Biochar–Nitrogen Composites: Synthesis, Properties, and Use as Fertilizer for Maize
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil and Feedstock Characterization
2.2. Acidification of Feedstock and the Composite Syntehsis
2.3. Laboratory Analysis
2.4. Nitrogen Release in Soil Solution
2.5. Infrared Spectroscopy
2.6. Maize Cultivation Conditions
2.7. Hydrolyzable N in Composites and Biochars
2.8. Statistical Analysis
3. Results and Discussion
3.1. Biochar Properties
3.2. Infrared Spectrsocopy
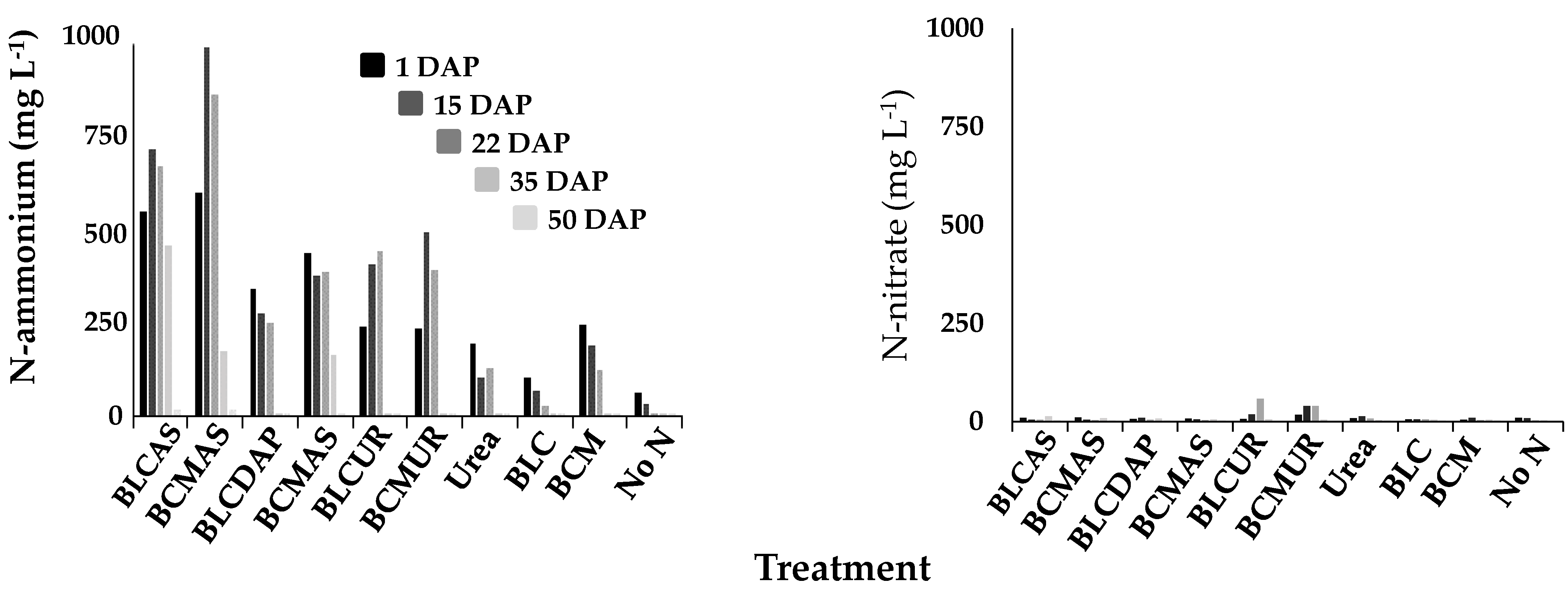
3.3. Nitrogen in Oxisol solution
3.4. Carbon in Oxisol Solution
3.5. Nitrogen Availability in Oxisol
3.6. Water- and HCl-Soluble N in Composite and Biochar
3.7. Composite Agronomic Effectiveness
3.8. N in Maize Shoot
3.9. Agronomic and Environmental Implications
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beig, B.; Niazi, M.B.K.; Jahan, Z.; Hussain, A.; Zia, M.H.; Mehran, M.T. Coating materials for slow release of nitrogen from urea fertilizer: A review. J. Plant Nutr. 2010, 43, 1510–1533. [Google Scholar] [CrossRef]
- Barbosa, W.S.S. Milho Cultivado Sob Diferentes Lâminas de Irrigação e Adubação Nitrogenada. Master’s Thesis, Universidade Federal de Alagoas, Delmiro Gouveia, Brazil, 2017. [Google Scholar]
- Lyra, G.B.; Rocha, A.E.Q.; Lyra, G.B.; Souza, J.L.; Teodoro, I. Crescimento e produtividade do milho, submetido a doses de nitrogênio nos Tabuleiros Costeiros de Alagoas. Rev. Ceres 2014, 61, 578–586. [Google Scholar] [CrossRef]
- Pino, R.C.; Cayuela, M.L.; García, M.S.; Monedero, M.A.S. Nitrogen availability in biochar-based fertilizers depending on activation treatment and nitrogen source. Waste Manag. 2023, 158, 76–83. [Google Scholar] [CrossRef]
- Puga, A.P.; Grutzmacher, P.; Cerri, C.E.P.; Ribeirinho, V.S.; Andrade, C.A. Biochar-based nitrogen fertilizers: Greenhouse gas emissions, use efficiency, and maize yield in tropical soils. Sci. Total Environ. 2020, 704, 135375. [Google Scholar] [CrossRef]
- Joseph, S.; Cowie, A.L.; Van Zwieten, L.; Bolan, N.; Budai, A.; Buss, W.; Cayuela, M.L.; Graber, E.R.; Ippolito, J.A.; Kuzyakov, Y.; et al. How biochar works, and when it doesn’t: A review of mechanisms controlling soil and plant responses to biochar. GCB Bioenergy 2021, 13, 1731–1764. [Google Scholar] [CrossRef]
- Melo, L.C.A.; Lehmann, J.; da Silva Carneiro, J.S.; Camps-Arbestain, M. Biochar-based fertilizer effects on crop productivity: A meta-analysis. Plant Soil 2022, 472, 45–58. [Google Scholar] [CrossRef]
