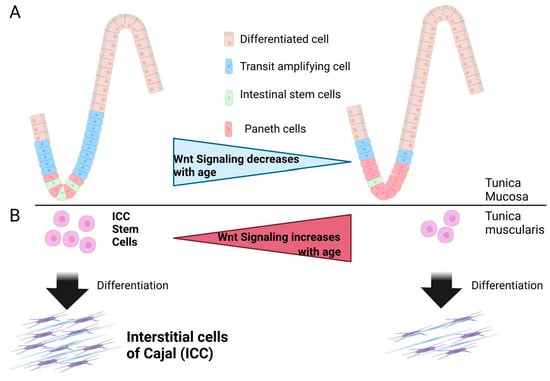
Figure 2.
Proposed Link Between Wnt (Wingless-Type MMTV Integration Site) Signaling, Intestinal Stem Cell Aging, and Interstitial Cell of Cajal (ICC) Stem Cell Aging. (A) In the tunica mucosa, aging-related reduced Wnt signaling leads to decreased numbers of intestinal stem cells and proliferating cells, whereas Paneth cells are increased. (B) The Wnt signaling pathway is overactivated in the aged gastric tunica muscularis and leads to decreased ICC and ICC stem cells.
7. Conclusions and Future Directions
As summarized above, Wnt signaling has a critical role in development, cancers, inflammation and aging in the GI tract. Although the detailed function and specificity of Wnt signaling are still unclear in some human organs, a growing body of research suggests a strong correlation between uncontrolled Wnt signaling and many types of human diseases and disorders. Wnt signaling has been shown to be tied with the regulation of GI stem cells, which can lead to aberrant intestinal crypt-villus structure due to either downregulation or upregulation. Wnt upregulation due to inflammatory factors coincides with cancer development due to Wnt’s relationship with metastasis and chemotherapeutic resistance. Combining these two functions of Wnt signaling shows that it is a major maintenance pathway that, when disrupted, can lead to hardy tumors. Despite intense efforts, the intervention of targeting Wnt signaling has not been translated into the clinic due to widespread effects of Wnt signaling in normal and diseased states. Hence, further investigation of Wnt signaling should focus on the exploration and characterization of tissue-specific Wnt signaling pathway and its crosstalk with other signaling pathways. This is critical for a better understanding of many pathophysiologic pathways and for developing novel treatments to prevent or reverse various human diseases and disorders. Furthermore, cutting-edge sequencing technologies integrated with multi-omics methods may help us reveal the single cell profile and cell interaction of complex organs and tissues leading to the novel diagnosis and treatment of Wnt signaling-associated diseases and disorders.
Author Contributions
N.T.: conception and design, manuscript writing, E.L.C.: manuscript writing, V.T.T.N.: manuscript editing, A.C.: manuscript editing, Y.H.: conception and design, financial support, manuscript writing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported in part by the National Institutes of Health Grants R01 DK121766 (Y.H.) and P30 DK084567 (Mayo Clinic Center for Cell Signaling in Gastroenterology); Mayo Clinic Center for Biomedical Discovery Pilot Award (Y.H.); and American Gastroenterology Association-Allergan Foundation Pilot Research Award in Gastroparesis (Y.H.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The funding agencies had no role in the study analysis or writing of the manuscript. Its contents are solely the responsibility of the authors. The Scientific Publications staff at Mayo Clinic provided editorial consultation, proofreading, and administrative and clerical support.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| 5-FU | 5-fluorouracil |
