Abstract
(1) Background: The influence of estrogen on cognitive and perceptual functions is debated. Some research suggests that estrogen increases arousal, improving cognitive function, while others propose that increased arousal might reduce performance in certain tasks. This study investigates the effects of menstrual cycle phase and estrogen levels on lightness perception in cycling women and hormonal contraceptive (HC) users. (2) Methods: Sixteen women (nine with natural cycles and seven HC users) completed three sessions aligned with different menstrual cycle phases. During these sessions, participants adjusted the luminance of five test stimuli (representing blue, green, green-yellow, yellow, and red) until they matched a flickering reference white stimulus. Lightness was calculated as the ratio of the reference stimulus luminance (5 cd/m2) divided by the test luminance required to match. Estrogen levels were also determined for each participant from saliva samples collected on the morning of each session. The effects of wavelength and menstrual cycle phase on lightness perception were analyzed, followed by post hoc comparisons and correlations between lightness perception and estrogen levels for both cycling women and HC users. (3) Results: Lightness varied by menstrual phase (MCP) in cycling women and was slightly higher during the low estrogen menstrual phase compared to peri-ovulation or luteal phases. In HC users, lightness measures were equivalent across phases. For cycling women, lightness was negatively correlated with estrogen for the green and green-yellow stimuli. There were no such associations among HC users. (4) Conclusions: This report challenges the concept that high estrogen phases of the menstrual cycle always positively influence perception. Conversely, these results revealed that—at least in cycling, non-hormonal contraceptive users—lightness perception was both at a maximum during the low estrogen menstrual phase and negatively associated with estrogen levels across all tested wavelengths.
1. Introduction
Estrogen is a steroid sex hormone that is primarily associated with female reproductive functioning. However, it also plays a role in various physiological processes, including brain function [1]. While there is significant variation between individuals [2], it is well established that estrogen levels fluctuate during the menstrual cycle. Estrogen is typically at a minimum during the first week of the cycle (i.e., menstrual phase) and peaks around day 12 or 13 (i.e., pre-ovulation). Levels quickly fall off after ovulation but gradually increase to a smaller peak at approximately the midpoint of the luteal phase (~day 22). Investigators can then use the menstrual cycle phase as a surrogate for hormone levels when accounting for behavioral or perceptual changes in women [2]. However, great care should be taken to account for individual differences, unless hormones are directly measured. Even if precisely measured, the directional influence of hormonal changes on perception is debated. Some research has shown that estrogen increases cognitive arousal and suggests decreased cognitive or perceptual function during low estrogen (i.e., menstrual) phases of the cycle [3,4]. Kopell et al. further argued in favor of a “general arousal theory” whereby females’ increased sensitivity to visual cues from males during high estrogen ovulation increases the chance of mating during peak fertility [3]. While it is well established that sex differences are not equivalent to or even derived from changes across the menstrual cycle [5], an arousal theory is supported by evidence that male–female differences in color descriptions may come as much from increased attention in females (i.e., relative male ‘carelessness’) as it does psychological or physiological structures [6]. Other studies, however, have suggested that increased arousal may decrease performance in certain tasks, e.g., [7]. These alternate positions are based on the idea that increased sensitivity to light during higher arousal phases of the menstrual cycle (i.e., near ovulation) may cause retinal cells to become saturated with light [8]. The overall saturated retina results in increased difference thresholds and decreased sensitivity [7].
There is a rich historical, though equivocal, body of evidence suggesting that changes across the menstrual cycle affect visual sensitivity [see [9,10] for reviews]. Experimental paradigms have varied significantly, and much of this research predates the first detection of estrogen receptors in mammalian and human retinas [11,12]. However, as far back as the late 19th and early 20th centuries, researchers found restricted color visual fields during the menstrual phase with specific changes in the middle (green and yellow) portions of the visual spectrum [13,14]. More recent evidence suggests little effect of menstrual cycle phase on achromatic (i.e., white on black) automated visual fields but decreased sensitivity to short-wavelength (i.e., blue) stimuli during the luteal phase [15,16]. Eisner et al. concluded that hormonal activation effects could alter retinal function across the short time span of a typical menstrual cycle [17]. They found increases and decreases in sensitivity near ovulation and pre-menstrually, respectively, that were most pronounced for short wavelength (blue) stimuli but also present in some subjects for middle wavelength (green) and long wavelength (red) sensitive mechanisms. At least one other study found greater color discrimination near ovulation than during menstrual or luteal phases, but they did not attempt to differentiate between psychological and hormonal roles [18]. Conversely, at least one study found faster color judgments during the menstrual phase when compared to peri-ovulation, particularly for non-cognitive blue and yellow stimuli [19].
Studies involving the influence of estrogen surrogates or modulators on color visibility have also produced equivocal results. For example, tamoxifen (a common treatment in breast cancer) primarily acts as a selective estrogen receptor modulator [SERM] by inhibiting the growth of estrogen-responsive breast cancer cells. However, tamoxifen can have various side effects, including those related to vision. Eisner and Incognito investigated these changes in short- and long-term tamoxifen users and found that tamoxifen use decreased the visibility of short-wavelength light [20]. Eisner et al. also demonstrated that long-term tamoxifen use decreased sensitivity during short wavelength (i.e., blue on yellow) automated perimetry [21].
