Comparative Reactivity of Different Polyols in the PET Saponification Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Method of PET Depolymerization
2.3. Method of Product Mixture Separation
2.4. Methods of Products Analysis
3. Results
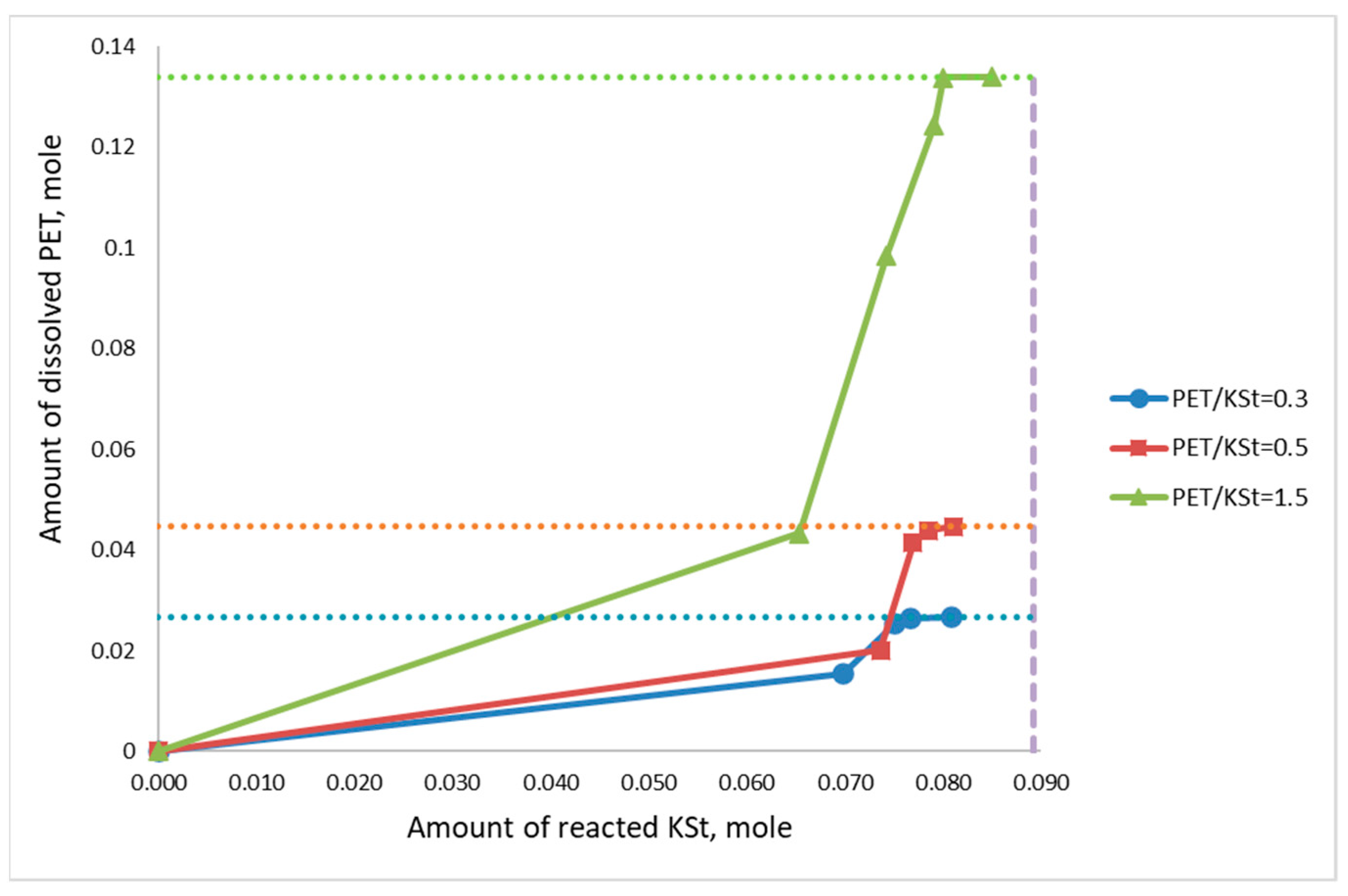
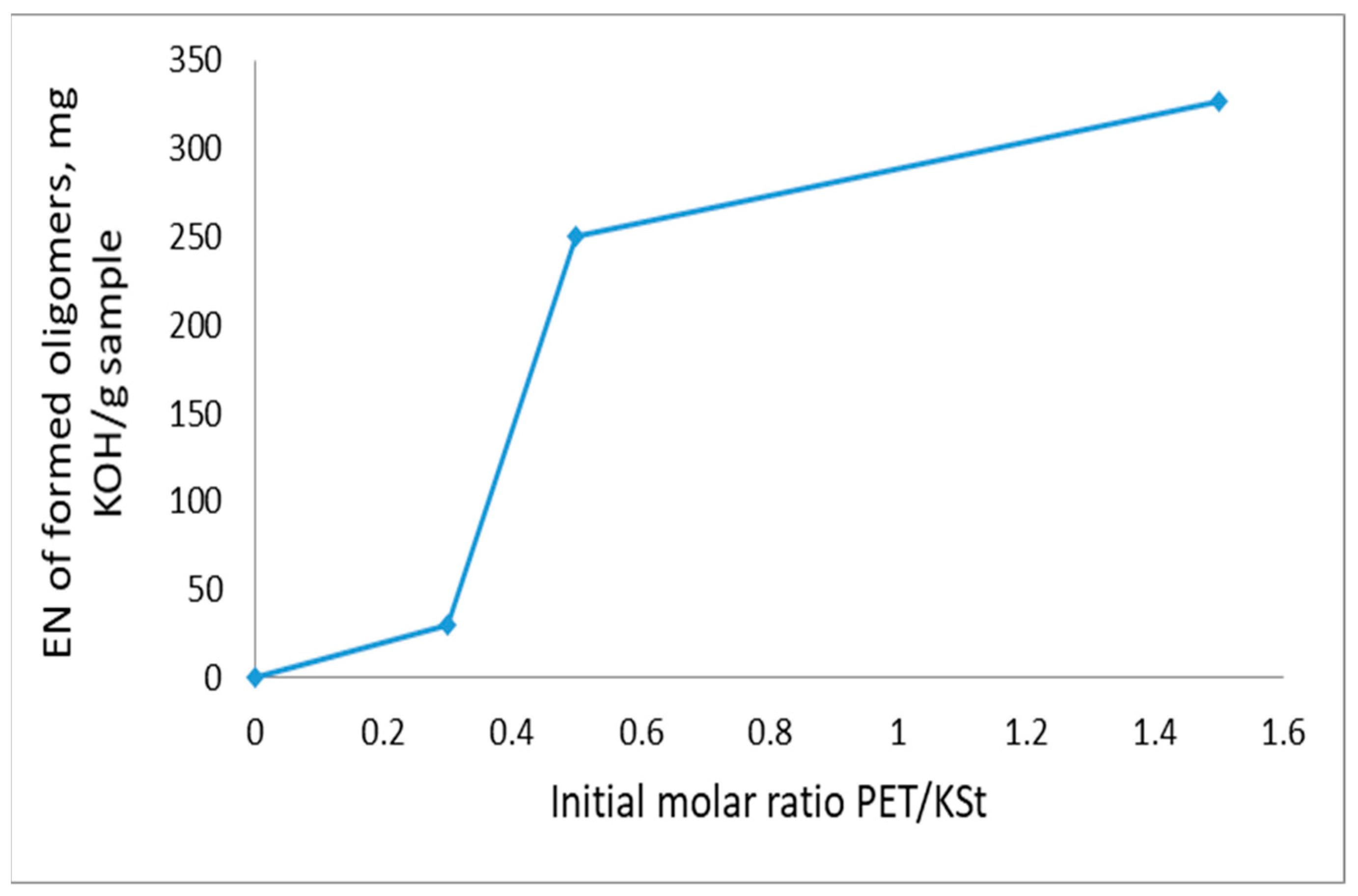
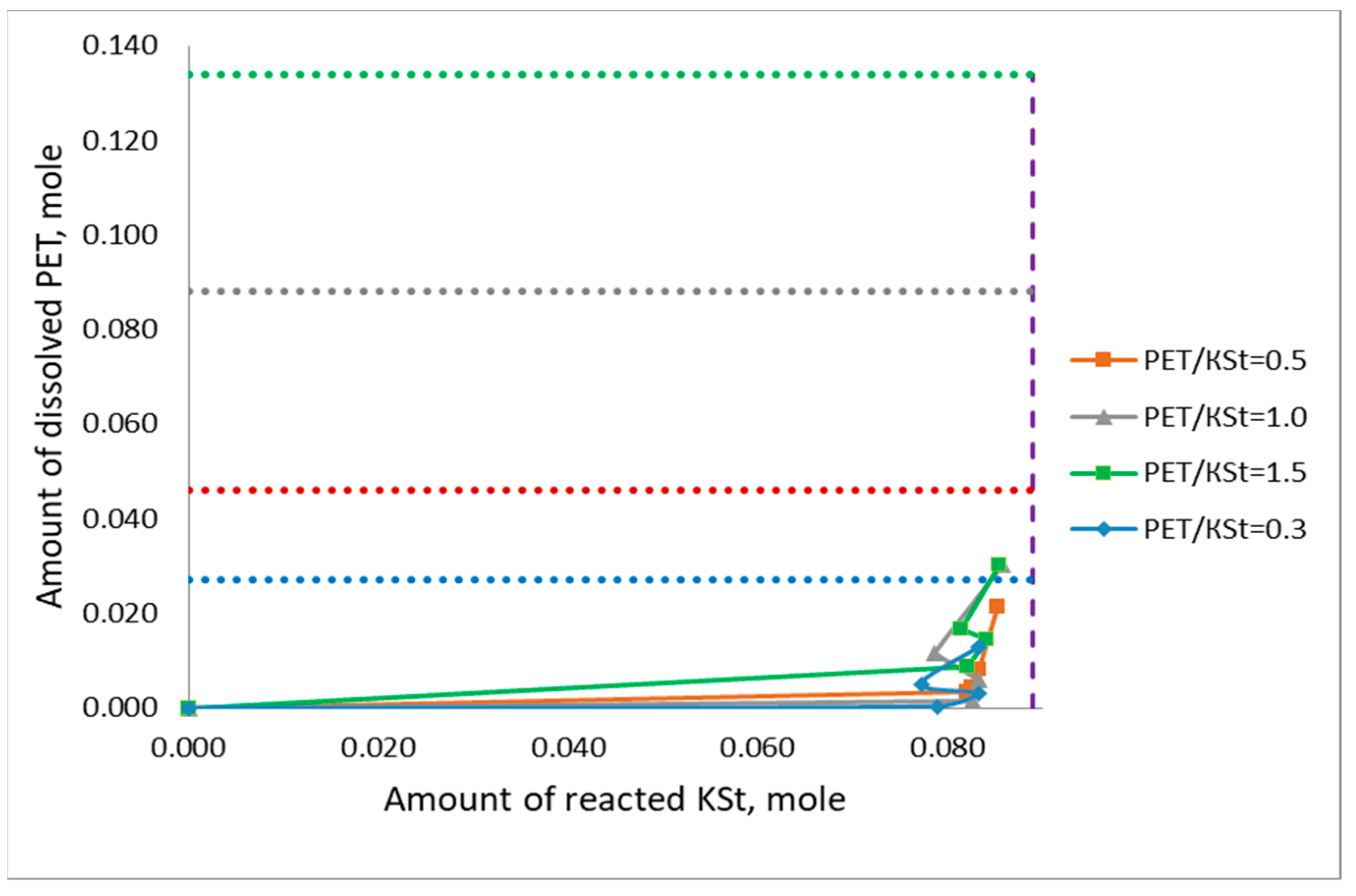
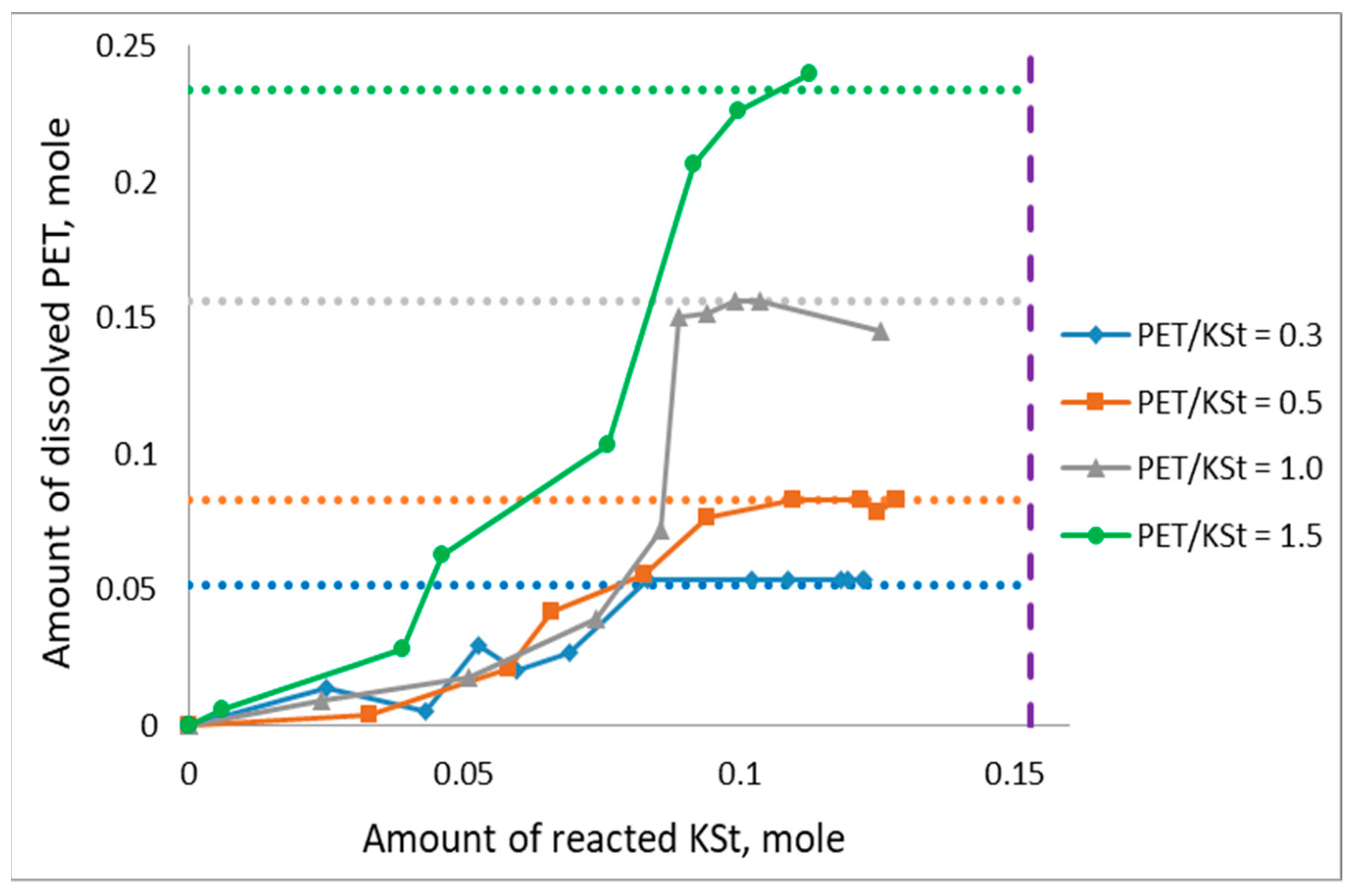