- Paiva, I.O.; Morais, E.; Jindo, K.; Silva, C.A. Biochar N Content, Pools and Aromaticity as Affected by Feedstock and Pyrolysis Temperature. Waste Biomass Valorization 2024. [Google Scholar] [CrossRef]
- Jia, Y.; Hu, Z.; Ba, Y.; Qi, W. Application of biochar-coated urea controlled loss of fertilizer nitrogen and increased nitrogen use efficiency. Chem. Biol. Technol. Agric. 2021, 8, 3. [Google Scholar] [CrossRef]
- Mandal, S.; Donner, E.; Vasileiadis, S.; Skinner, W.; Smith, E.; Lombi, E. The effect of biochar feedstock, pyrolysis temperature, and application rate on the reduction of ammonia volatilization from biochar-amended soil. Sci. Total Environ. 2018, 627, 942–950. [Google Scholar] [CrossRef]
- Zheng, J.; Han, J.; Liu, Z.; Xia, W.; Zhang, X.; Li, L.; Liu, X.; Bian, R.; Cheng, K.; Zheng, J.; et al. Biochar compound fertilizer increases nitrogen productivity and economic benefits but decreases carbon emission of maize production. Agric. Ecosyst. Environ. 2017, 241, 70–78. [Google Scholar] [CrossRef]
- He, Z.; Wang, C.; Cao, H.; Liang, J.; Pei, S.; Li, Z. Nitrate Absorption and Desorption by Biochar. Agronomy 2023, 13, 2440. [Google Scholar] [CrossRef]
- Banik, C.; Bakshi, S.; Laird, D.A.; Smith, R.G.; Brown, R.C. Impact of biochar-based slow-release N-fertilizers on maize growth and nitrogen recovery efficiency. J. Environ. Qual. 2023, 52, 630–640. [Google Scholar] [CrossRef]
- El-Gamal, E.; Saleh, M.; Elsokkary, I.; Rashad, M.; Abd El-Latif, M.M. Comparison between properties of biochar produced by traditional and controlled pyrolysis. ASEJ 2017, 38, 413–424. [Google Scholar]
- Novotny, E.H.; de Freitas Maia, C.M.B.; de Melo Carvalho, M.T.; Madari, B.E. Biochar: Pyrogenic Carbon for Agricultural Use—A Critical Review. Rev. Bras. Ciênc. Solo 2015, 39, 321–344. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Bio/Technol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Sohi, S.; Lopez-Capel, E.; Krull, E.; Bol, R. Biochar, climate change and soil: A review to guide future research. CSIRO Land. Water Sci. Rep. 2009, 5, 17–31. [Google Scholar]
- Chatterjee, R.; Sajjadi, B.; Chen, W.Y.; Mattern, D.L.; Hammer, N.; Raman, V.; Dorris, A. Effect of pyrolysis temperature on physicochemical properties and acoustic-based amination of biochar for efficient CO2 adsorption. Front. Energ. Res. 2020, 8, 85. [Google Scholar] [CrossRef]