The overall equivocal nature of these findings necessitates direct measurement of estrogen in lieu of categorical assumptions about hormone levels. A recent investigation by the present author involving chromatic brightness differences only produced negative menstrual cycle findings [22]. That report did, however, reveal that models of brightness (i.e., the apparent intensity of light) were associated with lightness (i.e., the apparent intensity of light relative to an area illuminated by white light) changes as well as changes in estrogen for normally cycling women but—to a lesser extent—hormonal contraceptive users [22]. However, while estrogen and menstrual cycle phase were both shown to affect the ratio of perceived brightness to perceived lightness (i.e., the Helmholtz–Kohlrausch effect), the effects of the menstrual cycle on lightness (i.e., the perceived intensity relative to that of white light) alone were never reported. The present work addresses this omission and uses the previously collected data to support this brief report on the effects of both menstrual cycle phase (MCP) and estrogen (EST) levels on lightness perception in both normally cycling women and hormonal contraceptive users.
2. Materials and Methods
2.1. Participants and Scheduling
The methods are fully reported in an investigation of the Helmholtz–Kohlrausch effect [22]. In brief, 16 women with normal color vision, normal visual acuity, free of any disease that might have affected vision (i.e., no diabetes, no hypertension, and no thyroid conditions), and no medication use other than oral contraception (ages 21–40 years; nine normally cycling [ages 25.8 ± 3.2 years] and seven using oral combination estradiol/progestin contraception [ages 26.0 ± 6.2 years]) were scheduled for three sessions coinciding with the menstrual (days 1–7), peri-ovulation (~day 12), and luteal (~day 21–22) phases of their menstrual cycle. All subjects collected saliva at home on the day of each session, and all samples were mailed for analysis the day they were received. Estrogen levels were analyzed by double antibody radioimmunoassay (RIA) within 21 days. HC users were all taking a triphasic (i.e., fixed ethinyl estradiol [EE] dose but three increasing doses of norgestimate [NG]) oral contraception combination except for one subject who was taking a monophasic EE/drospirenone (DRSP) combination. HC users were easier to schedule, as data collection simply coincided with their fixed 28-day pill cycle. Consequently, HC users completed 20 of 21 planned sessions. Cycling women were more difficult to accurately schedule. Two cycling subjects did not complete peri-ovulation sessions (completing two luteal sessions instead), and one cycling subject could only be scheduled during the menstrual phase. All participant data about age, contraception use, and session participation are shown in Table 1.

Table 1.
Individual participant information.
2.2. Data Collection Sessions
Subjects were not fully dark adapted; rather, they were adapted to a low background luminance of 0.4 cd/m2 for 30 min prior to each session, after which heterochromatic flicker matches (HFM) were used to measure lightness across five wavelengths (or colors)—450 (blue), 520 (green), 560 (green-yellow), 580 (yellow), and 650 nm (red). The test (or color) channel was produced by a narrow bandpass interference filter (NBIF) wheel producing each of the five test wavelengths. The reference channel was a spectrally broad (i.e., white) 5 cd/m2 circular stimulus that flickered against the test channel at 18 cycles/sec (Hz). The viewing stimulus subtended 2.5° at a viewing distance of 43 cm. Subjects were asked to adjust the intensity of the test light (while the white light luminance was held constant at 5 cd/m2) until they perceived a steady, non-flickering light. This was repeated four times for each of the five wavelengths at each session. The luminance values for the four trials for each wavelength were averaged, and the relative luminosity (RL or lightness) was derived by dividing the reference stimulus luminance (5 cd/m2) by this average. For example, if an observer required 50 cd/m2 on average at 450 nm to match the 5 cd/m2 white stimulus, the RL at 450 nm is (5 cd/m2)/(50 cd/m2) or 0.10 at 450 nm. Overall, the lighter the test color, the less luminance required to match the white stimulus. RL functions by HFM typically peak around 560 nm and are at a minimum near the low and high ends of the visual spectrum [24].
In this investigation, repeated measures analysis of variance (RM ANOVA) was used to determine the within-subject effects of wavelength and MCP on lightness (RL) for both normally cycling women and hormonal contraceptive (HC) users. Post hoc comparisons were used to determine pairwise differences in RL between menstrual, peri-ovulation, and luteal phases and—where appropriate—for each wavelength. Lastly, correlations were calculated between RL and estrogen levels at each wavelength. These were calculated separately for cycling women and HC users.
3. Results
3.1. Salivary Estrogen Measures
Data were collected on the following days for cycling women: menstrual (3.7 ± 2.0), periovulation (11.5 ± 0.8), and luteal (19.7 ± 1.6) and for HC users: menstrual (4.0 ± 2.8), peri-ovulation (11.8 ± 1.8), and luteal (19.4 ± 1.5). Kolmogorov–Smirnov tests showed that estrogen levels were not normally distributed (p = 0.009). Log-transformed estrogen (p = 0.200) levels were then used for all analyses. All other measures were normally distributed.
