Phycoremediation: Use of Algae to Sequester Heavy Metals
Abstract
:1. Introduction
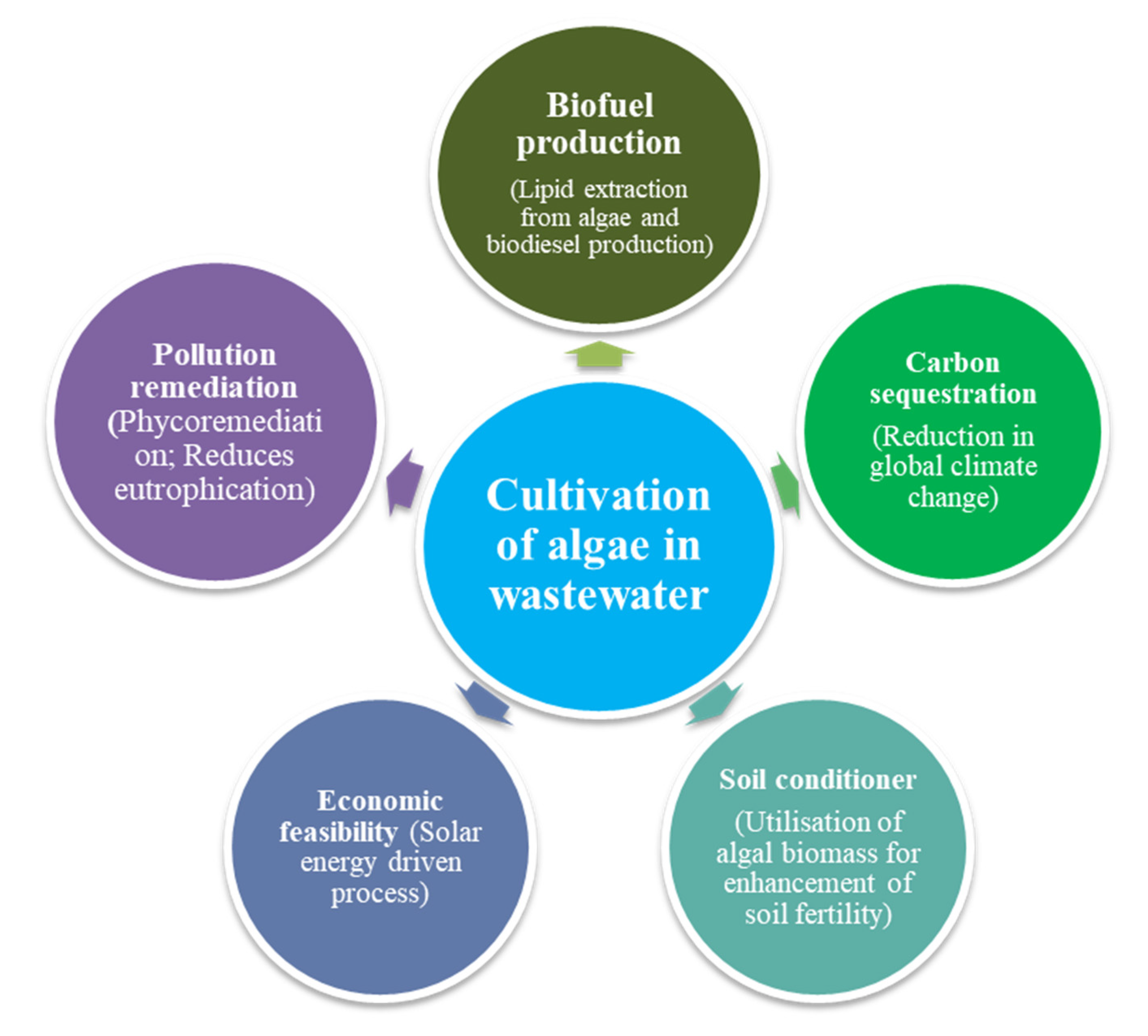
Other Ecosystem Services of Algae
2. Heavy Metal as a Potential Contaminant of Aquatic Ecosystems
3. Major Sources and Adverse Effects of Heavy Metals
4. Phytoremediation Potential of Algae
5. Mechanism of Phytoremediation by Algae
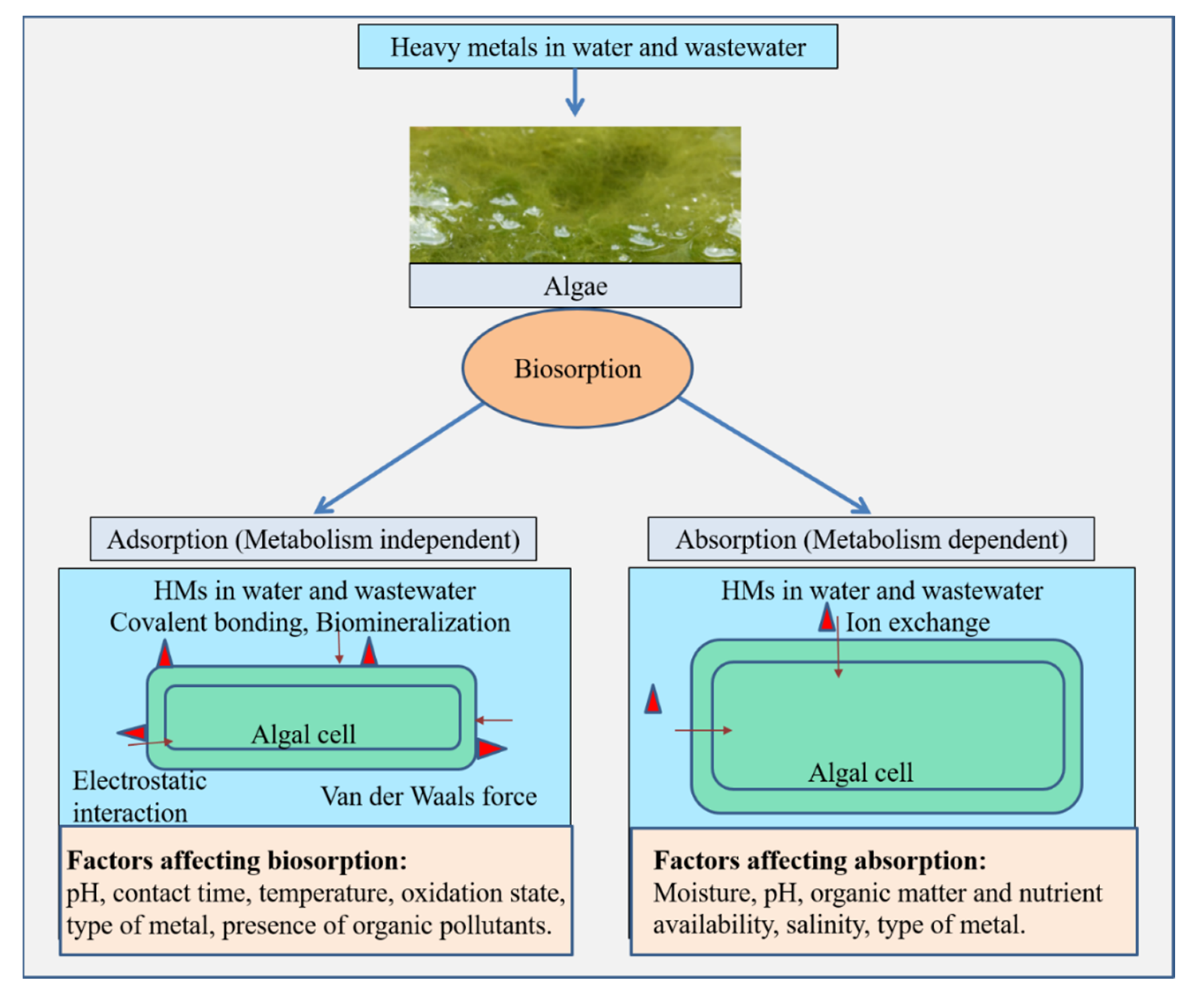
5.1. Biosorption
5.2. Bioaccumulation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kobielska, P.A.; Howarth, A.J.; Farha, O.K.; Nayak, S. Metal-organic frameworks for heavy metal removal from water. Coord. Chem. Rev. 2018, 358, 92–107. [Google Scholar] [CrossRef]
- Gupta, A.; Joia, J.; Sood, A.; Sood, R.; Sidhu, C. Microbes as potential tool for remediation of heavy metals: A review. J. Microb. Biochem. Technol. 2016, 8, 364–372. [Google Scholar] [CrossRef] [Green Version]
- Masindi, V.; Muedi, K.L. Environmental contamination by heavy metals. Heavy Met. 2018, 10, 115–132. [Google Scholar]