| APC | adenomatous polyposis coli |
| BRAF | B-RAF proto-oncogene |
| CDC42 | cell division control protein 42 |
| DKK | dickkopf |
| ENSC | enteric neural progenitor/stem cell |
| FDA | Food and Drug Administration |
| GI | gastrointestinal |
| GIST | gastrointestinal stromal tumor |
| IBD | inflammatory bowel disease |
| ICC | interstitial cells of Cajal |
| ICC-SC | ICC stem cell |
| ISC | intestinal stem cell |
| JNKs | c-Jun N-terminal kinases |
| KRAS | KRAS proto-oncogene |
| LRP5/6 | lipoprotein receptor-related protein5/6 |
| MDR1 | multidrug resistance mutation 1 |
| PARP | poly-ribose polymerase |
| PCP | planar cell polarity |
| PI3K | phosphoinositide 3-kinase |
| RAC | Ras-related C3 botulinum toxin substrate |
| RhoA | Ras homolog gene family member A |
| siRNA | small interfering RNA |
| TCF/LEF | T-cell specific factor/lymphoid enhancer |
| TGFβ | transforming growth factor-beta |
| TNFα | tumor necrosis factor-alpha |
| Wnt | wingless-type MMTV integration site |
References
- Nusslein-Volhard, C.; Wieschaus, E. Mutations affecting segment number and polarity in Drosophila. Nature 1980, 287, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Godoy-Parejo, C.; Deng, C.; Xu, J.; Zhang, Z.; Ren, Z.; Ai, N.; Liu, W.; Ge, W.; Deng, C.; Xu, X.; et al. Protein Kinase C Modulation Determines the Mesoderm/Extraembryonic Fate under BMP4 induction from Human Pluripotent Stem Cells. Stem Cells 2023, sxad006. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.C.; Zhang, J.Q.; Yan, T.H.; Miao, M.X.; Cao, Y.M.; Cao, Y.B.; Zhang, L.C.; Li, L. Novel strategies to reverse chemoresistance in colorectal cancer. Cancer Med. 2023. [Google Scholar] [CrossRef]
- Amirhosseini, M.; Madsen, R.V.; Escott, K.J.; Bostrom, M.P.; Ross, F.P.; Fahlgren, A. GSK-3beta inhibition suppresses instability-induced osteolysis by a dual action on osteoblast and osteoclast differentiation. J. Cell. Physiol. 2018, 233, 2398–2408. [Google Scholar] [CrossRef] [PubMed]
- Spiegelman, V.S.; Slaga, T.J.; Pagano, M.; Minamoto, T.; Ronai, Z.; Fuchs, S.Y. Wnt/beta-catenin signaling induces the expression and activity of betaTrCP ubiquitin ligase receptor. Mol. Cell 2000, 5, 877–882. [Google Scholar] [CrossRef]
- Liu, H.; Fergusson, M.M.; Castilho, R.M.; Liu, J.; Cao, L.; Chen, J.; Malide, D.; Rovira, I.I.; Schimel, D.; Kuo, C.J.; et al. Augmented Wnt signaling in a mammalian model of accelerated aging. Science 2007, 317, 803–806. [Google Scholar] [CrossRef]
- Yamamoto, S.; Nishimura, O.; Misaki, K.; Nishita, M.; Minami, Y.; Yonemura, S.; Tarui, H.; Sasaki, H. Cthrc1 selectively activates the planar cell polarity pathway of Wnt signaling by stabilizing the Wnt-receptor complex. Dev. Cell 2008, 15, 23–36. [Google Scholar] [CrossRef]
