Extraction of Carotenoids from Pumpkin (Cucurbita moschata) and Spinach (Spinacia oleracea) Using Environmentally Friendly Deep Eutectic Solvents (DESs)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Deep Eutectic Solvents (DESs)
2.3. Calibration Curve Study for β-Carotene in Deep Eutectic Solvents (DESs)
2.4. Mechanical Mixing Assisted Extraction (MMAE) of β-Carotene
2.5. Homogenization-Assisted Extraction (HAE) of β-Carotene
2.6. Analysis of Samples with a UV/Visible Spectrophotometer for Total Carotenoid Content
2.7. Statistical Design of Experiments
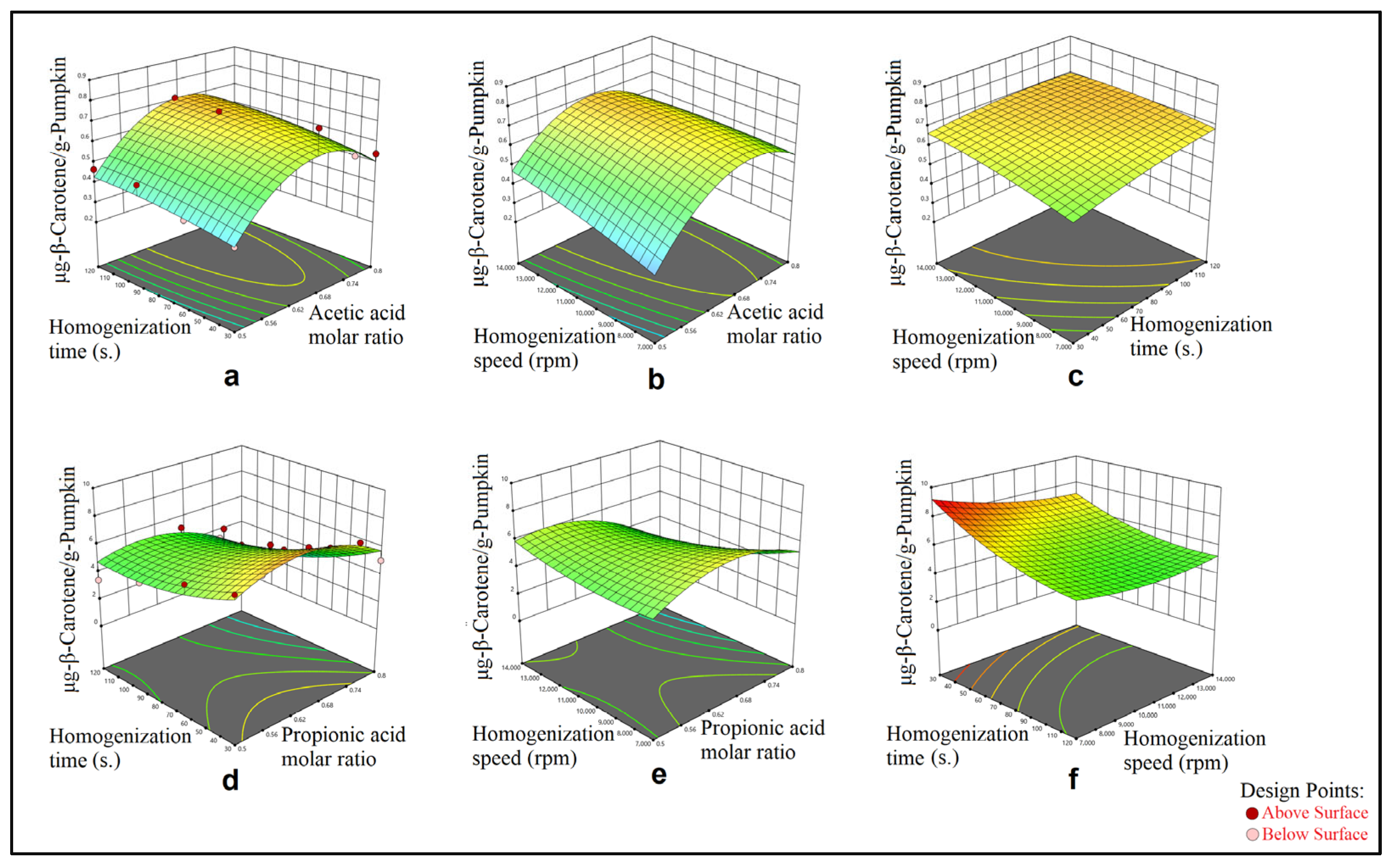
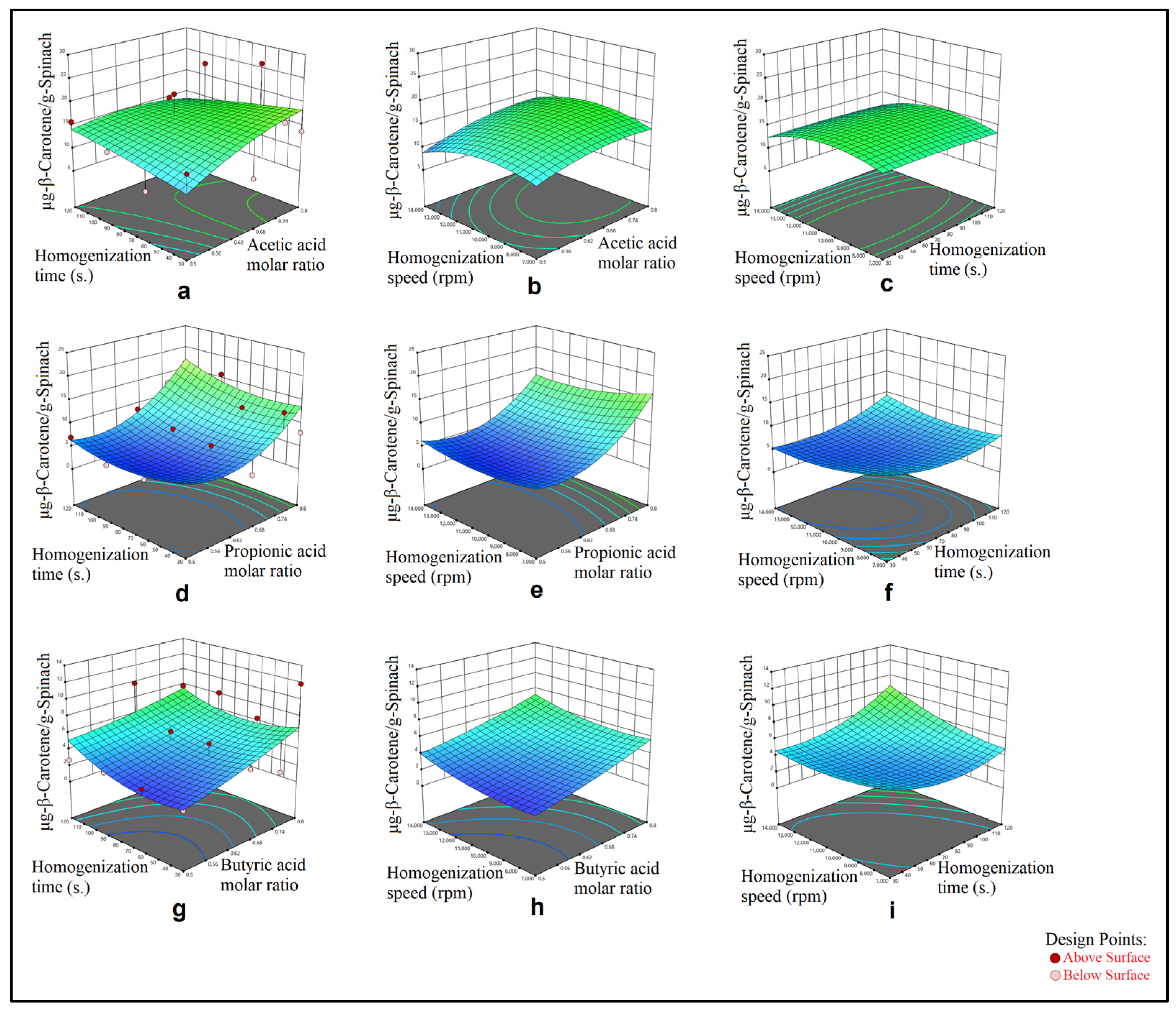
3. Results
3.1. Mechanical Mixing–Assisted Extraction (MMAE) and Homogenization-Assisted Extraction (HAE) of β-Carotene
3.2. Modeling
4. Discussion
4.1. Evaluation of ANOVA Analyses Results
4.2. Optimization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Şanal, İ.S.; Güvenç, A.; Salgın, U.; Mehmetoğlu, Ü.; Çalımlı, A. Recycling of apricot pomace by supercritical CO2 extraction. J. Supercrit. Fluids 2004, 32, 221–230. [Google Scholar] [CrossRef]
- Sandmann, G. Carotenoids and their biosynthesis in fungi. Molecules 2022, 27, 1431. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.D.; Ross, C.A.; Caballero, B.; Cousins, R.J.; Tucker, K.L.; Ziegler, T.R. 31-Carotenoids. In Modern Nutrition in Health and Disease, 11th ed.; Lippincott Williams & Wilkins: Philederphia, PA, USA, 2014; pp. 427–439. [Google Scholar]
- Ozturk, B.; Parkinson, C.; Gonzalez-Miquel, M. Extraction of polyphenolic antioxidants from orange peel waste using deep eutectic solvents. Sep. Purif. Technol. 2018, 206, 1–13. [Google Scholar] [CrossRef]
- α-Carotene, β-Carotene, β-Cryptoxanthin, Lycopene, Lutein, and Zeaxanthin. Available online: https://lpi.oregonstate.edu/mic/dietary-factors/phytochemicals/carotenoids#introduction (accessed on 11 November 2024).
- Craft, N.E.; Soares, J.H., Jr. Relative Solubility, Stability, and absorptivity of lutein and β-Carotene in organic solvents. J. Agric. Food. Chem. 1992, 40, 431–434. [Google Scholar] [CrossRef]