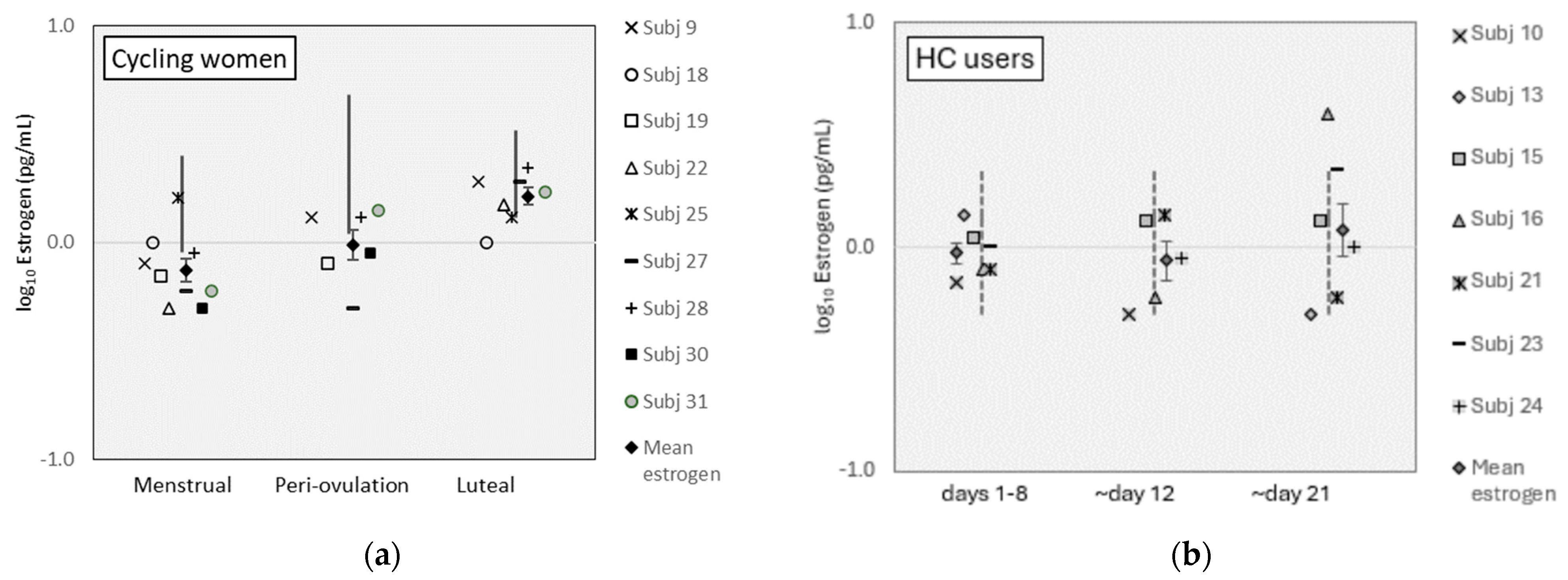
Mean estrogen levels were lower than reference ranges for cycling women during the menstrual and periovulation phases but within reference ranges for the luteal phase (Figure 1a; see www.zrtlab.com/resources/referencedocuments/saliva-reference-ranges [accessed on 27 October 2023] for published ranges). Estrogen levels were within reference ranges for HC users (Figure 1b). Estrogen levels were equivalent between cycling women and HC users (F [1,7] = 0.49, p = 0.507). Estrogen varied significantly across MC phases for all participants (F [2,14] = 5.68, p = 0.016, η2 = 0.45) and was higher on pairwise comparisons during the luteal phase than the menstrual phase for all women (t [16] = 3.88, p = 0.003). For cycling women, estrogen levels were also different across MC phases (F [2,8] = 13.01, p = 0.003, η2 = 0.77), where luteal levels were higher than menstrual phase levels (t [8] = 7.10, p = 0.0001). Estrogen levels were equivalent between MC phases for HC users (F [12,6] = 0.713, p = 0.519).
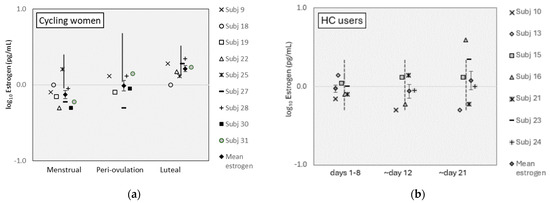
Figure 1.
Estrogen levels for (a) cycling women and (b) HC users. (Error bars represent ±1 SE).
3.2. Effects of Menstrual Cycle Phase (MCP) on Lightness (RL)
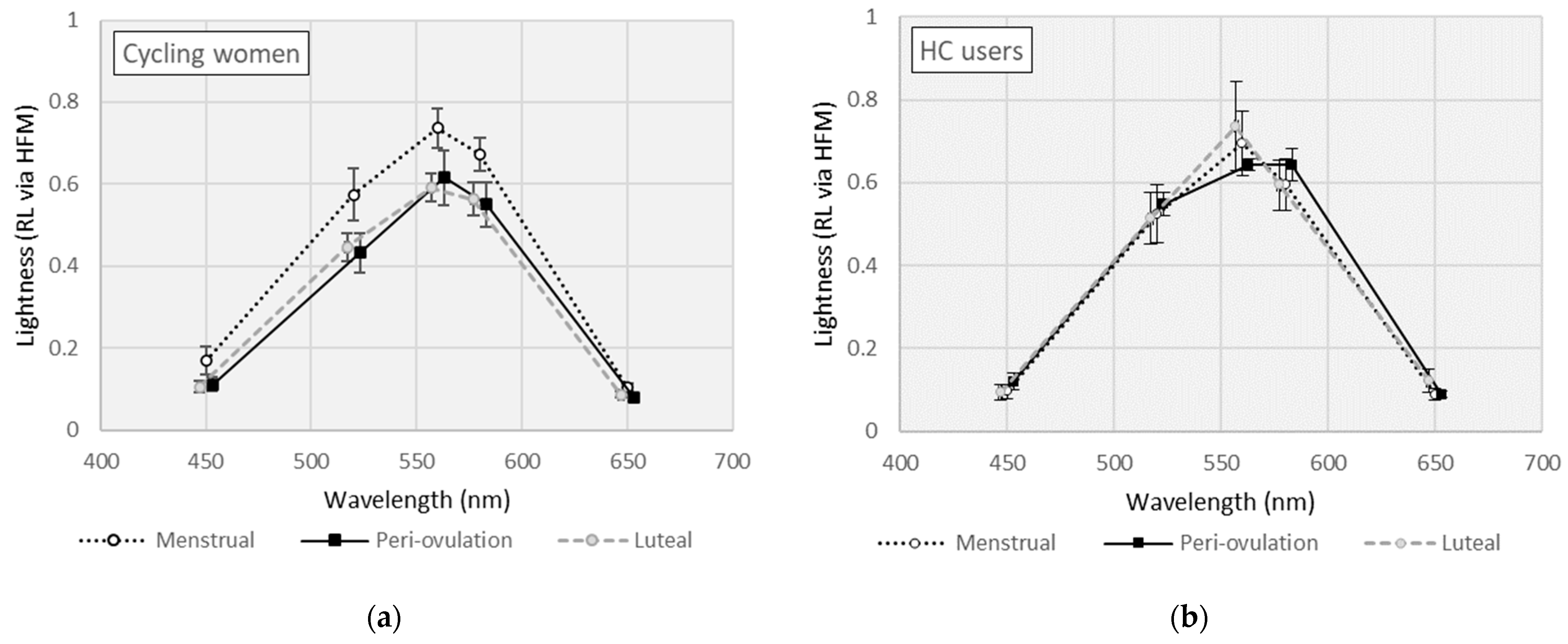