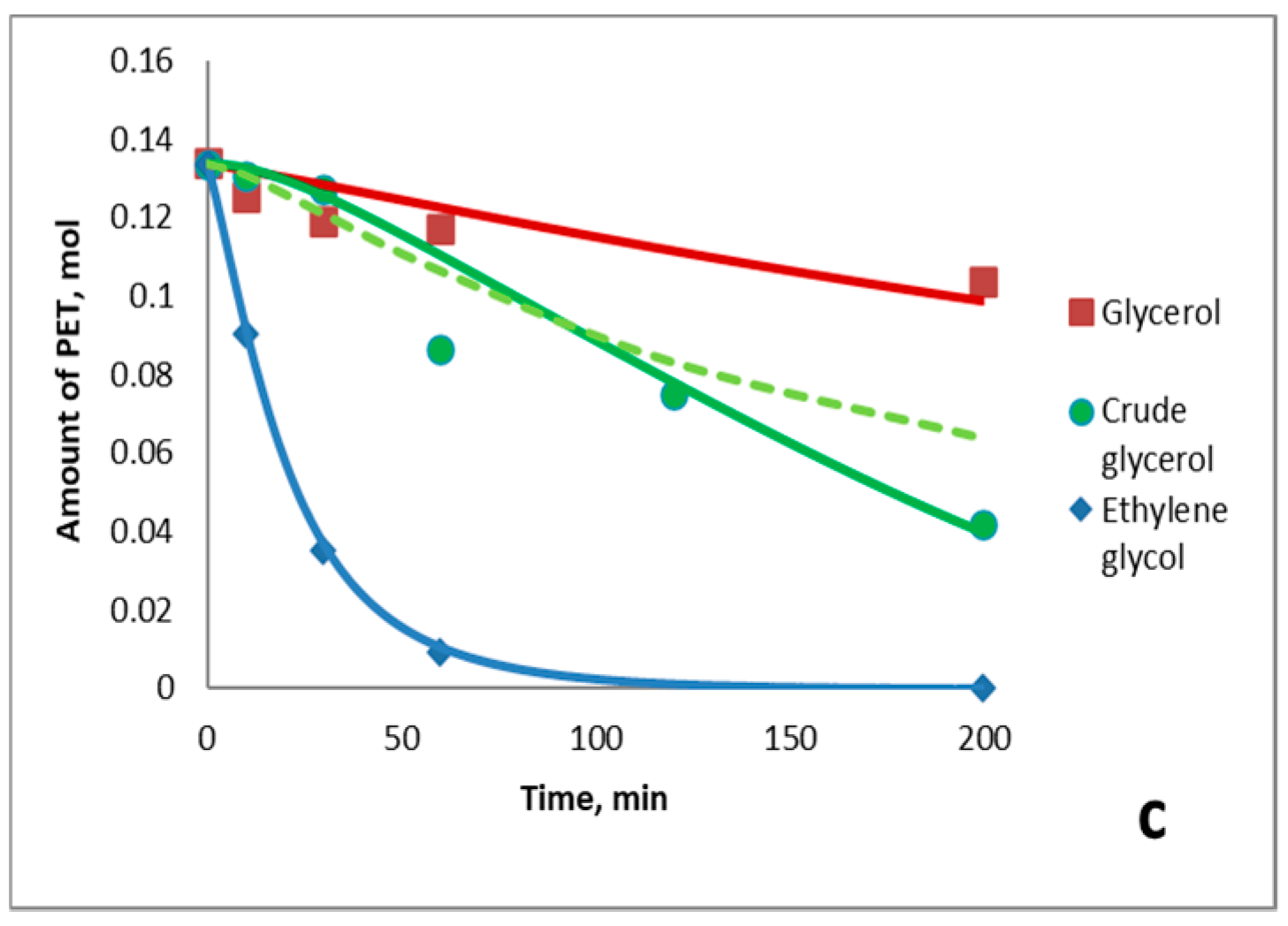
3.1. General Patterns of PET Depolymerization Process
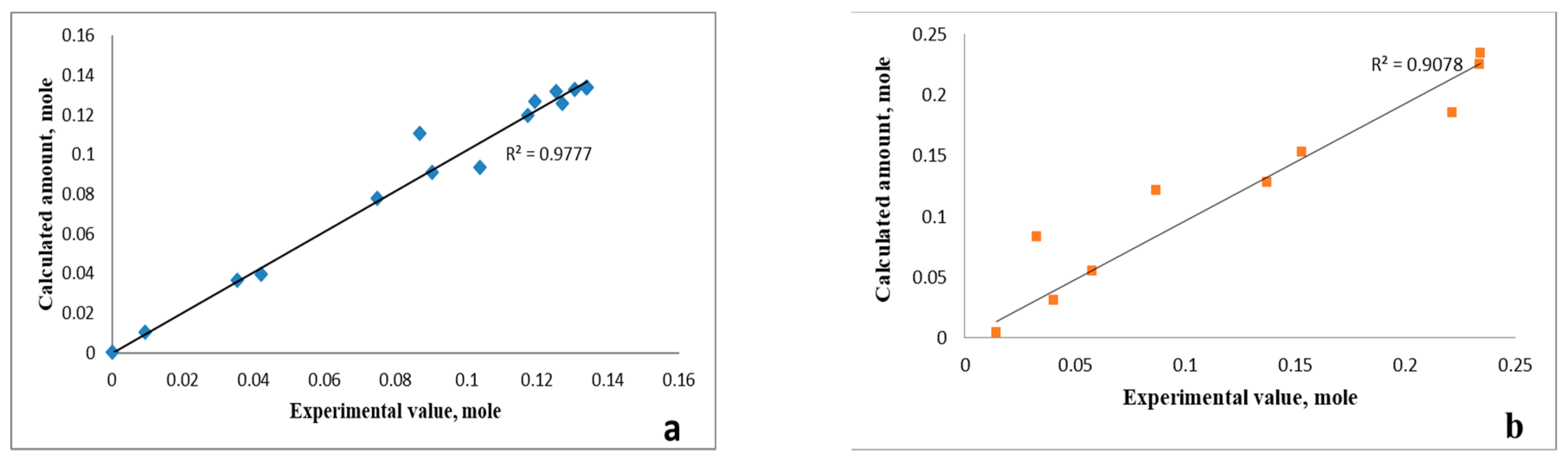
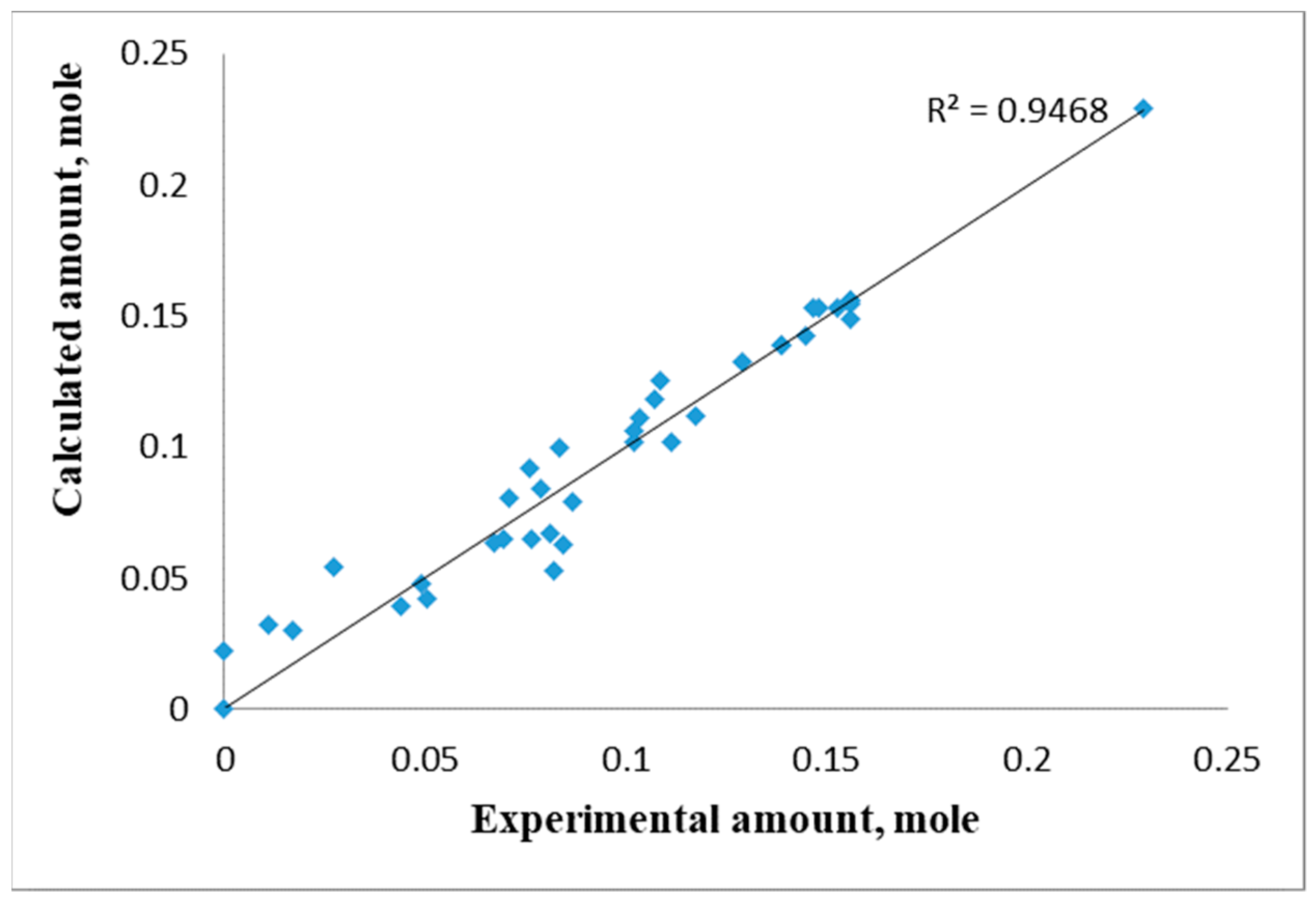
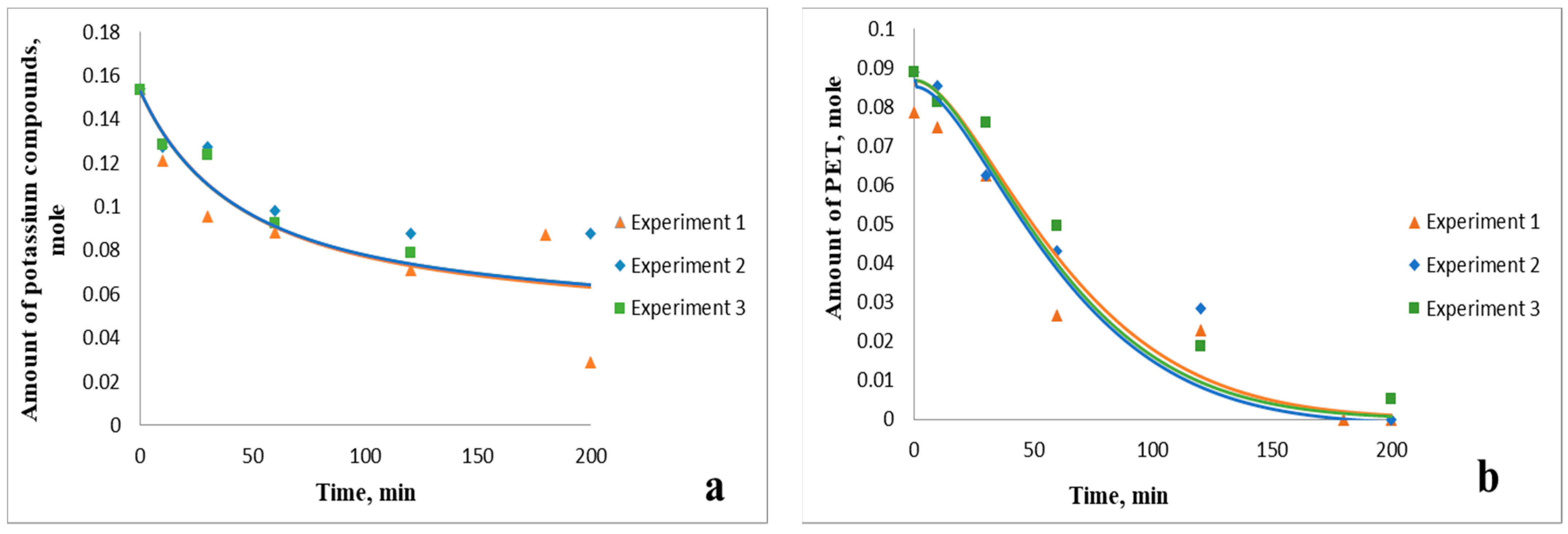
3.2. Kinetic Patterns of PET Depolymerization Process
- (1)
- PET saponification by potassium compounds through intercalate formation;
- (2)
- PET saponification by potassium compounds through the outer surface;
- (3)
- Transesterification of FAEGEs with glycerol with the formation of ethylene glycol (EG) and fatty acid glycerol esters (FAGEs);
- (4)
- PET glycolysis via in situ forming ethylene glycol.