- Kim, S.E.; Huang, H.; Zhao, M.; Zhang, X.; Zhang, A.; Semonov, M.V.; MacDonald, B.T.; Zhang, X.; Garcia Abreu, J.; Peng, L.; et al. Wnt stabilization of beta-catenin reveals principles for morphogen receptor-scaffold assemblies. Science 2013, 340, 867–870. [Google Scholar] [CrossRef]
- Bertrand, F.E. The cross-talk of NOTCH and GSK-3 signaling in colon and other cancers. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118738. [Google Scholar] [CrossRef]
- O’Brien, C.A.; Kreso, A.; Jamieson, C.H. Cancer stem cells and self-renewal. Clin. Cancer Res. 2010, 16, 3113–3120. [Google Scholar] [CrossRef]
- Ritsma, L.; Ellenbroek, S.I.J.; Zomer, A.; Snippert, H.J.; de Sauvage, F.J.; Simons, B.D.; Clevers, H.; van Rheenen, J. Intestinal crypt homeostasis revealed at single-stem-cell level by in vivo live imaging. Nature 2014, 507, 362–365. [Google Scholar] [CrossRef]
- Ireland, H.; Kemp, R.; Houghton, C.; Howard, L.; Clarke, A.R.; Sansom, O.J.; Winton, D.J. Inducible Cre-mediated control of gene expression in the murine gastrointestinal tract: Effect of loss of beta-catenin. Gastroenterology 2004, 126, 1236–1246. [Google Scholar] [CrossRef]
- San Roman, A.K.; Jayewickreme, C.D.; Murtaugh, L.C.; Shivdasani, R.A. Wnt secretion from epithelial cells and subepithelial myofibroblasts is not required in the mouse intestinal stem cell niche in vivo. Stem Cell Rep. 2014, 2, 127–134. [Google Scholar] [CrossRef]
- Pinto, D.; Gregorieff, A.; Begthel, H.; Clevers, H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 2003, 17, 1709–1713. [Google Scholar] [CrossRef] [PubMed]
- Kuhnert, F.; Davis, C.R.; Wang, H.T.; Chu, P.; Lee, M.; Yuan, J.; Nusse, R.; Kuo, C.J. Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc. Natl. Acad. Sci. USA 2004, 101, 266–271. [Google Scholar] [CrossRef] [PubMed]
- van de Wetering, M.; Sancho, E.; Verweij, C.; de Lau, W.; Oving, I.; Hurlstone, A.; van der Horn, K.; Batlle, E.; Coudreuse, D.; Haramis, A.P.; et al. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell 2002, 111, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Bottcher, A.; Buttner, M.; Tritschler, S.; Sterr, M.; Aliluev, A.; Oppenlander, L.; Burtscher, I.; Sass, S.; Irmler, M.; Beckers, J.; et al. Non-canonical Wnt/PCP signalling regulates intestinal stem cell lineage priming towards enteroendocrine and Paneth cell fates. Nat. Cell Biol. 2021, 23, 23–31. [Google Scholar] [CrossRef]
- Hu, D.J.; Yun, J.; Elstrott, J.; Jasper, H. Non-canonical Wnt signaling promotes directed migration of intestinal stem cells to sites of injury. Nat. Commun. 2021, 12, 7150. [Google Scholar] [CrossRef]
- Kaur, P.; Chua, E.H.Z.; Lim, W.K.; Liu, J.; Harmston, N.; Tolwinski, N.S. Wnt Signaling Rescues Amyloid Beta-Induced Gut Stem Cell Loss. Cells 2022, 11, 281. [Google Scholar] [CrossRef]
- Obata, Y.; Castano, A.; Fallesen, T.L.; Bon-Frauches, A.C.; Boeing, S.; Huseynova, A.; McCallum, S.; Lasrado, R.; Heanue, T.A.; Pachnis, V. Molecular profiling of enteric nervous system cell lineages. Nat. Protoc. 2022, 17, 1789–1817. [Google Scholar] [CrossRef]