Mean RL measures across wavelength by MCP are shown separately for cycling women and HC users in Figure 2. For cycling women (see Figure 2a), RL measures varied across wavelength (F [4,20] = 163, p < 0.001, η2 = 0.970) and by MCP (F [2,10] = 4.98, p = 0.032, η2 = 0.499). RL measures were slightly higher in cycling women during the menstrual phase, but the paired comparisons (i.e., mean differences [MD]) did not reach statistical significance (MD [menstrual − ovulation] = 0.110, p = 0.080; MD [menstrual − luteal] = 0.122, p = 0.071). In HC users (see Figure 2b), RL measures varied across wavelength (F [4,20] = 428, p < 0.001, η2 = 0.988) but were equivalent by MCP (F [2,10] = 0.165, p = 0.850).
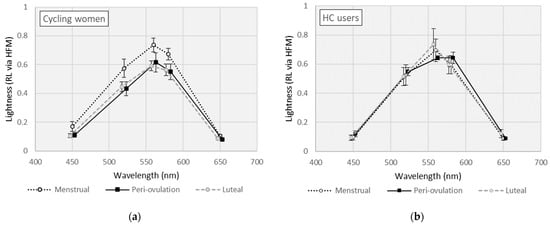
Figure 2.
Lightness (or relative luminosity) curves for (a) cycling women; (b) hormonal contraceptive (HC) users. (Error bars represent ±1 SE).
3.3. Relationships between Lightness (RL) and Estrogen (EST) Levels
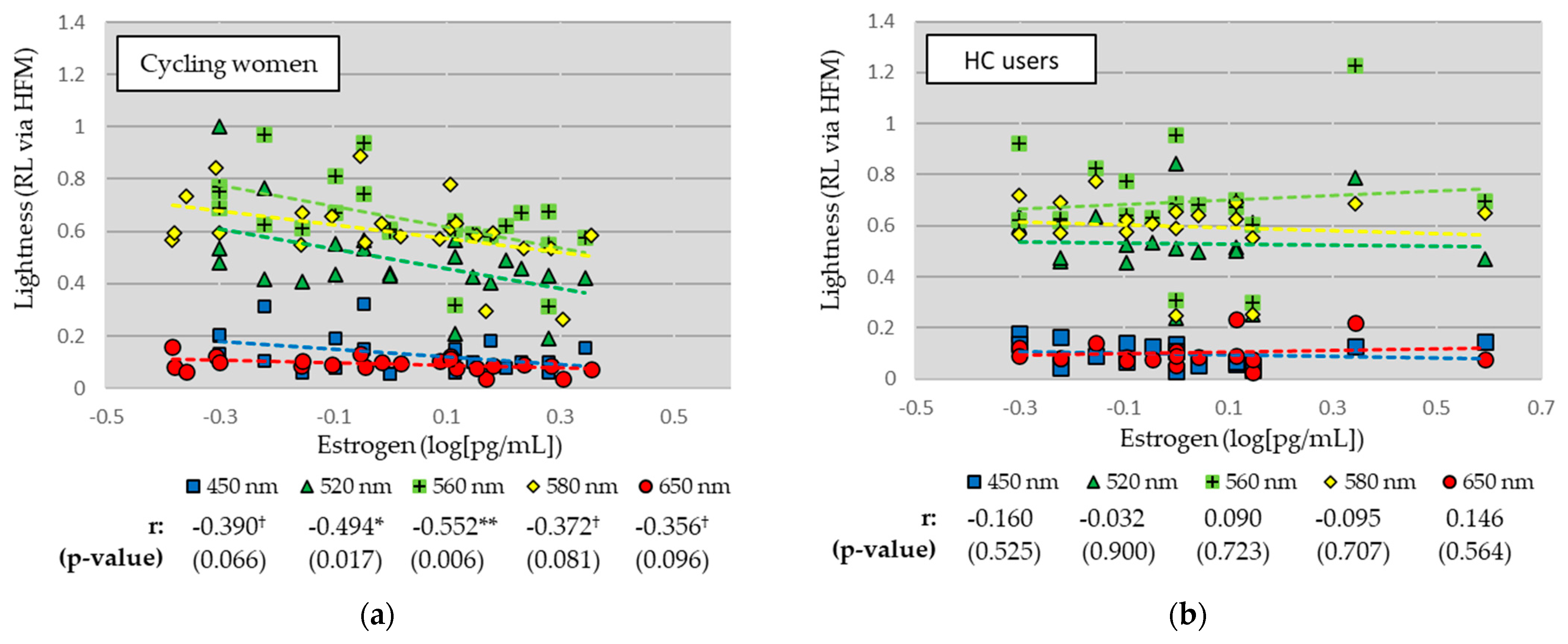
Linear relationships between RL measures and EST are shown separately for cycling women and HC users in Figure 3. For cycling women (see Figure 3a), RL measures were negatively correlated with EST for all wavelengths, and significantly so for the green (520 nm; r = −0.494, p = 0.006) and green-yellow (560 nm; r = −0.552, p = 0.006) stimuli. There were strong trends for the blue (450 nm), yellow (580 nm), and red (650 nm) stimuli. For HC users (see Figure 3b), there was little relationship between RL measures and EST levels across all wavelengths.