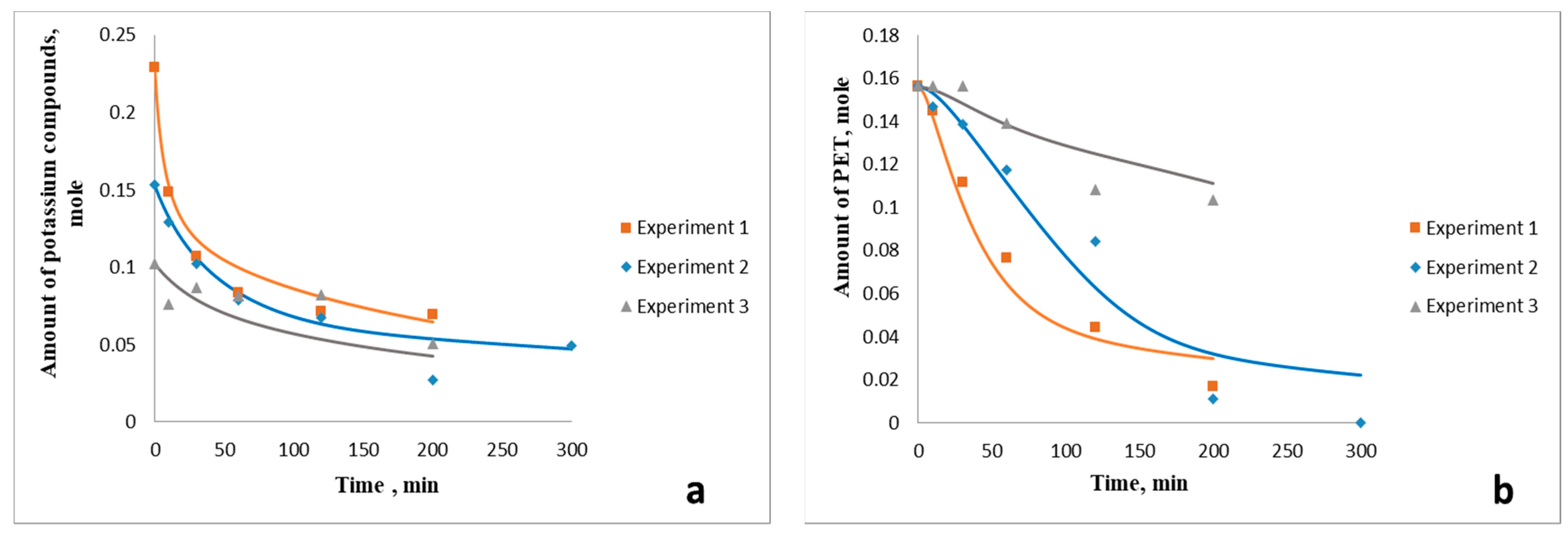
3.3. Influence of Crude Glycerol Composition on the Process Kinetics
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sarker, A.; Deepo, D.M.; Nandi, R.; Rana, J.; Islam, S.; Rahman, S.; Hossain, M.N.; Islam, M.S.; Baroi, A.; Kim, J.E. A review of microplastics pollution in the soil and terrestrial ecosystems: A global and Bangladesh perspective. Sci. Total Environ. 2020, 733, 139296. [Google Scholar] [CrossRef] [PubMed]
- Kirstein, I.V.; Gomiero, A.; Vollertsen, J. Microplastic pollution in drinking water. Curr. Opin. Toxicol. 2021, 28, 70–75. [Google Scholar] [CrossRef]
- James, A.; Rahman, M.R.; Khusairy Bin Bakri, M.; Mahbubul Matin, M. Introduction to Recycled Plastic Biocomposites. In Recycled Plastic Biocomposites; Woodhead Publishing: Sawston, UK; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Rahman, M.R.; Khusairy Bin Bakri, M.; Mahbubul Matin, M.; Law Nyuk Khui, P. Micro and Nano Effects of Recycled Plastic Waste to Reinforce and Enhance in Biocomposites. In Recycled Plastic Biocomposites; Woodhead Publishing: Sawston, UK, 2022. [Google Scholar]
- Masmoudi, F.; Alix, S.; Buet, S.; Mehri, A.; Bessadok, A.; Jaziri, M.; Ammar, E. Design and Characterization of a New Food Packaging Material by Recycling Blends Virgin and Recovered polyethylene terephthalate. Polym. Eng. Sci. 2020, 60, 250–256. [Google Scholar] [CrossRef]
- Nait-Ali, K.L.; Bergeret, A.; Ferry, L.; Colin, X. Chain branching detection by Cole-Cole modeling of rheological properties changes during PET mechanical recycling. Polym. Test. 2012, 31, 500–504. [Google Scholar] [CrossRef]
- Lee, H.L.; Chiu, C.W.; Lee, T. Engineering terephthalic acid product from recycling of PET bottles waste for downstream operations. Chem. Eng. J. Adv. 2021, 5, 100079. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, C.; Fang, C.; Yu, R.; Li, Y.; Lei, W. Structure and thermal properties of various alcoholysis products from waste poly(ethylene terephthalate). Waste Manag. 2019, 85, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Xin, J.; Zhang, Q.; Huang, J.; Huang, R.; Jaffery, Q.Z.; Yan, D.; Zhou, Q.; Xu, J.; Lu, X. Progress in the catalytic glycolysis of polyethylene terephthalate. J. Environ. Manag. 2021, 296, 113267. [Google Scholar] [CrossRef] [PubMed]
- Nikles, D.E.; Farahat, M.S. New motivation for the depolymerization products derived from poly(ethylene terephthalate) (PET) waste: A review. Macromol. Mater. Eng. 2005, 290, 13–30. [Google Scholar] [CrossRef]
- Hughes, A. Building and Constructions Plastic Market—An Analysis. Reinf. Plast. 2021, 65, 194–198. [Google Scholar] [CrossRef]
- Das, A.; Mahanwar, P. A brief discussion on advances in polyurethane applications. Adv. Ind. Eng. Polym. Res. 2020, 3, 93–101. [Google Scholar] [CrossRef]
- Cevher, D.; Sürdem, S. Polyurethane adhesive based on polyol monomers BHET and BHETA depolymerised from PET waste. Int. J. Adhes. Adhes. 2021, 105, 102799. [Google Scholar] [CrossRef]
- Jamdar, V.; Kathalewar, M.; Dubey, K.A.; Sabnis, A. Recycling of PET wastes using Electron beam radiations and preparation of polyurethane coatings using recycled material. Prog. Org. Coat. 2017, 107, 54–63. [Google Scholar] [CrossRef]
- Paraskar, P.M.; Prabhudesai, M.S.; Hatkar, V.M.; Kulkarni, R.D. Vegetable oil based polyurethane coatings—A sustainable approach: A review. Prog. Org. Coat. 2021, 156, 106267. [Google Scholar] [CrossRef]