- Shi, W.; Ju, Y.; Bian, R.; Li, L.; Joseph, S.; Mitchell, D.R.G.; Munroe, P.; Taherymoosavi, S.; Pan, G. Biochar bound urea boosts plant growth and reduces nitrogen leaching. Sci. Total Environ. 2020, 701, 134424. [Google Scholar] [CrossRef]
- Steiner, C.; Das, K.C.; Melear, N.; Lakly, D. Reducing nitrogen loss during poultry litter composting using biochar. J. Environ. Qual. 2010, 39, 1236. [Google Scholar] [CrossRef]
- Zhou, J.; Qu, T.; Li, Y.; Zwieten, L.; Wang, H.; Chen, J.; Song, X.; Lin, Z.; Zhang, X.; Luo, Y.; et al. Biochar-based fertilizer decreased while chemical fertilizer increased soil N2O emissions in a subtropical Moso bamboo plantation. CATENA 2021, 202, 105257. [Google Scholar] [CrossRef]
- Wang, L.; Ok, Y.S.; Tsang, D.C.W.; Alessi, D.S.; Rinklebe, J.; Wang, H.; Hou, D. New trends in biochar pyrolysis and modification strategies: Feedstock, pyrolysis conditions, sustainability concerns and implications for soil amendment. Soil Use Manag. 2020, 36, 358–386. [Google Scholar] [CrossRef]
- Domingues, R.R.; Trugilho, P.F.; Silva, C.A.; De Melo, I.C.N.A.; Melo, L.C.A.; Magriotis, Z.M.; Sánchez-Monedero, M.A. Properties of biochar derived from wood and high-nutrient biomasses with the aim of agronomic and environmental benefits. PLoS ONE 2017, 12, e0176884. [Google Scholar] [CrossRef]
- Carmo, D.L.; Silva, C.A.; Lima, J.M.; Pinheiro, G.L. Electrical Conductivity and Chemical Composition of Soil Solution: Comparison of Solut ion Samplers in Tropical Soils. Rev. Bras. Ciênc. Solo 2016, 40, e0140795. [Google Scholar] [CrossRef]
- Singh, B.; Camps-Arbestain, M.; Lehmann, J. Biochar: A Guide to Analytical Methods; CSIRO Publishing: Clayton, Australia, 2017; ISBN 1486305105. [Google Scholar]
- Maluf, H.J.G.M.; Silva, C.A.; Morais, E.G.D.; Paula, L.H.D.D. Is Composting a Route to Solubilize Low-Grade Phosphate Rocks and Improve MAP-Based Composts? Rev. Bras. Ciênc. Solo 2018, 42, e0170079. [Google Scholar] [CrossRef]
- Morais, E.G.; Silva, C.A.; Maluf, H.J.G.M.; Guilherme, L.R.G. Slow-Release Humic Acid-Based Zinc Fertilizers Improve Growth and Nutrition of Maize and Brachiaria Grass Successively Grown in Oxisols. ACS Agric. Sci. Technol. 2022, 3, 17–32. [Google Scholar] [CrossRef]
- Boguta, P.; Sokołowska, Z. Interactions of Zn (II) Ions with Humic Acids Isolated from Various Type of Soils. Effect of pH, Zn Concentrations and Humic Acids Chemical Properties. PLoS ONE 2016, 11, e0153626. [Google Scholar]