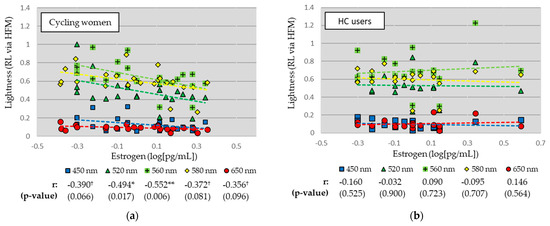
Figure 3.
Linear relationships between lightness and estrogen levels for (a) cycling women and (b) hormonal contraceptive (HC) users. Correlation coefficients (Pearson’s r) and significance (p-value) reported. ** p < 0.01, * p < 0.05, † p < 0.10.
4. Discussion
4.1. Notable Results
This current study investigated the relationship between estrogen levels and lightness perception separately by hormonal contraceptive (HC) use across different menstrual cycle phases. The results revealed several important findings and implications.
Analysis across wavelengths for cycling women and HC users added little to the discussion, as RL measures varied significantly across wavelengths in both cycling women and HC users. However, the effect of menstrual cycle phase on RL measures was only statistically significant in cycling women (and not HC users), predicting almost 50% of the variance in lightness. RL measures were slightly elevated in cycling women during the menstrual phase across all wavelengths. While the observed mean differences did not reach statistical significance on pairwise comparisons between menstrual and luteal or peri-ovulatory phases, these findings imply a perceptual advantage during menstruation. This result is a challenge to previous implications that high hormone phases of the menstrual cycle (i.e., peri-ovulation) produce perceptual advantages [3]. There have been previous challenges to the theory of perceptual disadvantages during low estrogen menstrual phases. For example, Cockrell et al. failed to find decreased perceptual task performance during menstruation, in spite of impaired mood and cognitive functioning [25]. At least one review has concluded against any menstrual cycle effect on perceptual or psychophysical measures [26], but there are even a few previous results that compare with the present finding of a perceptual advantage during menstruation [7,19].
The investigation into the relationships between RL and estrogen levels revealed intriguing patterns. In cycling women, a negative correlation was observed between RL measures and estrogen levels for several wavelengths. Notably, significant correlations were found for the green (520 nm; r = −0.494) and green-yellow (560 nm; r = −0.552) stimuli, with strong trends apparent for the blue (450 nm), yellow (580 nm), and red (650 nm) stimuli. These findings suggesting decreased lightness perception with increased estrogen levels provide the strongest challenge to a perceptual “arousal hypothesis” of estrogen. One explanation for the present perceptual advantage during low estrogen phases is that human retinas become more saturated for broadband (i.e., white) than narrowband (i.e., color) stimuli during excited (i.e., high estrogen) phases [7]. Therefore, the present measure of lightness (i.e., sensitivity to color/sensitivity to white) would be lower during high estrogen phases.
Perhaps the most interesting present finding is the lack of menstrual cycle influence on lightness in HC users. RL measures were essentially equivalent between phases. HC users also exhibited weak relationships between RL measures and estrogen levels across all wavelengths. This discrepancy in the relationship between RL and estrogen for cycling women versus HC users could further be attributed to the hormonal modulation introduced by contraceptive use, which may decouple the typical hormonal fluctuations observed in natural menstrual cycles. While the mechanisms varied significantly from this study, multiple previous results suggest a similar perceptual dimorphism between cycling women and HC users [22,27].
Overall, the observed negative correlations between lightness and estrogen levels in cycling women raise intriguing questions about the underlying mechanisms linking estrogen and lightness perception. This study provides evidence that variations in estrogen levels might indeed negatively influence the perception of lightness, particularly at specific wavelengths.