- Schwartz, G.E.; Hower, J.C.; Phillips, A.L.; Rivera, N.; Vengosh, A.; Hsu-Kim, H. Ranking coal ash materials for their potential to leach arsenic and selenium: Relative importance of ash chemistry and site biogeochemistry. Environ. Eng. Sci. 2018, 35, 728–738. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Duan, X.; Wang, L. Spatial distribution and source analysis of heavy metals in soils influenced by industrial enterprise distribution: Case study in Jiangsu Province. Sci. Total Environ. 2020, 710, 134953. [Google Scholar] [CrossRef]
- Wu, W.; Qu, S.; Nel, W.; Ji, J. The impact of natural weathering and mining on heavy metal accumulation in the karst areas of the Pearl River Basin, China. Sci. Total Environ. 2020, 734, 139480. [Google Scholar] [CrossRef]
- Wei, J.; Zheng, X.; Liu, J.; Zhang, G.; Zhang, Y.; Wang, C.; Liu, Y. The Levels, Sources, and Spatial Distribution of Heavy Metals in Soils from the Drinking Water Sources of Beijing, China. Sustainability 2021, 13, 3719. [Google Scholar] [CrossRef]
- Warmate, A.; Ideriah, T.; Tamunobereton ARI, I.T.; Inyang, U.U.; Ibaraye, T. Concentrations of heavy metals in soil and water receiving used engine oil in Port Harcourt, Nigeria. J. Ecol. Nat. Environ. 2011, 3, 54–57. [Google Scholar]