- Lin-Vien, D.; Colthup, N.B.; Fateley, W.G.; Grasselli, J.G. The 1233 Handbook of Infrared and Raman Characteristic Frequencies of Organic 1234 Molecules; Elsevier: Oxford, UK, 1991. [Google Scholar]
- Gautam, R.; Vanga, S.; Ariese, F.; Umapathy, S. Review of Multidimensional Data Processing Approaches for Raman and Infrared Spectroscopy. EPJ Tech. Instrum. 2015, 2, 1–38. [Google Scholar] [CrossRef]
- Parikh, S.J.; Goyne, K.W.; Margenot, A.J.; Mukome FN, D.; Calderon, F.J. Soil Chemical Insights Provided through Vibrational Spectroscopy. Adv. Agron. 2014, 126, 1–148. [Google Scholar]
- Wang, T.; Camps Arbestain, M.; Hedley, M.; Bishop, P. Chemical and bioassay characterization of nitrogen availability in biochar produced from dairy manure and biosolids. Org. Geochem. 2012, 51, 45–54. [Google Scholar] [CrossRef]
- Ferreira, D.F. Sisvar: A computer statistical analysis system. Ciência E Agrotecnologia 2011, 35, 1039–1042. [Google Scholar] [CrossRef]
- Joseph, S.; Peacocke, C.; Lehmann, J.; Munroe, P. Developing a biochar classification and test methods. Biochar Environ. Manag. Sci. Technol. 2009, 1, 107–126. [Google Scholar]
- Cantrell, K.B.; Hunt, P.G.; Uchimiya, M.; Novak, J.M.; Ro, K.S. Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresour. Technol. 2012, 107, 419–428. [Google Scholar] [CrossRef]
- Claoston, N.; Samsuri, A.; Ahmad Husni, M.; Mohd Amran, M. Effects of pyrolysis temperature on the physicochemical properties of empty fruit bunch and rice husk biochars. Waste Manag. Res. 2014, 32, 331–339. [Google Scholar] [CrossRef]
- Rehrah, D.; Reddy, M.R.; Novak, J.M.; Bansode, R.R.; Schimmel, K.A.; Yu, J.; Watts, D.W.; Ahmedna, M. Production and characterization of biochars from agricultural by-products for use in soil quality enhancement. J. Anal. Appl. Pyrolysis 2014, 108, 301–309. [Google Scholar] [CrossRef]
- Borchard, N.; Schirmann, M.; Cayuela, M.L.; Kammann, C.; Wrage-M¨onnig, N.; Estavillo, J.M.; Novak, J. Biochar, soil and land-use interactions that reduce nitrate leaching and N2O emissions: A meta-analysis. Sci. Total Environ. 2019, 651, 2354–2364. [Google Scholar] [CrossRef]
- Wang, Y.; Villamil, M.B.; Davidson, P.C.; Akdeniz, N. A quantitative understanding of the role of co-composted biochar in plant growth using metaanalysis. Sci. Total Environ. 2019, 685, 741–752. [Google Scholar] [CrossRef]