- Pan, W.; Rahman, A.A.; Stavely, R.; Bhave, S.; Guyer, R.; Omer, M.; Picard, N.; Goldstein, A.M.; Hotta, R. Schwann Cells in the Aganglionic Colon of Hirschsprung Disease Can Generate Neurons for Regenerative Therapy. Stem Cells Transl. Med. 2022, 11, 1232–1244. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Seid, K.; Obermayr, F.; Just, L.; Neckel, P.H. Activation of Wnt Signaling Increases Numbers of Enteric Neurons Derived From Neonatal Mouse and Human Progenitor Cells. Gastroenterology 2017, 153, 154–165.e159. [Google Scholar] [CrossRef]
- Sasselli, V.; Boesmans, W.; Vanden Berghe, P.; Tissir, F.; Goffinet, A.M.; Pachnis, V. Planar cell polarity genes control the connectivity of enteric neurons. J. Clin. Investig. 2013, 123, 1763–1772. [Google Scholar] [CrossRef] [PubMed]
- Drokhlyansky, E.; Smillie, C.S.; Van Wittenberghe, N.; Ericsson, M.; Griffin, G.K.; Eraslan, G.; Dionne, D.; Cuoco, M.S.; Goder-Reiser, M.N.; Sharova, T.; et al. The Human and Mouse Enteric Nervous System at Single-Cell Resolution. Cell 2020, 182, 1606–1622.e1623. [Google Scholar] [CrossRef] [PubMed]
- Morarach, K.; Mikhailova, A.; Knoflach, V.; Memic, F.; Kumar, R.; Li, W.; Ernfors, P.; Marklund, U. Diversification of molecularly defined myenteric neuron classes revealed by single-cell RNA sequencing. Nat. Neurosci. 2021, 24, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Bradford, E.M.; Ryu, S.H.; Singh, A.P.; Lee, G.; Goretsky, T.; Sinh, P.; Williams, D.B.; Cloud, A.L.; Gounaris, E.; Patel, V.; et al. Epithelial TNF Receptor Signaling Promotes Mucosal Repair in Inflammatory Bowel Disease. J. Immunol. 2017, 199, 1886–1897. [Google Scholar] [CrossRef]
- Frei, S.M.; Hemsley, C.; Pesch, T.; Lang, S.; Weber, A.; Jehle, E.; Ruhl, A.; Fried, M.; Rogler, G.; Scharl, M. The role for dickkopf-homolog-1 in the pathogenesis of Crohn’s disease-associated fistulae. PLoS ONE 2013, 8, e78882. [Google Scholar] [CrossRef]
- Kim, M.J.; Choe, Y.H. Correlation of Dickkopf-1 with Inflammation in Crohn Disease. Indian Pediatr. 2019, 56, 929–932. [Google Scholar] [CrossRef]
- Uchiyama, K.; Takagi, T.; Mizushima, K.; Asaeda, K.; Kajiwara, M.; Kashiwagi, S.; Minagawa, Y.; Hotta, Y.; Tanaka, M.; Inoue, K.; et al. Investigation on the Inhibitory Effect of Wnt-5a on Colonic Mucosal Inflammation in Patients with Ulcerative Colitis. Dig. Dis. Sci. 2022, 67, 4760–4769. [Google Scholar] [CrossRef]
- Surdea-Blaga, T.; Popovici, E.; Fadgyas Stanculete, M.; Dumitrascu, D.L.; Scarpignato, C. Eosinophilic Esophagitis: Diagnosis and Current Management. J. Gastrointest. Liver Dis. 2020, 29, 85–97. [Google Scholar] [CrossRef]
- Lyros, O.; Rafiee, P.; Nie, L.; Medda, R.; Jovanovic, N.; Schmidt, J.; Mackinnon, A.; Venu, N.; Shaker, R. Dickkopf-1, the Wnt antagonist, is induced by acidic pH and mediates epithelial cellular senescence in human reflux esophagitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 306, G557–G574. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Gurumurthy, R.K.; Prakash, P.G.; Kurian, S.M.; Wentland, C.; Brinkmann, V.; Mollenkopf, H.-J.; Krammer, T.; Toussaint, C.; Saliba, A.-E.; et al. Spatial organisation and homeostasis of epithelial lineages at the gastroesophageal junction is regulated by the divergent Wnt mucosal microenvironment. bioRxiv 2021. [Google Scholar] [CrossRef]