- Chen, K.; Ren, Q.; Li, J.; Chen, D.; Li, C. A highly stretchable and self-healing hydroxy-terminated polybutadiene elastomer. J. Saudi Chem. Soc. 2020, 24, 1034–1041. [Google Scholar] [CrossRef]
- Djapovic, M.; Milivojevic, D.; Ilic-Tomic, T.; Lješević, M.; Nikolaivits, E.; Topakas, E.; Maslak, V.; Nikodinovic-Runic, J. Synthesis and characterization of polyethylene terephthalate (PET) precursors and potential degradation products: Toxicity study and application in discovery of novel PETases. Chemosphere 2021, 275, 130005. [Google Scholar] [CrossRef]
- Bhogle, C.S.; Pandit, A.B. Ultrasound-Assisted Alkaline Hydrolysis of Waste Poly(Ethylene Terephthalate) in Aqueous and Non-aqueous Media at Low Temperature. Indian Chem. Eng. 2018, 60, 122–140. [Google Scholar] [CrossRef]
- Dzhabarov, G.V.; Sapunov, V.N.; Shadrina, V.V.; Voronov, M.S.; Nhi, T.D.; Staroverov, D.V. A kinetic study on the depolymerization of polyethylene terephthalate waste with crude glycerol. Chem. Pap. 2021, 75, 6035–6046. [Google Scholar] [CrossRef]
- Panasyuk, G.P.; Azarova, L.A.; Khaddaj, M.; Budova, G.P.; Voroshilov, I.L.; Grusha, T.V.; Izotov, A.D. Preparation and Properties of Sodium, Potassium, Magnesium, Calcium, and Aluminum Terephthalates. Inorg. Mater. 2003, 39, 1292–1297. [Google Scholar] [CrossRef]
- Dzhabarov, G.; Sapunov, V.; Kozlovskiy, R.; Makarova, E.; Dinh, P.; Voronov, M.; Shadrina, V.; Nhi, T.D.; Kurneshova, T. A Method of Polyethylene Terephthalate Depolymerization by Biodiesel Wastes. Pet. Coal 2020, 62, 19–26. [Google Scholar]
- Sapunov, V.N.; Voronov, M.S.; Dzhabarov, G.V.; Makarova, E.M.; Suchkov, Y.P.; Shadrina, V.V.; Orel, P.A.; Kurneshova, T.A.; Korovina, N.S. Method for Processing Polyethylene Terephthalate Waste. Patent RU 2754972, 8 September 2021. [Google Scholar]







| Compound | Content, % wt. | Composition of FAMEs Fraction | |
|---|---|---|---|
| Fatty acid methyl ethers (FAMEs) | 4.5 | C16:0palmitic | 10.2 |
| C18:0+1stearic + oleic | 67.4 | ||
| C18:2linoleic | 21.9 | ||
| C18:3linolenic | 0.5 | ||
| Glycerol | 50.4 | ||
| Potassium salts of fatty acids * (KSt) | 45.1 | ||
| № | Polyol Type | [PET]0/[K+]0 | k1 mol−1*min−1 | k11 min−1 | k12 mol−1*min−1 | k2 mol−1 *min−1 | k21 min−1 | k22 mol−1*min−1 | k3 mol−1*min−1 | k4 mol−1*min−1 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Ethylene glycol | 0.3 | 0.210 | 0.090 | 6.000 | 9.500 | 0.080 | 7.000 | - | 0.300 |
| 2 | 0.5 | 0.140 | 0.090 | 5.500 | 7.000 | 0.080 | 5.000 | - | 0.250 | |
| 3 | 1.5 | 0.030 | 0.090 | 0.600 | 0.850 | 0.080 | 0.310 | - | 0.025 | |
| 4 | Glycerol | 0.3 | 0.75 | 0.022 | 2.8 | 0.99 | 0.0001 | 0.65 | 1.5 | 0.0005 |
| 5 | 0.5 | 0.68 | 0.022 | 2.5 | 0.92 | 0.0001 | 0.5 | 1.5 | 0.001 | |
| 6 | 1.5 | 0.14 | 0.022 | 1.5 | 0.71 | 0.0001 | 0 | 1.5 | 0.022 |
| № | t, °C | [PET]0/[K+]0 | [KOH]0 mol | [KSt]0 mol | k1 mol−1*min−1 | k11 min−1 | k12 mol−1*min−1 | k2 mol−1 *min−1 | k21 min−1 | k22 mol−1*min−1 | k3 mol−1*min−1 | k4 mol−1*min−1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 170 | 0.3 | 0.047 | 0.11 | 0.11 | 0.03 | 0.040 | 1.2 | 0.035 | 0.05 | 0.0105 | 0.65 |
| 2 | 0.5 | 0.047 | 0.11 | 0.11 | 0.03 | 0.008 | 0.2 | 0.035 | 0.008 | 0.0085 | 0.53 | |
| 3 | 1.0 | 0.047 | 0.11 | 0.114 | 0.03 | 0.006 | 0.12 | 0.035 | 0.005 | 0.0065 | 0.36 | |
| 4 | 1.5 | 0.047 | 0.11 | 0.015 | 0.03 | 0 | 0.042 | 0.035 | 0 | 0.0045 | 0.25 | |
| 5 | 190 | 0.4 | 0.049 | 0.117 | 1.5 | 0.06 | 1.9 | 7.5 | 0.07 | 1.5 | 0.95 | 2 |