4.2. Reliability and Individual Differences
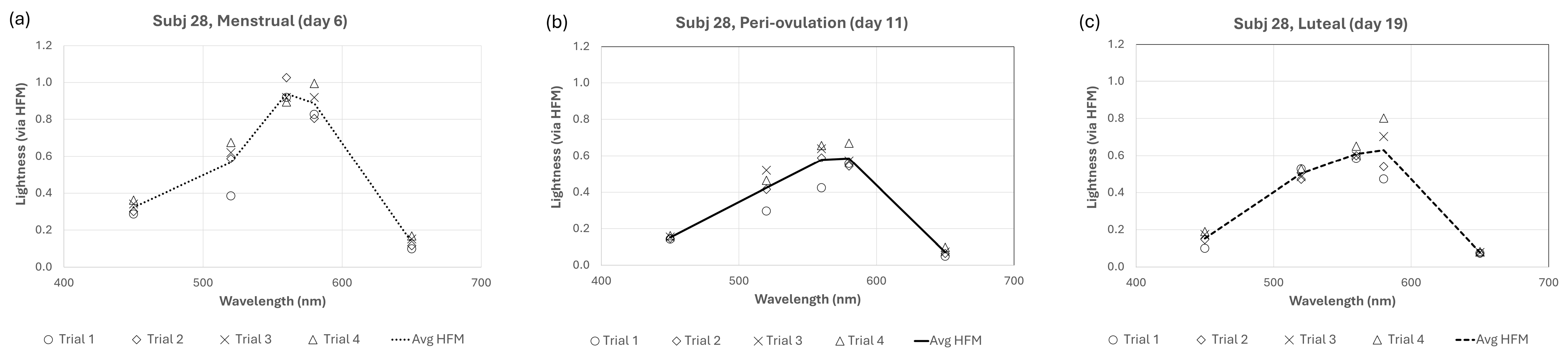
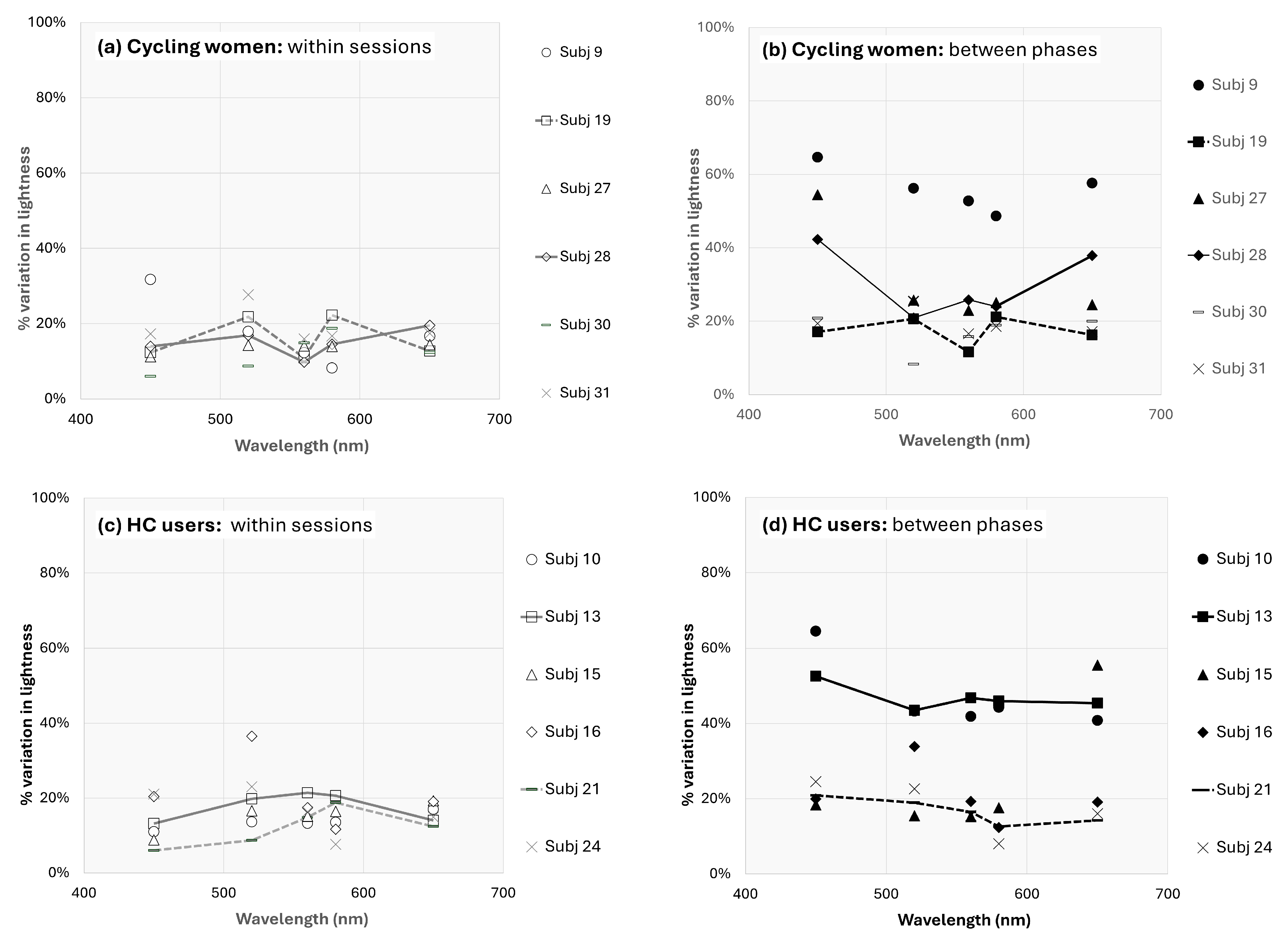
It is well known that subjective lightness matches from heterochromatic flicker (such as those used in the present experiment) are easier to make and more reliable than other brightness or lightness matching techniques [28,29]. In addition to this inherent advantage in flicker matches, great care was taken to control for unwanted variation by allowing for 30 min of monitored practice and requiring subjects to demonstrate consistent matches at each wavelength before starting experimental sessions. Lastly, subject lightness “expectations” were controlled by randomly resetting the test stimulus intensity after each trial. Despite these measures, there was certainly some within-trial variation across different cycles for all subjects. Figure 4 shows all data for one representative cycling subject. Data (including figures) for all subjects are published separately (see Data Availability Statement).
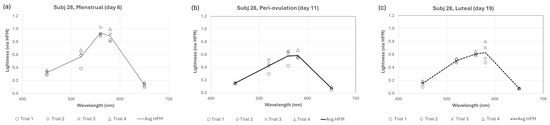
Figure 4.
Individual trial data for a representative cycling subject (Subj 28) for (a) menstrual, (b) pre-ovulation, and (c) luteal phases.