- Saha, A.; Basak, B.B.; Gajbhiye, N.A.; Kalariya, K.A.; Manivel, P. Sustainable fertilization through co-application of biochar and chemical fertilizers improvers yield, quality of Andrographis paniculate and soil health. Ind. Crop. Prod. 2019, 140, 111607. [Google Scholar] [CrossRef]
- Han, L.F.; Sun, K.; Yang, Y.; Xia, X.H.; Li, F.B.; Yang, Z.F.; Xing, B.S. Biochar’s stability and effect on the content, composition and turnover of soil organic carbon. Geoderma 2020, 364, 114184. [Google Scholar] [CrossRef]
- Xu, A.X.; Li, L.L.; Xie, J.H.; Wang, X.Z.; Coulter, J.; Liu, C.; Wang, L.L. Effect of long-term nitrogen addition on wheat yield, nitrogen use efficiency, and residual soil nitrate in a semiarid area of the Loess Plateau of China. Sustainability 2020, 12, 1735. [Google Scholar] [CrossRef]
- Gao, Y.; Shao, G.C.; Yang, Z.; Zhang, K.; Lu, J.; Wang, Z.Y.; Wu, S.Q.; Xu, D. Influences of soil and biochar properties and amount of biochar and fertilizer on the performance of biochar in improving plant photosynthetic rate: A meta-analysis. Eur. J. Agron. 2021, 130, 126345. [Google Scholar] [CrossRef]
- Dong, L.; Yang, X.; Shi, L.; Shen, Y.; Wang, L.; Wang, J.; Li, C.; Zhang, H. Biochar and nitrogen fertilizer co-application changed SOC content and fraction composition in Huang-Huai-Hai plain, China. Chemosphere 2022, 291, 132925. [Google Scholar] [CrossRef]
- Zhang, J.N.; Zhou, S.; Sun, H.F.; Lü, F.; He, P.J. The soluble fraction from straw-derived biochar supplies nutrients and affects carbon storage of coastal mudflat soil in rice paddy. Environ. Sci. Pollut. Res. 2020, 27, 18079–18088. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.Y.; Zhang, L.; Riaz, M.; Zhang, M.Y.; Xia, H.; Lv, B.; Jiang, C.C. Assessing the potential of biochar and aged biochar to alleviate aluminum toxicity in an acid soil for achieving cabbage productivity. Ecotoxicol. Environ. Saf. 2018, 161, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Laird, D.; Fleming, P.; Wang, B.Q.; Horton, R.; Karlen, D.L. Biochar impact on nutrient leaching from a Midwestern agricultural soil. Geoderma 2010, 158, 436–442. [Google Scholar] [CrossRef]
- Liao, P.; Huang, S.; van Gestel, N.C.; Zeng, Y.J.; Wu, Z.M.; van Groenigen, K.J. Liming and straw retention interact to increase nitrogen uptake and grain yield in a double rice-cropping system. Field Crop. Res. 2018, 216, 217–224. [Google Scholar] [CrossRef]