- Manresa, M.C.; Wu, A.; Nhu, Q.M.; Chiang, A.W.T.; Okamoto, K.; Miki, H.; Kurten, R.; Pham, E.; Duong, L.D.; Lewis, N.E.; et al. LIGHT controls distinct homeostatic and inflammatory gene expression profiles in esophageal fibroblasts via differential HVEM and LTbetaR-mediated mechanisms. Mucosal Immunol. 2022, 15, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Kamboj, A.K.; Cotter, T.G.; Oxentenko, A.S. Helicobacter pylori: The Past, Present, and Future in Management. Mayo Clin. Proc. 2017, 92, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Toller, I.M.; Neelsen, K.J.; Steger, M.; Hartung, M.L.; Hottiger, M.O.; Stucki, M.; Kalali, B.; Gerhard, M.; Sartori, A.A.; Lopes, M.; et al. Carcinogenic bacterial pathogen Helicobacter pylori triggers DNA double-strand breaks and a DNA damage response in its host cells. Proc. Natl. Acad. Sci. USA 2011, 108, 14944–14949. [Google Scholar] [CrossRef]
- Neal, J.T.; Peterson, T.S.; Kent, M.L.; Guillemin, K.H. Pylori virulence factor CagA increases intestinal cell proliferation by Wnt pathway activation in a transgenic zebrafish model. Dis. Model. Mech. 2013, 6, 802–810. [Google Scholar] [CrossRef]
- Song, X.; Xin, N.; Wang, W.; Zhao, C. Wnt/β-catenin, an oncogenic pathway targeted by H. pylori in gastric carcinogenesis. Oncotarget 2015, 6, 35579–35588. [Google Scholar] [CrossRef] [PubMed]
- Zuo, W.; Yang, H.; Li, N.; Ouyang, Y.; Xu, X.; Hong, J. Helicobacter pylori infection activates Wnt/β-catenin pathway to promote the occurrence of gastritis by upregulating ASCL1 and AQP5. Cell Death Discov. 2022, 8, 257. [Google Scholar] [CrossRef]
- Koushyar, S.; Powell, A.G.; Vincan, E.; Phesse, T.J. Targeting Wnt Signaling for the Treatment of Gastric Cancer. Int. J. Mol. Sci. 2020, 21, 3927. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012, 487, 330–337. [Google Scholar] [CrossRef]
- Rosin-Arbesfeld, R.; Cliffe, A.; Brabletz, T.; Bienz, M. Nuclear export of the APC tumour suppressor controls beta-catenin function in transcription. EMBO J. 2003, 22, 1101–1113. [Google Scholar] [CrossRef] [PubMed]
- Parker, T.W.; Neufeld, K.L. APC controls Wnt-induced beta-catenin destruction complex recruitment in human colonocytes. Sci. Rep. 2020, 10, 2957. [Google Scholar] [CrossRef]
- van Neerven, S.M.; de Groot, N.E.; Nijman, L.E.; Scicluna, B.P.; van Driel, M.S.; Lecca, M.C.; Warmerdam, D.O.; Kakkar, V.; Moreno, L.F.; Vieira Braga, F.A.; et al. Apc-mutant cells act as supercompetitors in intestinal tumour initiation. Nature 2021, 594, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Kakugawa, S.; Langton, P.F.; Zebisch, M.; Howell, S.; Chang, T.H.; Liu, Y.; Feizi, T.; Bineva, G.; O’Reilly, N.; Snijders, A.P.; et al. Notum deacylates Wnt proteins to suppress signalling activity. Nature 2015, 519, 187–192. [Google Scholar] [CrossRef]