- Huff, J. Benzene-induced cancers: Abridged history and occupational health impact. Int. J. Occup. Environ. Health. 2007, 13, 213–221. [Google Scholar] [CrossRef]
- ICH Guideline Q3C (R9) on Impurities: Guideline for Residual Solvents (EMA/CHMP/ICH/82260/2006). Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-q3c-r9-guideline-impurities-guideline-residual-solvents-step-5_en.pdf (accessed on 11 November 2024).
- Waste and Resources Action Programme—Why we need to take action on food waste. Available online: https://www.wrap.ngo/taking-action/food-drink/actions/action-on-food-waste#:~:text=Producing%20food%20requires%20significant%20resources,greenhouse%20gas%20(GHG)%20emissions (accessed on 20 October 2024).
- United Nations Environment Programme. Food Waste Index Report 2024. Think Eat Save: Tracking Progress to Halve Global Food Waste; Knowledge Repository—UNEP. UNEP.: Nairobi, Kenya, 2024; p. 12. [Google Scholar]
- The World Counts—Food is lost in every step of the food “life cycle”. Available online: https://www.theworldcounts.com/challenges/people-and-poverty/hunger-and-obesity/food-waste-statistics (accessed on 21 November 2024).
- Kee, P.E.; Cheng, Y.-S.; Chang, J.-S.; Yim, H.S.; Tan, J.C.Y.; Lam, S.S.; Lan, J.C.-W.; Ng, H.S.; Khoo, K.S. Insect biorefinery: A circular economy concept for biowaste conversion to value-added products. Environ. Res. 2023, 221, 115284. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations—Food Wastage Footprint & Climate Change. Available online: https://openknowledge.fao.org/server/api/core/bitstreams/7fffcaf9-91b2-4b7b-bceb-3712c8cb34e6/content (accessed on 22 October 2024).
- Food and Agriculture Organization of the United Nations (Crops and livestock productions Area: World + (Total)/Item: Pumpkins, Squash and Gourds/Year: 2022-2022). Available online: https://www.fao.org/faostat/en/#data/QCL/visualize (accessed on 11 November 2024).
- Oh My Gourd! 1.3B Pounds of Pumpkins Reach Landfills Every Year. Available online: https://www.wastedive.com/news/oh-my-gourd-13b-pounds-of-pumpkins-reach-landfills-every-year/408341/ (accessed on 8 November 2024).