| 6 | 0.5 | 0.049 | 0.117 | 1.35 | 0.06 | 0.5 | 3.1 | 0.07 | 0.55 | 0.55 | 1.7 | |
| 7 | 1.0 | 0.047 | 0.11 | 0.185 | 0.06 | 0 | 0.45 | 0.07 | 0 | 0.085 | 0.5 | |
| 8 | 1.5 | 0.047 | 0.11 | 0.068 | 0.06 | 0 | 0.12 | 0.07 | 0 | 0.0105 | 0.4 |
| № | t, °C | [PET]0 | [KOH]0 mol | [KSt]0 mol | k1 mol−1*min−1 | k11 min−1 | k12 mol−1*min−1 | k2 mol−1 *min−1 | k21 min−1 | k22 mol−1*min−1 | k3 mol−1*min−1 | k4 mol−1*min−1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 170 | 0.156 | 0.119 | 0.11 | 0.317 | 0.03 | 0.016 | 0.12 | 0.035 | 0.011 | 0.0065 | 0.58 |
| 2 | 0.047 | 0.11 | 0.114 | 0.03 | 0.006 | 0.12 | 0.035 | 0.005 | 0.0065 | 0.36 | ||
| 3 | 0.029 | 0.073 | 0.076 | 0.03 | 0.011 | 0.08 | 0.035 | 0.007 | 0.0043 | 0.05 |
| № | t, °C | [PET]0 | [KOH]0 mol | [KSt]0 mol | k′1 mol−1*min−1 | k′11 min−1 | k′12 mol−1*min−1 | k′2 mol−1 *min−1 | k′21 min−1 | k′22 mol−1*min−1 | k′3 mol−1*min−1 | k4 mol−1*min−1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 170 | 0.156 | 0.119 | 0.11 | 2.646 | 0.030 | 0.150 | 1.089 | 0.035 | 0.101 | 0.059 | 2.646 |
| 2 | 0.047 | 0.11 | 2.654 | 0.030 | 0.145 | 1.091 | 0.035 | 0.100 | 0.059 | 2.654 | ||
| 3 | 0.029 | 0.073 | 2.651 | 0.030 | 0.055 | 1.091 | 0.035 | 0.045 | 0.059 | 2.651 |
| № | t, °C | [PET]0/[K+]0 | [KOH]0 mol | [KSt]0 mol | k′1 mol−1*min−1 | k′11 min−1 | k′12 mol−1*min−1 | k′2 mol−1 *min−1 | k′21 min−1 | k′22 mol−1*min−1 | k′3 mol−1*min−1 | k4 mol−1*min−1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 170 | 0.3 | 0.047 | 0.11 | 3.953 | 0.03 | 0.025 | 1.229 | 0.035 | 0.049 | 0.095 | 0.65 |
| 2 | 0.5 | 0.047 | 0.11 | 2.558 | 0.03 | 0.010 | 0.255 | 0.035 | 0.010 | 0.077 | 0.53 | |
| 3 | 1.0 | 0.047 | 0.11 | 2.651 | 0.03 | 0.008 | 0.169 | 0.035 | 0.007 | 0.059 | 0.36 | |
| 4 | 1.5 | 0.047 | 0.11 | 0.349 | 0.03 | 0.000 | 0.065 | 0.035 | 0.000 | 0.041 | 0.25 | |
| 5 | 190 | 0.4 | 0.049 | 0.117 | 30.612 | 0.06 | 2.236 | 8.825 | 0.07 | 1.765 | 8.120 | 2.00 |
| 6 | 0.5 | 0.049 | 0.117 | 27.551 | 0.06 | 0.602 | 3.734 | 0.07 | 0.663 | 4.701 | 1.70 | |
| 7 | 1.0 | 0.043 | 0.11 | 4.302 | 0.06 | 0.000 | 0.634 | 0.07 | 0.000 | 0.773 | 0.70 | |
| 8 | 1.5 | 0.043 | 0.11 | 1.581 | 0.06 | 0.000 | 0.185 | 0.07 | 0.000 | 0.095 | 0.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sapunov, V.N.; Dzhabarov, G.V.; Shadrina, V.V.; Voronov, M.S.; Kozlovskiy, R.A.; Orel, P.A.; Magorina, L.N.; Izmailova, T.D.; Boldina, E.V. Comparative Reactivity of Different Polyols in the PET Saponification Process. AppliedChem 2023, 3, 153-168. https://doi.org/10.3390/appliedchem3010011
Sapunov VN, Dzhabarov GV, Shadrina VV, Voronov MS, Kozlovskiy RA, Orel PA, Magorina LN, Izmailova TD, Boldina EV. Comparative Reactivity of Different Polyols in the PET Saponification Process. AppliedChem. 2023; 3(1):153-168. https://doi.org/10.3390/appliedchem3010011
Chicago/Turabian StyleSapunov, Valentin N., Georgy V. Dzhabarov, Violetta V. Shadrina, Mikhail S. Voronov, Roman A. Kozlovskiy, Pavel A. Orel, Lubov N. Magorina, Tatiana D. Izmailova, and Elena V. Boldina. 2023. "Comparative Reactivity of Different Polyols in the PET Saponification Process" AppliedChem 3, no. 1: 153-168. https://doi.org/10.3390/appliedchem3010011
APA StyleSapunov, V. N., Dzhabarov, G. V., Shadrina, V. V., Voronov, M. S., Kozlovskiy, R. A., Orel, P. A., Magorina, L. N., Izmailova, T. D., & Boldina, E. V. (2023). Comparative Reactivity of Different Polyols in the PET Saponification Process. AppliedChem, 3(1), 153-168. https://doi.org/10.3390/appliedchem3010011