- Feng, W.; Yang, F.; Cen, R.; Liu, J.; Qu, Z.; Miao, Q.; Chen, H. Effects of straw biochar application on soil temperature, available nitrogen and growth of corn. J. Environ. Manag. 2021, 277, 111331. [Google Scholar] [CrossRef]
- Chimento, C.; Amaducci, S. Characterization of fine root system and potential contribution to soil organic carbon of six perennial bioenergy crops. Biomass Bioenergy 2015, 83, 116–122. [Google Scholar] [CrossRef]
- Somenahally, A.; Mclawrence, J.; DuPont, J.I.; Brady, J.; Sarkar, R.; Rouquette, M., Jr. Root-mycorrhizae interactions contributed to organic carbon density in the sandy soil profiles of adapted grazing lands. Appl. Soil Ecol. 2020, 154, 103656. [Google Scholar] [CrossRef]
- Fan, C.; Cui, Y.; Zhang, Q.; Yin, N.; Cai, X.; Yuan, X.; Senadheera, S.; Cho, Y. A critical review of the interactions between rhizosphere and biochar during the remediation of metal (loid) contaminated soils. Biochar 2023, 5, 1–33. [Google Scholar] [CrossRef]
- Xu, W.; Xu, H.; Delgado-Baquerizo, M.; Gundale, M.J.; Zou, X.; Ruan, H. Global meta-analysis reveals positive effects of biochar on soil microbial diversity. Geoderma 2023, 436, 116528. [Google Scholar] [CrossRef]
- Glaser, B.; Lehr, V.I. Biochar effects on phosphorus availability in agricultural soils: A meta-analysis. Sci. Rep. 2019, 9, 9338. [Google Scholar] [CrossRef] [PubMed]
- Gou, Z.; Zheng, H.; He, Z.; Su, Y.; Chen, S.; Chen, H.; Chen, G.; Ma, N.L.; Sun, Y. The combined action of biochar and nitrogen-fixing bacteria on microbial and enzymatic activities of soil N cycling. Environ. Pollut. 2023, 317, 120790. [Google Scholar] [CrossRef] [PubMed]
- Phares, C.A.; Atiah, K.; Frimpong, K.A.; Danquah, A.; Asare, A.T.; Aggor-Woananu, S. Application of biochar and inorganic phosphorus fertilizer influenced rhizosphere soil characteristics, nodule formation and phytoconstituents of cowpea grown on tropical soil. Heliyon 2020, 6, e05255. [Google Scholar] [CrossRef] [PubMed]


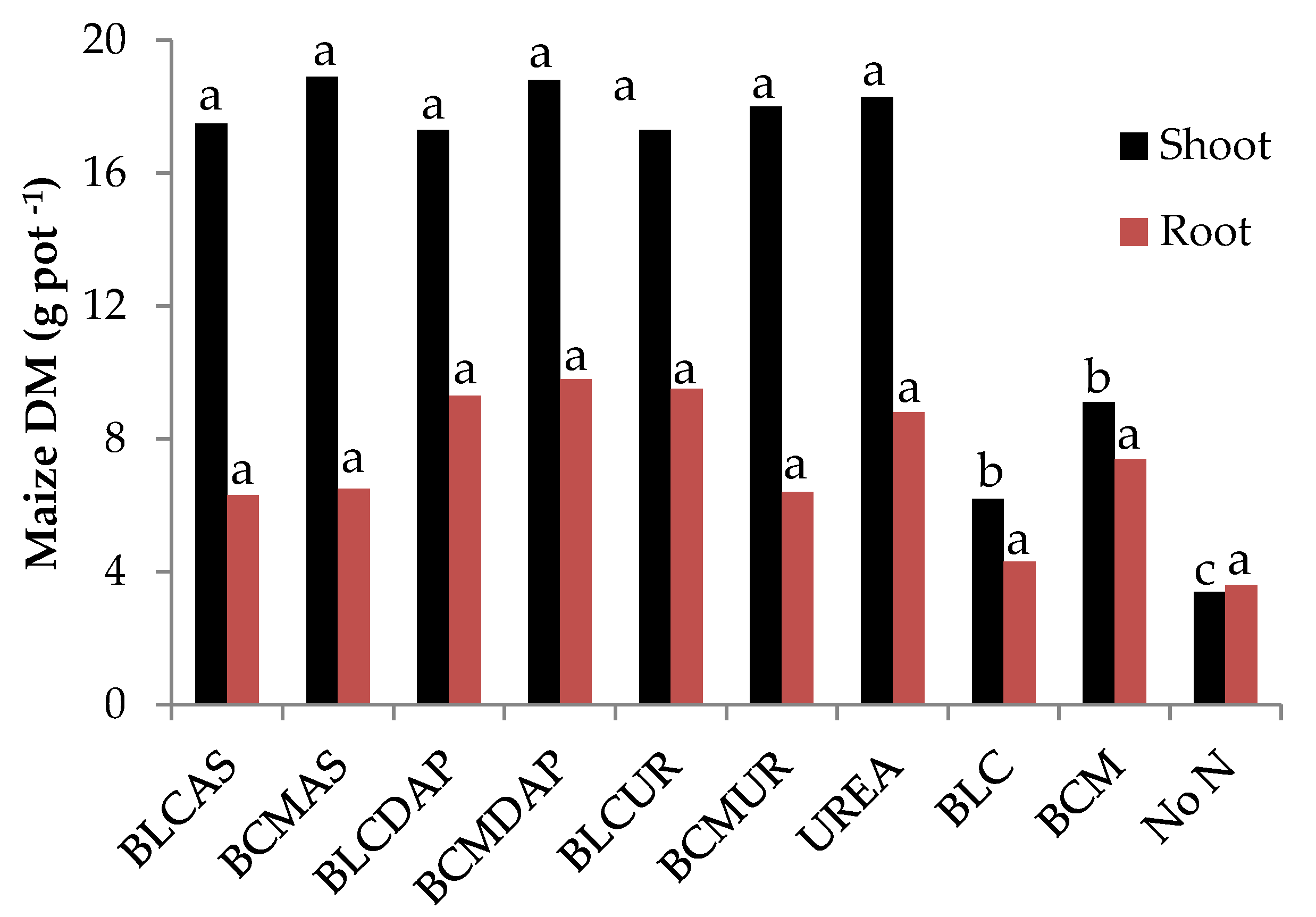

| Soil | pH | K | P | Ca | Mg | Al | H + Al | SB * | t | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (mg dm−3) | (cmolc dm−3) | |||||||||||
| Red-Yellow Latosol | 4.7 | 226 | 0.1 | 0.9 | 0.39 | 0.35 | 2.8 | 1.87 | 2.22 | |||
| T (cmolc dm−3) | BS | m | C | P-Rem (mg dm−3) | Zn | Fe | Mn | Cu | B | S | ||
| (%) | (mg dm−3) | |||||||||||