- United States Department of Energy—Office of Energy Efficiency & Renewable Energy—Reducing Waste and Harvesting Energy This Halloween. Available online: https://www.energy.gov/eere/articles/reducing-waste-and-harvesting-energy-halloween (accessed on 9 November 2024).
- United States Department of Agriculture. National Nutrient Database for Standard Reference. In USDA National Nutrient Database for Standard Reference 2015, Release 28, 4; United States Department of Agriculture: Washington, DC, USA, 2015. [Google Scholar]
- Lin, H.; Black, M.J.; Walsh, L.; Giordano, F.S.; Borrion, A. Life cycle assessment of baby leaf spinach: Reduction of waste through interventions in growing treatments and packaging. J. Clean. Prod. 2024, 449, 141723. [Google Scholar] [CrossRef]
- Frankowska, A.; Jeswani, H.K.; Azapagic, A. Environmental impacts of vegetables consumption in the UK, Science of the Total Environment. Sci. Total Environ. 2019, 682, 80–105. [Google Scholar] [CrossRef]
- Menthols—OECD SIDS Initial Assessment Report. Available online: https://hpvchemicals.oecd.org/ui/handler.axd?id=463ce644-e5c8-42e8-962d-3a917f32ab90 (accessed on 16 December 2024).
- Environmental Assessment for Food Contact Notification FCN 1783. Available online: https://www.fda.gov/files/food/published/Environmental-Assessment-for-Food-Contact-Notification-No.-1783.pdf (accessed on 16 December 2024).
- Gad, S.C. Propionic Acid. In Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Abdollahi, M., De Peyster, A., Gad, S.C., Greim, H., Harper, S., Moser, V.C., Ray, S., Tarazona, J., Wiegand, T.J., Eds.; Academic Press: California, CA, USA, 2014; Volume 1, pp. 1105–1107. [Google Scholar]
- N-Butyric Acid—OECD SIDS Initial Assessment Report. Available online: https://hpvchemicals.oecd.org/ui/handler.axd?id=ca33e5a6-65f4-412d-b22c-0f7ed839f886 (accessed on 16 December 2024).
- Hayyan, A.; Zainal-Abidin, M.H.; Putra, S.S.S.; Alanazi, Y.M.; Saleh, J.; Nor, M.R.M.; Hashim, M.A.; Gupta, B.S. Evaluation of biodegradability, toxicity and ecotoxicity of organic acid-based deep eutectic solvents. Sci. Total Environ. 2024, 948, 174758. [Google Scholar] [CrossRef] [PubMed]
- Sebdani, M.M.; Abbasi, H. Green extraction of carotenoids from pumpkin with ultrasound-assisted method; optimization using response surface methodology. Microchem. J. 2023, 193, 109092. [Google Scholar] [CrossRef]
- Stupar, A.; Šeregelj, V.; Ribeiro, B.D.; Pezo, L.; Cvetanović, A.; Mišan, A.; Marrucho, I. Recovery of β-carotene from pumpkin using switchable natural deep eutectic solvents. Ultrason. Sonochem. 2021, 76, 105638. [Google Scholar] [CrossRef]
- Zaib, Q.; Eckelman, M.J.; Yang, Y.; Kyung, D. Are deep eutectic solvents really green?: A life-cycle perspective. Green Chem. 2022, 24, 7924–7930. [Google Scholar] [CrossRef]
- Lazzarini, C.; Casadei, E.; Valli, E.; Tura, M.; Ragni, L.; Bendini, A.; Toschi, T.G. Sustainable Drying and Green Deep Eutectic Extraction of Carotenoids from Tomato Pomace. Foods 2022, 11, 405. [Google Scholar] [CrossRef]
- Terlidis, K.; Athanasiadis, V.; Chatzimitakos, T.; Bozinou, E.; Lalas, S.I. Carotenoids Extraction from Orange Peels Using a Thymol-Based Hydrophobic Eutectic Solvent. AppliedChem 2023, 3, 437–451. [Google Scholar] [CrossRef]
- Moradi, M.; Fazlzadehdavil, M.; Pirsaheb, M.; Mansouri, Y.; Khosravi, T.; Sharafi, K. Response surface methodology (RSM) and its application for optimization of ammonium ions removal from aqueous solutions by pumice as a natural and low cost adsorbent. Arch. Environ. Prot. 2016, 42, 33–43. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Bhat, B.; Vaid, S.; Habib, B.; Bajaj, B.K. Design of experiments for enhanced production of bioactive exopolysaccharides from indigenous probiotic lactic acid bacteria. Indian J. Biochem. Biophys. 2020, 57, 539–551. [Google Scholar]
- Florindo, C.; McIntosh, A.J.S.; Welton, T.; Branco, L.C.; Marrucho, I.M. A closer look into deep eutectic solvents: Exploring intermolecular interactions using solvatochromic probes. Phys. Chem. Chem. Phys. 2018, 20, 206–213. [Google Scholar] [CrossRef]
- Acetic Acid (glacial) 100% SDS. Available online: https://www.merckmillipore.com/DE/en/product/msds/MDA_CHEM-100056 (accessed on 13 November 2024).
- Propionic Acid for Synthesis SDS. Available online: https://www.merckmillipore.com/DE/en/product/msds/MDA_CHEM-800605?Origin=PDP (accessed on 13 November 2024).