- Yum, M.K.; Han, S.; Fink, J.; Wu, S.S.; Dabrowska, C.; Trendafilova, T.; Mustata, R.; Chatzeli, L.; Azzarelli, R.; Pshenichnaya, I.; et al. Tracing oncogene-driven remodelling of the intestinal stem cell niche. Nature 2021, 594, 442–447. [Google Scholar] [CrossRef]
- Sehgal, P.; Lanauze, C.; Wang, X.; Hayer, K.E.; Torres-Diz, M.; Leu, N.A.; Sela, Y.; Stanger, B.Z.; Lengner, C.J.; Thomas-Tikhonenko, A. MYC Hyperactivates Wnt Signaling in APC/CTNNB1-Mutated Colorectal Cancer Cells through miR-92a-Dependent Repression of DKK3. Mol. Cancer Res. 2021, 19, 2003–2014. [Google Scholar] [CrossRef]
- Emons, G.; Spitzner, M.; Reineke, S.; Moller, J.; Auslander, N.; Kramer, F.; Hu, Y.; Beissbarth, T.; Wolff, H.A.; Rave-Frank, M.; et al. Chemoradiotherapy Resistance in Colorectal Cancer Cells is Mediated by Wnt/beta-catenin Signaling. Mol. Cancer Res. 2017, 15, 1481–1490. [Google Scholar] [CrossRef]
- Gang, E.J.; Hsieh, Y.T.; Pham, J.; Zhao, Y.; Nguyen, C.; Huantes, S.; Park, E.; Naing, K.; Klemm, L.; Swaminathan, S.; et al. Small-molecule inhibition of CBP/catenin interactions eliminates drug-resistant clones in acute lymphoblastic leukemia. Oncogene 2014, 33, 2169–2178. [Google Scholar] [CrossRef]
- Chandra, A.; Lin, T.; Zhu, J.; Tong, W.; Huo, Y.; Jia, H.; Zhang, Y.; Liu, X.S.; Cengel, K.; Xia, B.; et al. PTH1-34 blocks radiation-induced osteoblast apoptosis by enhancing DNA repair through canonical Wnt pathway. J. Biol. Chem. 2015, 290, 157–167. [Google Scholar] [CrossRef]
- Fang, L.; Zhu, Q.; Neuenschwander, M.; Specker, E.; Wulf-Goldenberg, A.; Weis, W.I.; von Kries, J.P.; Birchmeier, W. A Small-Molecule Antagonist of the beta-Catenin/TCF4 Interaction Blocks the Self-Renewal of Cancer Stem Cells and Suppresses Tumorigenesis. Cancer Res. 2016, 76, 891–901. [Google Scholar] [CrossRef]
- Fukumoto, T.; Zhu, H.; Nacarelli, T.; Karakashev, S.; Fatkhutdinov, N.; Wu, S.; Liu, P.; Kossenkov, A.V.; Showe, L.C.; Jean, S.; et al. N(6)-Methylation of Adenosine of FZD10 mRNA Contributes to PARP Inhibitor Resistance. Cancer Res. 2019, 79, 2812–2820. [Google Scholar] [CrossRef]
- Cho, Y.H.; Ro, E.J.; Yoon, J.S.; Mizutani, T.; Kang, D.W.; Park, J.C.; Il Kim, T.; Clevers, H.; Choi, K.Y. 5-FU promotes stemness of colorectal cancer via p53-mediated WNT/beta-catenin pathway activation. Nat. Commun. 2020, 11, 5321. [Google Scholar] [CrossRef]
- Serebriiskii, I.G.; Pavlov, V.; Tricarico, R.; Andrianov, G.; Nicolas, E.; Parker, M.I.; Newberg, J.; Frampton, G.; Meyer, J.E.; Golemis, E.A. Comprehensive characterization of PTEN mutational profile in a series of 34,129 colorectal cancers. Nat. Commun. 2022, 13, 1618. [Google Scholar] [CrossRef]
- Ponce, D.P.; Maturana, J.L.; Cabello, P.; Yefi, R.; Niechi, I.; Silva, E.; Armisen, R.; Galindo, M.; Antonelli, M.; Tapia, J.C. Phosphorylation of AKT/PKB by CK2 is necessary for the AKT-dependent up-regulation of beta-catenin transcriptional activity. J. Cell. Physiol. 2011, 226, 1953–1959. [Google Scholar] [CrossRef]