| 4.67 | 40 | 16 | 1.06 | 36.6 | 0.2 | 36 | 3.5 | 0.1 | 0.1 | 1.2 | ||
| N Source | Yield, % | C, % | N, % | Ash, % | EC, dS m−1 | pH in Water |
|---|---|---|---|---|---|---|
| Biochar (CM) acidified—H3PO4 | ||||||
| 75 | 23 | 3.0 | 60 | 1.5 | 5.5 | |
| Biochar (CM)—H2SO4 | ||||||
| 66 | 24 | 3.4 | 51 | 2.6 | 6.1 | |
| Biochars without acidification | ||||||
| BCM | 73 | 30 | 4.4 | 35 | 1.2 | 8.5 |
| BLC | 76 | 50 | 9.1 | 5 | 2.4 | 6.2 |
| Composite | ||||||
| BCMAS | - | 9 | 15 | 15 | 65 | 5.1 |
| BLCAS | - | 17 | 15 | 9 | 50.2 | 5.5 |
| BCMDAP | - | 8 | 10 | 50 | 44.9 | 5.5 |
| BLCDAP | - | 16 | 10 | 35 | 41.5 | 5.9 |
| BCMUR | - | 23 | 20 | 44 | 8.1 | 6.3 |
| BLCUR | - | 36 | 20 | 10 | 8.3 | 5.2 |
| Treatment | Water-Soluble Carbon (mg L−1) | |
|---|---|---|
| 15 DAP | 22 DAP | |
| BLCAS | 806 | 883 |
| BCMAS | 911 | 768 |
| BLCDAP | 1424 | 1393 |
| BCMDAP | 1261 | 986 |
| BLCUR | 1381 | 1419 |
| BCMUR | 532 | 1681 |
| Urea | 814 | 960 |
| BLC | 945 | 1140 |
| BCM | 1397 | 1272 |
| No N | 809 | 704 |
| N Source | N Soluble in Water (%) | N Soluble in HCl (%) |
|---|---|---|
| BLCAS * | 6.72 | 10.4 |
| BCMAS | 8.57 | 14 |
| BLCDAP | 4.80 | 8.66 |
| BCMDAP | 6.07 | 9 |
| BLCUR | 0.19 | 4.55 |
| BCMUR | 0.22 | 7.57 |
| UREA | 0.43 | - |
| BLC | 0.01 | 0.65 |
| BCM | 0.01 | 1.24 |
| Biochar + H3PO4 | 0.09 | 0.69 |
| Biochar + H2SO4 | 0.18 | 0.37 |
| Treatment | N in Maize Shoot (g pot−1) |
|---|---|
| BLCAS * | 0.25 |
| BCMAS | 0.24 |
| BLCDAP | 0.17 |
| BCMDAP | 0.23 |
| BLCUR | 0.21 |
| BCMUR | 0.23 |
| UREA | 0.18 |
| BLC | 0.03 |
| BCM | 0.08 |
| No N fertilization | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mota, C.P.; Silva, C.A. Biochar–Nitrogen Composites: Synthesis, Properties, and Use as Fertilizer for Maize. AppliedChem 2024, 4, 157-173. https://doi.org/10.3390/appliedchem4020011
Mota CP, Silva CA. Biochar–Nitrogen Composites: Synthesis, Properties, and Use as Fertilizer for Maize. AppliedChem. 2024; 4(2):157-173. https://doi.org/10.3390/appliedchem4020011
Chicago/Turabian StyleMota, Caio Pereira, and Carlos Alberto Silva. 2024. "Biochar–Nitrogen Composites: Synthesis, Properties, and Use as Fertilizer for Maize" AppliedChem 4, no. 2: 157-173. https://doi.org/10.3390/appliedchem4020011
APA StyleMota, C. P., & Silva, C. A. (2024). Biochar–Nitrogen Composites: Synthesis, Properties, and Use as Fertilizer for Maize. AppliedChem, 4(2), 157-173. https://doi.org/10.3390/appliedchem4020011