- Kwan, Y.H.; Tung, Y.K.; Kochhar, J.S.; Li, H.; Poh, A.L.; Kang, L. 6-Esstial Monographs. In Handbook of Cosmeceutical Excipients and their Safeties, 1st ed.; Woodhead Publishing: Kidlington, UK, 2014; p. 105. [Google Scholar]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 1, 70–71. [Google Scholar] [CrossRef]

| Hydrogen Bond Acceptor (HBA) | Hydrogen Bond Donor (HBD) | Molar Ratio (HBA:HBD) | Abbreviations |
|---|---|---|---|
| L-menthol | Acetic acid | 1:1 | M1ACA1 |
| L-menthol | Acetic acid | 1:2 | M1ACA2 |
| L-menthol | Acetic acid | 1:3 | M1ACA3 |
| L-menthol | Acetic acid | 1:4 | M1ACA4 |
| L-menthol | Propionic acid | 1:1 | M1PRA1 |
| L-menthol | Propionic acid | 1:2 | M1PRA2 |
| L-menthol | Propionic acid | 1:3 | M1PRA3 |
| L-menthol | Propionic acid | 1:4 | M1PRA4 |
| L-menthol | Butyric acid | 1:1 | M1BTA1 |
| L-menthol | Butyric acid | 1:2 | M1BTA2 |
| L-menthol | Butyric acid | 1:3 | M1BTA3 |
| L-menthol | Butyric acid | 1:4 | M1BTA4 |
| DES 1 | Mixing Time (min.) | μg-β-Carotene /g-Pumpkin (Mean Value ± S.D.) 2,3 | μg-β-Carotene /g-Spinach (Mean Value ± S.D.) 2,3 |
|---|---|---|---|
| M1ACA1 | 15 | 0.196 c,A ± 0.043 | 0.714 a,A ± 0.025 |
| M1ACA1 | 30 | 0.254 c,A ± 0.011 | 5.093 a,A ± 0.032 |
| M1ACA1 | 45 | 0.229 c,A ± 0.012 | 1.083 a,A ± 0.016 |
| M1ACA1 | 60 | 0.290 c,A ± 0.026 | 1.959 a,A ± 0.031 |
| M1ACA2 | 15 | 0.637 a,A ± 0.011 | 13.867 a,A ± 0.069 |
| M1ACA2 | 30 | 0.603 a,A ± 0.009 | 23.238 a,A ± 0.075 |
| M1ACA2 | 45 | 0.566 a,A ± 0.006 | 9.348 a,A ± 0.108 |
| M1ACA2 | 60 | 0.610 a,A ± 0.004 | 6.186 a,A ± 0.026 |
| M1ACA3 | 15 | 0.639 a,A ± 0.020 | 4.447 a,A ± 0.029 |
| M1ACA3 | 30 | 0.558 a,A ± 0.012 | 10.994 a,A ± 0.029 |
| M1ACA3 | 45 | 0.572 a,A ± 0.010 | 6.863 a,A ± 0.040 |
| M1ACA3 | 60 | 0.561 a,A ± 0.003 | 5.058 a,A ± 0.028 |
| M1ACA4 | 15 | 0.442 b,A ± 0.008 | 2.202 a,A ± 0.039 |
| M1ACA4 | 30 | 0.483 b,A ± 0.010 | 12.089 a,A ± 0.035 |
| M1ACA4 | 45 | 0.510 b,A ± 0.007 | 7.820 a,A ± 0.069 |
| M1ACA4 | 60 | 0.486 b,A ± 0.009 | 20.168 a,A ± 0.044 |
| M1PRA1 | 15 | 7.873 c,A ± 0.023 | 0.866 b,A ± 0.019 |
| M1PRA1 | 30 | 6.452 c,A ± 0.040 | 1.803 b,A ± 0.009 |
| M1PRA1 | 45 | 3.960 c,A ± 0.019 | 1.869 b,A ± 0.009 |
| M1PRA1 | 60 | 2.631 c,A ± 0.014 | 1.476 b,A ± 0.005 |
| M1PRA2 | 15 | 10.816 a,A ± 0.044 | 3.567 b,A ± 0.020 |
| M1PRA2 | 30 | 10.585 a,A ± 0.045 | 4.508 b,A ± 0.026 |
| M1PRA2 | 45 | 11.542 a,A ± 0.034 | 5.044 b,A ± 0.027 |
| M1PRA2 | 60 | 11.566 a,A ± 0.044 | 8.986 b,A ± 0.033 |
| M1PRA3 | 15 | 8.796 a,b,A ± 0.051 | 3.256 b,A ± 0.011 |
| M1PRA3 | 30 | 8.234 a,b,A ± 0.042 | 5.889 b,A ± 0.019 |
| M1PRA3 | 45 | 9.264 a,b,A ± 0.023 | 6.944 b,A ± 0.017 |
| M1PRA3 | 60 | 8.939 a,b,A ± 0.072 | 5.915 b,A ± 0.023 |
| M1PRA4 | 15 | 7.917 b,c,A ± 0.041 | 9.312 a,A ± 0.049 |
| M1PRA4 | 30 | 8.063 b,c,A ± 0.031 | 19.156 a,A ± 0.122 |
| M1PRA4 | 45 | 7.757 b,c,A ± 0.047 | 17.580 a,A ± 0.043 |
| M1PRA4 | 60 | 7.465 b,c,A ± 0.043 | 18.990 a,A ± 0.057 |
| M1BTA1 | 15 | 1.552 a,C ± 0.045 | |
| M1BTA1 | 30 | 1.944 a,B,C ± 0.039 | |
| M1BTA1 | 45 | 2.774 a,A,B ± 0.031 | |
| M1BTA1 | 60 | 3.925 a,A ± 0.024 | |
| M1BTA2 | 15 | 2.193 a,C ± 0.040 | |
| M1BTA2 | 30 | 1.718 a,B,C ± 0.044 | |
| M1BTA2 | 45 | 4.321 a,A,B ± 0.036 | |
| M1BTA2 | 60 | 4.142 a,A ± 0.029 | |
| M1BTA3 | 15 | 1.593 a,C ± 0.032 | |
| M1BTA3 | 30 | 2.280 a,B,C ± 0.026 | |
| M1BTA3 | 45 | 2.720 a,A,B ± 0.021 | |
| M1BTA3 | 60 | 7.036 a,A ± 0.032 | |
| M1BTA4 | 15 | 1.319 a,C ± 0.024 | |
| M1BTA4 | 30 | 2.226 a,B,C ± 0.028 | |
| M1BTA4 | 45 | 5.109 a,A,B ± 0.029 | |