- Ma, L.; Cao, Y.; Hu, J.J.; Chu, M.L. Casein kinase 2 interacting protein 1 positively regulates caudal-related homeobox 1 in intestinal-type gastric cancer. Chin. Med. J. 2020, 133, 154–164. [Google Scholar] [CrossRef]
- Bian, J.; Dannappel, M.; Wan, C.; Firestein, R. Transcriptional Regulation of Wnt/beta-Catenin Pathway in Colorectal Cancer. Cells 2020, 9, 2125. [Google Scholar] [CrossRef]
- Schmitt, C.A.; Fridman, J.S.; Yang, M.; Baranov, E.; Hoffman, R.M.; Lowe, S.W. Dissecting p53 tumor suppressor functions in vivo. Cancer Cell 2002, 1, 289–298. [Google Scholar] [CrossRef]
- Hirota, S.; Isozaki, K.; Moriyama, Y.; Hashimoto, K.; Nishida, T.; Ishiguro, S.; Kawano, K.; Hanada, M.; Kurata, A.; Takeda, M.; et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 1998, 279, 577–580. [Google Scholar] [CrossRef]
- Bardsley, M.R.; Horvath, V.J.; Asuzu, D.T.; Lorincz, A.; Redelman, D.; Hayashi, Y.; Popko, L.N.; Young, D.L.; Lomberk, G.A.; Urrutia, R.A.; et al. Kitlow stem cells cause resistance to Kit/platelet-derived growth factor alpha inhibitors in murine gastrointestinal stromal tumors. Gastroenterology 2010, 139, 942–952. [Google Scholar] [CrossRef]
- Hayashi, Y.; Bardsley, M.R.; Toyomasu, Y.; Milosavljevic, S.; Gajdos, G.B.; Choi, K.M.; Reid-Lombardo, K.M.; Kendrick, M.L.; Bingener-Casey, J.; Tang, C.M.; et al. Platelet-Derived Growth Factor Receptor-alpha Regulates Proliferation of Gastrointestinal Stromal Tumor Cells With Mutations in KIT by Stabilizing ETV1. Gastroenterology 2015, 149, 420–432.e416. [Google Scholar] [CrossRef]
- Hayashi, Y.; Asuzu, D.T.; Bardsley, M.R.; Gajdos, G.B.; Kvasha, S.M.; Linden, D.R.; Nagy, R.A.; Saravanaperumal, S.A.; Syed, S.A.; Toyomasu, Y.; et al. Wnt-induced, TRP53-mediated Cell Cycle Arrest of Precursors Underlies Interstitial Cell of Cajal Depletion During Aging. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 117–145. [Google Scholar] [CrossRef]
- Zeng, S.; Seifert, A.M.; Zhang, J.Q.; Cavnar, M.J.; Kim, T.S.; Balachandran, V.P.; Santamaria-Barria, J.A.; Cohen, N.A.; Beckman, M.J.; Medina, B.D.; et al. Wnt/beta-catenin Signaling Contributes to Tumor Malignancy and Is Targetable in Gastrointestinal Stromal Tumor. Mol. Cancer Ther. 2017, 16, 1954–1966. [Google Scholar] [CrossRef]
- Hayashi, Y.; Nguyen, V.T.T. A narrative review of imatinib-resistant gastrointestinal stromal tumors. Gastrointest. Stromal Tumor 2021, 4, 6. [Google Scholar] [CrossRef]
- McKee, C.M.; Chapski, D.J.; Wehling-Henricks, M.; Rosa-Garrido, M.; Kuro, O.M.; Vondriska, T.M.; Tidball, J.G. The anti-aging protein Klotho affects early postnatal myogenesis by downregulating Jmjd3 and the canonical Wnt pathway. FASEB J. 2022, 36, e22192. [Google Scholar] [CrossRef]
- Miao, J.; Huang, J.; Luo, C.; Ye, H.; Ling, X.; Wu, Q.; Shen, W.; Zhou, L. Klotho retards renal fibrosis through targeting mitochondrial dysfunction and cellular senescence in renal tubular cells. Physiol. Rep. 2021, 9, e14696. [Google Scholar] [CrossRef]