One can see in Figure 4 that lightness for subject 28 is approximately 30–50% higher across wavelengths during the menstrual phase than peri-ovulation or luteal phases. This equates to approximately 0.2–0.3 log units, but what portion of that difference is due to observational variations and what portion is due to fluctuations from menstrual cycle changes? For subject 28, the average within-session percent variation (i.e., 100% × standard deviation of observations/mean observation; or PV) ranged from 9.8% (for 560 nm stimulus) to 19.5% (for 650 nm stimulus). A good rule of thumb is that error measurements are good if the percent variation is less than 10% and acceptable if between 10 and 20%, but acceptable error depends highly on what one is measuring. In the absence of an absolute value of lightness, it is useful to also consider the between-phase percent variation. These have been calculated for all 12 subjects who completed sessions during all three menstrual phases and are shown for all subjects in Figure 5. In some cycling women (subjects 9, 27, 28) and HC users (subjects 10, 13), the between-phase variation is much larger and represents a large effect of menstrual cycle phase (and potentially, estrogen) on lightness differences. The solid lines in Figure 5 (subjects 13 and 28) are representative of this effect. However, the effect for other cycling women (subjects 19, 30, 31) and HC users (subjects 15, 16, 21, 24) was more limited (see data for subjects 19 and 21).
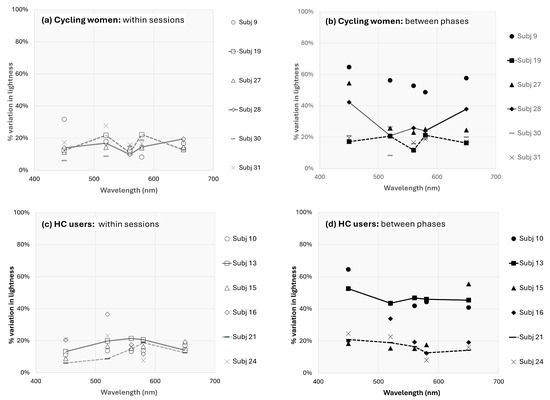
Figure 5.
Percent variation in lightness perception for all subjects. The top two figures are the (a) within session and (b) between phases percent variation in cycling women, and the bottom two figures represent the (c) within session and (d) between phases percent variation in HC users. The representative solid (and dashed) lines in the right figures (b,d) should be compared with the solid (and dashed) lines in the left figures (a,c).
While the data supporting the present findings are not homogenous, they are reliable. That is, the variation in lightness measurements is within normal limits and appears largely due to menstrual phase differences in many subjects.
4.3. Limitations
It is important to acknowledge the limitations of this report, largely concerning the relatively small sample size. Due to the high participant expectations (e.g., multiple sessions, and sharing sensitive information about their menstrual cycle and contraceptive status), this was difficult to avoid. Second, the retrospective analysis of estrogen’s effects on lightness certainly limits the inferences from this report. Again, this was unplanned and unavoidable.
Perhaps the most limiting factor and potentially confounding limitation was the missing estrogen peaks predicted for the peri-ovulatory phase in cycling women (see Figure 1a). This result, however, is common in menstrual cycle research (e.g., [30]) but is avoidable with inexpensive over-the-counter urine tests that help to verify peri-ovulation timing and estrogen peaks [31]. Lastly, while salivary hormone levels may well represent circulating bioavailable hormones [32], commercially available salivary hormone tests (as used in the present work) have been shown to underestimate hormones in HC users [2]. While the present estrogen values were within normal reference ranges for HC users, their under- or over-estimation in cycling women could severely limit our inference that estrogen’s effect on lightness perception varies by contraceptive use. This limitation would not, however, affect the categorical menstrual phase differences present in cycling women (but not present in HC users).
Further investigations could examine the neural pathways and mechanisms through which estrogen influences sensory perception, shedding light on the observed correlations. Prior to a more careful investigation and structured methodology—including larger and more diverse samples along with a more refined prospective design—most of the present conclusions must be made with caution.
5. Conclusions
In conclusion, this current study highlights the complex interplay between lightness perception and estrogen levels across different menstrual cycle phases in naturally cycling women. The results surprisingly suggest a negative association between estrogen and lightness perception, contributing to the growing body of literature exploring the effects of estrogen and other sex hormones on sensory perception and cognitive processes.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the University of Missouri—St Louis (#2006-05-06).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are openly available in FigShare at doi: 10.6084/m9.figshare.25043855.v1.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Li, R.; Shen, Y. Estrogen and brain: Synthesis, function and diseases. Front. Biosci. 2005, 10, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Hampson, E. A brief guide to the menstrual cycle and oral contraceptive use for researchers in behavioral endocrinology. Horm. Behav. 2020, 119, 104655. [Google Scholar] [CrossRef] [PubMed]
- Kopell, B.S.; Lunde, D.T.; Clayton, R.B.; Moos, R.H. Variations in some measures of arousal during the menstrual cycle. J. Nerv. Ment. Dis. 1969, 48, 180–187. [Google Scholar] [CrossRef]
- Parlee, M.B. Menstrual rhythms in sensory processes: A review of fluctuations in vision, olfaction, audition, taste, and touch. Psychol. Bull. 1983, 93, 539–548. [Google Scholar] [CrossRef]
- Cahill, L. Why sex matters for neuroscience. Nat. Rev. Neurosci. 2006, 7, 477–484. [Google Scholar] [CrossRef]
- Griffin, L.D. Males are ‘noisy females’ when it comes to reporting the psychological structure of the basic colours. Perception 2002, 32, 387. [Google Scholar]
- Scher, D.; Purcell, D.G.; Caputo, S.J. Visual acuity at two phases of the menstrual cycle. Bull. Psychon. Soc. 1985, 23, 119–121. [Google Scholar] [CrossRef]
- Werblin, F.S. The control of sensitivity in the retina. Sci. Am. 1973, 228, 70. [Google Scholar] [CrossRef]
- Guttridge, N.M. Changes in ocular and visual variables during the menstrual cycle. Ophthalmic Physiol. Opt. 1994, 14, 38–48. [Google Scholar] [CrossRef]
- Handa, R.J.; McGivern, R.F. Steroid hormones, receptors, and perceptual and cognitive sex differences in the visual system. Curr. Eye Res. 2015, 40, 110–127. [Google Scholar] [CrossRef]
- Kobayashi, K.; Kobayashi, H.; Ueda, M.; Honda, Y. Estrogen receptor expression in bovine and rat retinas. Investig. Ophthalmol. Vis. Sci. 1998, 39, 2105–2110. Available online: https://pubmed.ncbi.nlm.nih.gov/9761289/ (accessed on 27 October 2023).