| M1BTA4 | 60 | 4.468 a,A ± 0.017 |
| DES 1 | Hmj. 1 Time (s.) | Hmj. 1 Speed (rpm) | μg-β-Carotene /g-Pumpkin (Mean Value ± S.D.) 2,3 | μg-β-Carotene /g-Spinach (Mean Value ± S.D.) 2,3 |
| M1ACA1 | 30 | 7000 | 0.287 c,A,A ± 0.002 | 8.830 a,A,A ± 0.027 |
| M1ACA1 | 60 | 7000 | 0.295 c,A,A ± 0.002 | 13.459 a,A,A ± 0.084 |
| M1ACA1 | 90 | 7000 | 0.241 c,A,A ± 0.005 | 12.948 a,A,A ± 0.056 |
| M1ACA1 | 120 | 7000 | 0.291 c,A,A ± 0.003 | 11.703 a,A,A ± 0.012 |
| M1ACA1 | 30 | 10,500 | 0.359 c,A,A ± 0.003 | 14.116 a,A,A ± 0.054 |
| M1ACA1 | 60 | 10,500 | 0.390 c,A,A ± 0.002 | 7.225 a,A,A ± 0.021 |
| M1ACA1 | 90 | 10,500 | 0.471 c,A,A ± 0.005 | 12.260 a,A,A ± 0.047 |
| M1ACA1 | 120 | 10,500 | 0.467 c,A,A ± 0.004 | 15.822 a,A,A ± 0.044 |
| M1ACA1 | 30 | 14,000 | 0.464 c,A,A ± 0.002 | 7.731 a,A,A ± 0.078 |
| M1ACA1 | 60 | 14,000 | 0.449 c,A,A ± 0.002 | 7.511 a,A,A ± 0.070 |
| M1ACA1 | 90 | 14,000 | 0.441 c,A,A ± 0.003 | 7.239 a,A,A ± 0.020 |
| M1ACA1 | 120 | 14,000 | 0.506 c,A,A ± 0.002 | 9.139 a,A,A ± 0.106 |
| M1ACA2 | 30 | 7000 | 0.553 a,A,A ± 0.039 | 19.093 a,A,A ± 0.074 |
| M1ACA2 | 60 | 7000 | 0.666 a,A,A ± 0.019 | 13.158 a,A,A ± 0.067 |
| M1ACA2 | 90 | 7000 | 0.637 a,A,A ± 0.028 | 9.728 a,A,A ± 0.056 |
| M1ACA2 | 120 | 7000 | 0.811 a,A,A ± 0.032 | 12.469 a,A,A ± 0.056 |
| M1ACA2 | 30 | 10,500 | 0.762 a,A,A ± 0.044 | 7.622 a,A,A ± 0.014 |
| M1ACA2 | 60 | 10,500 | 0.652 a,A,A ± 0.041 | 15.598 a,A,A ± 0.053 |
| M1ACA2 | 90 | 10,500 | 0.707 a,A,A ± 0.008 | 20.129 a,A,A ± 0.041 |
| M1ACA2 | 120 | 10,500 | 0.711 a,A,A ± 0.002 | 14.714 a,A,A ± 0.029 |
| M1ACA2 | 30 | 14,000 | 0.562 a,A,A ± 0.038 | 18.474 a,A,A ± 0.017 |
| M1ACA2 | 60 | 14,000 | 0.632 a,A,A ± 0.020 | 9.334 a,A,A ± 0.016 |
| M1ACA2 | 90 | 14,000 | 0.771 a,A,A ± 0.006 | 11.925 a,A,A ± 0.081 |
| M1ACA2 | 120 | 14,000 | 0.670 a,A,A ± 0.006 | 17.460 a,A,A ± 0.107 |
| M1ACA3 | 30 | 7000 | 0.434 a,b,A,A ± 0.003 | 6.767 a,A,A ± 0.027 |
| M1ACA3 | 60 | 7000 | 0.639 a,b,A,A ± 0.005 | 8.553 a,A,A ± 0.031 |
| M1ACA3 | 90 | 7000 | 0.651 a,b,A,A ± 0.054 | 16.257 a,A,A ± 0.048 |
| M1ACA3 | 120 | 7000 | 0.640 a,b,A,A ± 0.000 | 15.457 a,A,A ± 0.098 |
| M1ACA3 | 30 | 10,500 | 0.571 a,b,A,A ± 0.002 | 16.982 a,A,A ± 0.024 |
| M1ACA3 | 60 | 10,500 | 0.563 a,b,A,A ± 0.010 | 15.152 a,A,A ± 0.015 |
| M1ACA3 | 90 | 10,500 | 0.645 a,b,A,A ± 0.003 | 24.930 a,A,A ± 0.057 |
| M1ACA3 | 120 | 10,500 | 0.588 a,b,A,A ± 0.002 | 14.775 a,A,A ± 0.042 |
| M1ACA3 | 30 | 14,000 | 0.606 a,b,A,A ± 0.018 | 13.767 a,A,A ± 0.053 |
| M1ACA3 | 60 | 14,000 | 0.611 a,b,A,A ± 0.002 | 11.293 a,A,A ± 0.028 |
| M1ACA3 | 90 | 14,000 | 0.689 a,b,A,A ± 0.000 | 15.814 a,A,A ± 0.039 |
| M1ACA3 | 120 | 14,000 | 0.644 a,b,A,A ± 0.000 | 13.564 a,A,A ± 0.027 |
| M1ACA4 | 30 | 7000 | 0.449 b,A,A ± 0.012 | 22.831 a,A,A ± 0.056 |
| M1ACA4 | 60 | 7000 | 0.548 b,A,A ± 0.020 | 20.749 a,A,A ± 0.062 |
| M1ACA4 | 90 | 7000 | 0.629 b,A,A ± 0.029 | 12.540 a,A,A ± 0.014 |
| M1ACA4 | 120 | 7000 | 0.579 b,A,A ± 0.005 | 7.834 a,A,A ± 0.041 |
| M1ACA4 | 30 | 10,500 | 0.545 b,A,A ± 0.004 | 13.751 a,A,A ± 0.063 |
| M1ACA4 | 60 | 10,500 | 0.534 b,A,A ± 0.008 | 26.006 a,A,A ± 0.092 |
| M1ACA4 | 90 | 10,500 | 0.572 b,A,A ± 0.005 | 11.626 a,A,A ± 0.014 |
| M1ACA4 | 120 | 10,500 | 0.554 b,A,A ± 0.011 | 9.568 a,A,A ± 0.038 |
| M1ACA4 | 30 | 14,000 | 0.581 b,A,A ± 0.003 | 18.883 a,A,A ± 0.055 |
| M1ACA4 | 60 | 14,000 | 0.525 b,A,A ± 0.017 | 14.477 a,A,A ± 0.080 |
| M1ACA4 | 90 | 14,000 | 0.581 b,A,A ± 0.050 | 7.578 a,A,A ± 0.053 |
| M1ACA4 | 120 | 14,000 | 0.543 b,A,A ± 0.006 | 7.993 a,A,A ± 0.069 |