- Chen, G.; Liu, Y.; Goetz, R.; Fu, L.; Jayaraman, S.; Hu, M.C.; Moe, O.W.; Liang, G.; Li, X.; Mohammadi, M. Alpha-Klotho is a non-enzymatic molecular scaffold for FGF23 hormone signalling. Nature 2018, 553, 461–466. [Google Scholar] [CrossRef]
- Suzuki, Y.; Kuzina, E.; An, S.J.; Tome, F.; Mohanty, J.; Li, W.; Lee, S.; Liu, Y.; Lax, I.; Schlessinger, J. FGF23 contains two distinct high-affinity binding sites enabling bivalent interactions with alpha-Klotho. Proc. Natl. Acad. Sci. USA 2020, 117, 31800–31807. [Google Scholar] [CrossRef]
- Matsumura, Y.; Aizawa, H.; Shiraki-Iida, T.; Nagai, R.; Kuro-o, M.; Nabeshima, Y. Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem. Biophys. Res. Commun. 1998, 242, 626–630. [Google Scholar] [CrossRef]
- Kuro-o, M.; Matsumura, Y.; Aizawa, H.; Kawaguchi, H.; Suga, T.; Utsugi, T.; Ohyama, Y.; Kurabayashi, M.; Kaname, T.; Kume, E.; et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 1997, 390, 45–51. [Google Scholar] [CrossRef]
- Cheng, Y.W.; Hung, C.C.; Fang, W.H.; Chen, W.L. Association between Soluble alpha-Klotho Protein and Metabolic Syndrome in the Adult Population. Biomolecules 2022, 12, 70. [Google Scholar] [CrossRef]
- Kurosu, H.; Yamamoto, M.; Clark, J.D.; Pastor, J.V.; Nandi, A.; Gurnani, P.; McGuinness, O.P.; Chikuda, H.; Yamaguchi, M.; Kawaguchi, H.; et al. Suppression of aging in mice by the hormone Klotho. Science 2005, 309, 1829–1833. [Google Scholar] [CrossRef]
- Brack, A.S.; Conboy, M.J.; Roy, S.; Lee, M.; Kuo, C.J.; Keller, C.; Rando, T.A. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science 2007, 317, 807–810. [Google Scholar] [CrossRef]
- Izbeki, F.; Asuzu, D.T.; Lorincz, A.; Bardsley, M.R.; Popko, L.N.; Choi, K.M.; Young, D.L.; Hayashi, Y.; Linden, D.R.; Kuro-o, M.; et al. Loss of Kitlow progenitors, reduced stem cell factor and high oxidative stress underlie gastric dysfunction in progeric mice. J. Physiol. 2010, 588, 3101–3117. [Google Scholar] [CrossRef]
- Nalapareddy, K.; Nattamai, K.J.; Kumar, R.S.; Karns, R.; Wikenheiser-Brokamp, K.A.; Sampson, L.L.; Mahe, M.M.; Sundaram, N.; Yacyshyn, M.B.; Yacyshyn, B.; et al. Canonical Wnt Signaling Ameliorates Aging of Intestinal Stem Cells. Cell Rep. 2017, 18, 2608–2621. [Google Scholar] [CrossRef]
- Nguyen, V.T.T.; Taheri, N.; Chandra, A.; Hayashi, Y. Aging of enteric neuromuscular systems in gastrointestinal tract. Neurogastroenterol. Motil. 2022, 34, e14352. [Google Scholar] [CrossRef]
- Pentinmikko, N.; Iqbal, S.; Mana, M.; Andersson, S.; Cognetta, A.B., 3rd; Suciu, R.M.; Roper, J.; Luopajarvi, K.; Markelin, E.; Gopalakrishnan, S.; et al. Notum produced by Paneth cells attenuates regeneration of aged intestinal epithelium. Nature 2019, 571, 398–402. [Google Scholar] [CrossRef]
- Reya, T.; Duncan, A.W.; Ailles, L.; Domen, J.; Scherer, D.C.; Willert, K.; Hintz, L.; Nusse, R.; Weissman, I.L. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature 2003, 423, 409–414. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).