- Ogueta, S.B.; Schwartz, S.D.; Yamashita, C.K.; Farber, D.B. Estrogen receptor in the human eye: Influence of gender and age on gene expression. Investig. Ophthalmol. Vis. Sci. 1999, 40, 1906–1911. Available online: https://pubmed.ncbi.nlm.nih.gov/10440242/ (accessed on 27 October 2023).
- Finkelstein, L.O. On sensory disorders in diseases, and on changes of the fields of vision in menstruation. Opth. Rev. Rec. Ophthal. Sci. 1887, 6, 323–326. [Google Scholar]
- Lorenzetti, F. Contributo allo Studio del Campo Visivo e del Senso Cromatico Della Donna Durante i Periodi Catameniale e Puerperale (Contribution to the study of the visual field and chromatic sense of the woman during the menstrual and post-partum periods). La Clin. Ostet. (Obstet. Clin.) 1926, 48, 345–349. [Google Scholar]
- Akar, Y.; Yucel, I.; Akar, M.E.; Taskin, O. Menstrual cycle-dependent changes in visual field analysis of healthy women. Ophthalmologica 2005, 219, 30–35. [Google Scholar] [CrossRef]
- Yucel, I.; Akar, M.E.; Dora, B.; Akar, Y.; Taskin, O.; Ozer, H.O. Effect of the menstrual cycle on standard achromatic and blue-on-yellow visual field analysis of women with migraine. Can. J. Ophthalmol. 2005, 40, 51–57. [Google Scholar] [CrossRef]
- Eisner, A.; Burke, S.N.; Toomey, M.D. Visual sensitivity across the menstrual cycle. Vis. Neurosci. 2004, 21, 513–531. [Google Scholar] [CrossRef]
- Giuffrè, G.; Di Rosa, L.; Fiorino, F. Changes in colour discrimination during the menstrual cycle. Ophthalmologica 2007, 221, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Iriguchi, M.; Koda, H.; Koyama, T.; Masataka, N. Colour-odour correspondences in women during the menstrual cycle: Comparative analysis between the menstrual and ovulation phases. Color Res. Appl. 2020, 45, 178–182. [Google Scholar] [CrossRef]
- Eisner, A.; Incognito, L.J. The color appearance of stimuli detected via short-wavelength-sensitive cones for breast cancer survivors using tamoxifen. Vis. Res. 2006, 46, 1816–1822. [Google Scholar] [CrossRef]
- Eisner, A.; Austin, D.F.; Samples, J.R. Short wavelength automated perimetry and tamoxifen use. Br. J. Ophthalmol. 2004, 88, 125–130. [Google Scholar] [CrossRef][Green Version]
- Foutch, B.K. Sex hormones influence the Helmholtz-Kohlrausch Effect. J. Ophthalmic Vis. Res. 2024, 19, 71–81. [Google Scholar] [CrossRef]
- Foutch, B.K.; Bassi, C.J. Is the Helmholtz-Kohlrausch Effect more robust in women? Perception 2020, 49, 636–657. [Google Scholar] [CrossRef] [PubMed]
- Lennie, P.; Pokorny, J.; Smith, V.C. Luminance. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 1993, 10, 1283–1293. [Google Scholar] [CrossRef]
- Cockerill, I.M.; Wormington, J.A.; Nevill, A.M. Menstrual-cycle effects on mood and perceptual-motor performance. J. Psychosom. Res. 1994, 38, 763–771. [Google Scholar] [CrossRef]
- Sommer, B. How does menstruation affect cognitive competence and psychophysiological response? Women Health 1983, 8, 53–90. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.A.; Anfe, T.E.; Matos, A.B.; Vieira, G.F. Influence of gender, anxiety and depression symptoms, and use of oral contraceptive in color perception. J. Esthet. Restor. Dent. 2015, 27 (Suppl. S1), S74–S79. [Google Scholar] [CrossRef] [PubMed]
- Ives, H.E. Studies in the photometry of different colours. I. Spectral luminosity curves obtained by the equality of the brightness photometer and the flicker photometer under similar conditions. Phil. Mag. 1912, 24, 149–188. [Google Scholar] [CrossRef]
- Ikeda, M.; Shimozono, H. Luminous efficiency functions determined by successive brightness matching. J. Am. Opt. Soc. 1978, 68, 1767–1771. [Google Scholar] [CrossRef]
- Draper, C.F.; Duisters, K.; Weger, B.; Chakrabarti, A.; Harms, A.C.; Brennan, L.; Hankemeier, T.; Goulet, L.; Konz, T.; Martin, F.P.; et al. Menstrual cycle rhythmicity: Metabolic patterns in healthy women. Sci. Rep. 2018, 8, 14568, Erratum in Sci. Rep. 2019, 9, 5797. [Google Scholar] [CrossRef]
- National Library of Medicine. Ovulation Home Test. Retrieved from MedlinePlus. 2021. Available online: https://medlineplus.gov/ency/article/007062.htm (accessed on 27 October 2023).
- Lewis, J.G. Steroid analysis in saliva: An overview. Clin. Biochem. Rev. 2006, 27, 139–146. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).