| M1PRA1 | 30 | 7000 | 7.675 a,A,A ± 0.030 | 6.081 b,A,A ± 0.039 |
| M1PRA1 | 60 | 7000 | 5.030 a,A,A ± 0.023 | 7.450 b,A,A ± 0.020 |
| M1PRA1 | 90 | 7000 | 3.769 a,b,B,A ± 0.013 | 5.234 b,A,A ± 0.014 |
| M1PRA1 | 120 | 7000 | 4.698 a,b,B,A ± 0.023 | 8.447 b,A,A ± 0.034 |
| M1PRA1 | 30 | 10,500 | 6.896 a,b,A,A ± 0.013 | 11.252 b,A,A ± 0.027 |
| M1PRA1 | 60 | 10,500 | 6.088 a,b,A,B,A ± 0.021 | 4.241 b,A,A ± 0.019 |
| M1PRA1 | 90 | 10,500 | 4.712 a,b,B,A ± 0.019 | 4.010 b,A,A ± 0.019 |
| M1PRA1 | 120 | 10,500 | 3.456 a,b,B,A ± 0.014 | 6.955 b,A,A ± 0.013 |
| M1PRA1 | 30 | 14,000 | 6.120 a,b,A,A ± 0.024 | 8.539 b,A,A ± 0.020 |
| M1PRA1 | 60 | 14,000 | 7.147 a,b,A,B,A ± 0.013 | 3.704 b,A,A ± 0.020 |
| M1PRA1 | 90 | 14,000 | 6.487 a,b,B,A ± 0.023 | 6.080 b,A,A ± 0.012 |
| M1PRA1 | 120 | 14,000 | 5.340 a,b,B,A ± 0.020 | 8.139 b,A,A ± 0.028 |
| M1PRA2 | 30 | 7000 | 8.762 a,A,A ± 0.029 | 4.731 b,A,A ± 0.082 |
| M1PRA2 | 60 | 7000 | 6.586 a,A,B,A ± 0.037 | 5.323 b,A,A ± 0.013 |
| M1PRA2 | 90 | 7000 | 8.579 a,B,A ± 0.043 | 6.669 b,A,A ± 0.021 |
| M1PRA2 | 120 | 7000 | 6.472 a,B,A ± 0.039 | 11.232 b,A,A ± 0.013 |
| M1PRA2 | 30 | 10,500 | 7.316 a,A,A ± 0.045 | 2.958 b,A,A ± 0.033 |
| M1PRA2 | 60 | 10,500 | 6.561 a,A,B,A ± 0.038 | 6.132 b,A,A ± 0.011 |
| M1PRA2 | 90 | 10,500 | 6.411 a,B,A ± 0.043 | 6.950 b,A,A ± 0.058 |
| M1PRA2 | 120 | 10,500 | 5.201 a,B,A ± 0.032 | 8.706 b,A,A ± 0.013 |
| M1PRA2 | 30 | 14,000 | 6.935 a,A,A ± 0.040 | 8.774 b,A,A ± 0.032 |
| M1PRA2 | 60 | 14,000 | 5.880 a,A,B,A ± 0.031 | 10.494 b,A,A ± 0.053 |
| M1PRA2 | 90 | 14,000 | 4.935 a,B,A ± 0.039 | 4.587 b,A,A ± 0.048 |
| M1PRA2 | 120 | 14,000 | 6.839 a,B,A ± 0.017 | 7.195 b,A,A ± 0.035 |
| M1PRA3 | 30 | 7000 | 8.596 b,A,A ± 0.021 | 14.763 a,A,A ± 0.034 |
| M1PRA3 | 60 | 7000 | 5.541 b,A,B,A ± 0.045 | 10.024 a,A,A ± 0.044 |
| M1PRA3 | 90 | 7000 | 3.802 b,B,A ± 0.043 | 17.005 a,A,A ± 0.031 |
| M1PRA3 | 120 | 7000 | 4.888 b,B,A ± 0.039 | 14.631 a,A,A ± 0.048 |
| M1PRA3 | 30 | 10,500 | 6.981 b,A,A ± 0.041 | 13.676 a,A,A ± 0.052 |
| M1PRA3 | 60 | 10,500 | 5.321 b,A,B,A ± 0.039 | 12.177 a,A,A ± 0.034 |
| M1PRA3 | 90 | 10,500 | 1.803 b,B,A ± 0.033 | 9.143 a,A,A ± 0.035 |
| M1PRA3 | 120 | 10,500 | 3.367 b,B,A ± 0.039 | 12.853 a,A,A ± 0.035 |
| M1PRA3 | 30 | 14,000 | 5.253 b,A,A ± 0.041 | 10.178 a,A,A ± 0.065 |
| M1PRA3 | 60 | 14,000 | 2.010 b,A,B,A ± 0.032 | 5.838 a,A,A ± 0.052 |
| M1PRA3 | 90 | 14,000 | 0.631 b,B,A ± 0.038 | 11.197 a,A,A ± 0.045 |
| M1PRA3 | 120 | 14,000 | 3.596 b,B,A ± 0.043 | 13.982 a,A,A ± 0.049 |
| M1PRA4 | 30 | 7000 | 8.898 b,A,A ± 0.031 | 24.598 a,A,A ± 0.126 |
| M1PRA4 | 60 | 7000 | 6.158 b,A,B,A ± 0.027 | 19.838 a,A,A ± 0.113 |
| M1PRA4 | 90 | 7000 | 7.714 b,B,A ± 0.031 | 15.597 a,A,A ± 0.069 |
| M1PRA4 | 120 | 7000 | 1.773 b,B,A ± 0.045 | 12.177 a,A,A ± 0.118 |
| M1PRA4 | 30 | 10,500 | 5.064 b,A,A ± 0.028 | 7.972 a,A,A ± 0.091 |
| M1PRA4 | 60 | 10,500 | 4.638 b,A,B,A ± 0.037 | 10.282 a,A,A ± 0.065 |
| M1PRA4 | 90 | 10,500 | 3.145 b,B,A ± 0.030 | 15.900 a,A,A ± 0.056 |
| M1PRA4 | 120 | 10,500 | 2.184 b,B,A ± 0.025 | 16.399 a,A,A ± 0.057 |
| M1PRA4 | 30 | 14,000 | 5.653 b,A,A ± 0.026 | 8.387 a,A,A ± 0.078 |
| M1PRA4 | 60 | 14,000 | 3.880 b,A,B,A ± 0.034 | 14.157 a,A,A ± 0.115 |
| M1PRA4 | 90 | 14,000 | 2.029 b,B,A ± 0.040 | 13.669 a,A,A ± 0.100 |
| M1PRA4 | 120 | 14,000 | 1.318 b,B,A ± 0.025 | 24.910 a,A,A ± 0.071 |
| M1BTA1 | 30 | 7000 | 2.563 c,A,A ± 0.033 | |
| M1BTA1 | 60 | 7000 | 3.240 c,A,A ± 0.032 | |
| M1BTA1 | 90 | 7000 | 3.129 c,A,A ± 0.033 | |
| M1BTA1 | 120 | 7000 | 4.082 c,A,A ± 0.035 | |
| M1BTA1 | 30 | 10,500 | 2.244 c,A,A ± 0.023 | |
| M1BTA1 | 60 | 10,500 | 2.768 c,A,A ± 0.032 | |
| M1BTA1 | 90 | 10,500 | 2.889 c,A,A ± 0.039 | |
| M1BTA1 | 120 | 10,500 | 2.701 c,A,A ± 0.021 | |
| M1BTA1 | 30 | 14,000 | 2.979 c,A,A ± 0.022 | |
| M1BTA1 | 60 | 14,000 | 4.285 c,A,A ± 0.029 | |
| M1BTA1 | 90 | 14,000 | 3.862 c,A,A ± 0.050 | |
| M1BTA1 | 120 | 14,000 | 6.460 c,A,A ± 0.038 | |
| M1BTA2 | 30 | 7000 | 4.171 a,b,A,A ± 0.046 | |
| M1BTA2 | 60 | 7000 | 4.609 a,b,A,A ± 0.038 | |
| M1BTA2 | 90 | 7000 | 4.188 a,b,A,A ± 0.027 | |
| M1BTA2 | 120 | 7000 | 6.461 a,b,A,A ± 0.026 | |
| M1BTA2 | 30 | 10,500 | 3.844 a,b,A,A ± 0.042 | |
| M1BTA2 | 60 | 10,500 | 5.263 a,b,A,A ± 0.042 | |
| M1BTA2 | 90 | 10,500 | 5.191 a,b,A,A ± 0.031 | |
| M1BTA2 | 120 | 10,500 | 9.820 a,b,A,A ± 0.077 | |
| M1BTA2 | 30 | 14,000 | 5.457 a,b,A,A ± 0.045 | |
| M1BTA2 | 60 | 14,000 | 7.097 a,b,A,A ± 0.050 | |
| M1BTA2 | 90 | 14,000 | 6.782 a,b,A,A ± 0.034 | |
| M1BTA2 | 120 | 14,000 | 13.221 a,b,A,A ± 0.044 | |
| M1BTA3 | 30 | 7000 | 5.708 b,c,A,A ± 0.045 | |
| M1BTA3 | 60 | 7000 | 4.283 b,c,A,A ± 0.020 | |
| M1BTA3 | 90 | 7000 | 3.736 b,c,A,A ± 0.032 | |
| M1BTA3 | 120 | 7000 | 3.884 b,c,A,A ± 0.020 | |
| M1BTA3 | 30 | 10,500 | 2.020 b,c,A,A ± 0.038 | |
| M1BTA3 | 60 | 10,500 | 2.917 b,c,A,A ± 0.021 | |
| M1BTA3 | 90 | 10,500 | 3.239 b,c,A,A ± 0.030 | |
| M1BTA3 | 120 | 10,500 | 3.332 b,c,A,A ± 0.027 | |
| M1BTA3 | 30 | 14,000 | 5.543 b,c,A,A ± 0.037 | |
| M1BTA3 | 60 | 14,000 | 4.595 b,c,A,A ± 0.029 | |
| M1BTA3 | 90 | 14,000 | 2.968 b,c,A,A ± 0.033 | |
| M1BTA3 | 120 | 14,000 | 8.684 b,c,A,A ± 0.027 | |
| M1BTA4 | 30 | 7000 | 8.584 a,A,A ± 0.028 | |
| M1BTA4 | 60 | 7000 | 6.231 a,A,A ± 0.037 | |
| M1BTA4 | 90 | 7000 | 9.738 a,A,A ± 0.049 | |
| M1BTA4 | 120 | 7000 | 4.637 a,A,A ± 0.037 | |
| M1BTA4 | 30 | 10,500 | 11.840 a,A,A ± 0.043 | |
| M1BTA4 | 60 | 10,500 | 6.343 a,A,A ± 0.024 | |
| M1BTA4 | 90 | 10,500 | 8.197 a,A,A ± 0.039 | |
| M1BTA4 | 120 | 10,500 | 7.814 a,A,A ± 0.046 | |
| M1BTA4 | 30 | 14,000 | 8.211 a,A,A ± 0.025 | |
| M1BTA4 | 60 | 14,000 | 5.023 a,A,A ± 0.033 | |
| M1BTA4 | 90 | 14,000 | 7.401 a,A,A ± 0.046 | |
| M1BTA4 | 120 | 14,000 | 12.534 a,A,A ± 0.023 |
| Extraction Setup | Independent Variables | Model Equation | R2 |
| Sample: Pumpkin Method: MMAE and L-menthol:acetic acid DESs | X1: Acetic acid molar ratio X2: Mixing time (min.) | −4.45150 + 14.57986X1 + 0.001912X2 − 0.005262X1X2 −10.44080X12 + 0.000024X22 | 0.9642 |
| Sample: Pumpkin Method: MMAE and L-menthol:propionic acid DESs | X1: Propionic acid molar ratio X2: Mixing time (min.) | −63.76949 + 241.35123X1 − 0.301289X2 + 0.401802X1X2 −191.69872X12 + 0.000041X22 | 0.9006 |
| Sample: Spinach Method: MMAE and L-menthol:acetic acid DESs | X1: Acetic acid molar ratio X2: Mixing time (min.) | −87.29436 + 291.71772X1 − 0.080113X2 + 0.815406X1X2 −232.20477X12 − 0.006091X22 | 0.3941 |
| Sample: Spinach Method: MMAE and L-menthol:propionic acid DESs | X1: Propionic acid molar ratio X2: Mixing time (min.) | 81.41602 − 286.71718X1 + 0.011693X2 +0.437977X1X2 + 239.97280 X12 − 0.002896X22 | 0.7835 |
| Sample: Spinach Method: MMAE and L-menthol:butyric acid DESs | X1: Butyric acid molar ratio X2: Mixing time (min.) | 0.019209 + 7.15429X1 − 0.089691X2 + 0.147515X1X2 −7.71617X12 + 0.000871X22 | 0.7688 |
| Sample: Pumpkin Method: HAE and L-menthol:acetic acid DESs | X1: Acetic acid molar ratio X2: Homogenization time (s.) X3: Homogenization speed (rpm) | −4.30138 + 12.54252X1 + 0.003089X2 + 0.000103X3 +0.000543X1X2 − 0.000081X1X3 − 1.29643 × 10−7X2X3 −8.63510X12 − 8.37963 × 10−6X22 − 1.42092 × 10−9X32 | 0.8418 |
| Sample: Pumpkin Method: HAE and L-menthol:propionic acid DESs | X1: Propionic acid molar ratio X2: Homogenization time (s.) X3: Homogenization speed (rpm) | −24.31624 + 109.68892X1 − 0.063963X2 + 0.000120X3 −0.068021X1X2 − 0.001957X1X3 + 2.65619 × 10−6X2X3 −69.07260X12 + 0.000334X22 + 3.75153 × 10−8X32 | 0.7052 |
| Sample: Spinach Method: HAE and L-menthol:acetic acid DESs | X1: Acetic acid molar ratio X2: Homogenization time (s.) X3: Homogenization speed (rpm) | −46.56104 + 104.57014X1 + 0.229675X2 + 0.003205X3 −0.333622X1X2 + 0.000977X1X3 + 2.93333 × 10−7X2X3 −59.45170X12 − 0.000142X22 − 1.93862 × 10−7X32 | 0.2725 |
| Sample: Spinach Method: HAE and L-menthol:propionic acid DESs | X1: Propionic acid molar ratio X2: Homogenization time (s.) X3: Homogenization speed (rpm) | 88.81933 − 218.95677X1 − 0.304985X2 − 0.002093X3 +0.146208X1X2 − 0.001599X1X3 + 7.80619 × 10−6X2X3 +195.82812X12 + 0.000968X22 + 1.13311 × 10−7X32 | 0.6540 |
| Sample: Spinach Method: HAE and L-menthol:butyric acid DESs | X1: Butyric acid molar ratio X2: Homogenization time (s.) X3: Homogenization speed (rpm) | 9.20900 + 1.75614X1 − 0.106600X2 − 0.001464X3 −0.073053X1X2 − 0.000142X1X3 + 7.09500 × 10−6X2X3 +12.70142X12 + 0.000667X22 + 5.99541 × 10−8X32 | 0.4718 |
| Extraction Method | DESs (HBA:HBD) | Optimum Extraction Conditions | μg-β-Carotene/g-Pumpkin | |
|---|---|---|---|---|
| Predicted 1 | Actual 2,3,4 | |||
| MMAE | L-menthol:propionic acid | HBD Molar Ratio: 0.6452 Mixing Time: 15.0 min. | 11.528 ± 0.946 | 10.816 a,A ± 0.044 |
| HAE | L-menthol:propionic acid | HBD Molar Ratio: 0.6198 Homogenization Time: 30.0 s. Homogenization Speed: 7061 rpm | 8.966 ± 1.252 | 8.762 a,A,A ± 0.029 |
| Extraction Method | DESs (HBA:HBD) | Optimum Extraction Conditions | μg-β-Carotene/g-spinach | |
|---|---|---|---|---|
| Predicted 1 | Actual 2,3,4 | |||
| MMAE | L-menthol:propionic acid | HBD Molar Ratio: 0.8000 Mixing Time: 60.0 min. | 16.924 ± 3.518 | 18.990 a,A ± 0.057 |
| HAE | L-menthol:propionic acid | HBD Molar Ratio: 0.8000 Homogenization Time: 120.0 s. Homogenization Speed: 7000 rpm | 18.870 ± 3.302 | 12.177 a,A,A ± 0.118 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanrıver, K.; Bilgin, M.; Şahin Sevgili, S.; Toprakçı Yüksel, İ.; Kurtulbaş Şahin, E. Extraction of Carotenoids from Pumpkin (Cucurbita moschata) and Spinach (Spinacia oleracea) Using Environmentally Friendly Deep Eutectic Solvents (DESs). AppliedChem 2025, 5, 2. https://doi.org/10.3390/appliedchem5010002
Tanrıver K, Bilgin M, Şahin Sevgili S, Toprakçı Yüksel İ, Kurtulbaş Şahin E. Extraction of Carotenoids from Pumpkin (Cucurbita moschata) and Spinach (Spinacia oleracea) Using Environmentally Friendly Deep Eutectic Solvents (DESs). AppliedChem. 2025; 5(1):2. https://doi.org/10.3390/appliedchem5010002
Chicago/Turabian StyleTanrıver, Koray, Mehmet Bilgin, Selin Şahin Sevgili, İrem Toprakçı Yüksel, and Ebru Kurtulbaş Şahin. 2025. "Extraction of Carotenoids from Pumpkin (Cucurbita moschata) and Spinach (Spinacia oleracea) Using Environmentally Friendly Deep Eutectic Solvents (DESs)" AppliedChem 5, no. 1: 2. https://doi.org/10.3390/appliedchem5010002
APA StyleTanrıver, K., Bilgin, M., Şahin Sevgili, S., Toprakçı Yüksel, İ., & Kurtulbaş Şahin, E. (2025). Extraction of Carotenoids from Pumpkin (Cucurbita moschata) and Spinach (Spinacia oleracea) Using Environmentally Friendly Deep Eutectic Solvents (DESs). AppliedChem, 5(1), 2. https://doi.org/10.3390/appliedchem5010002