- Kwaansa-Ansah, E.E.; Nti, S.O.; Opoku, F. Heavy metals concentration and human health risk assessment in seven commercial fish species from Asafo Market, Ghana. Food Sci. Biotechnol. 2019, 28, 569–579. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Y.; Wang, X.; Wang, M.; Liang, Y.; Zhu, G.; Jin, T. A nomogram for predicting the renal dysfunction in a Chinese population with reduction in cadmium exposure based on 8 years follow up study. Ecotoxicol. Environ. Saf. 2020, 191, 110251. [Google Scholar] [CrossRef]
- Huang, J.; Peng, S.; Mao, X.; Li, F.; Guo, S.; Shi, L.; Shi, Y.; Yu, H.; Zeng, G. Source apportionment and spatial and quantitative ecological risk assessment of heavy metals in soils from a typical Chinese agricultural county. Process Saf. Environ. Prot. 2019, 126, 339–347. [Google Scholar] [CrossRef]
- Dixit, S.; Singh, D.P. Phycoremediation: Future perspective of green technology. In Algae and Environmental Sustainability; Springer: New Delhi, India, 2015; pp. 9–21. [Google Scholar]
- Schuler, M.S.; Relyea, R.A. A review of the combined threats of road salts and heavy metals to freshwater systems. BioScience 2018, 68, 327–335. [Google Scholar] [CrossRef]
- Jordao, C.P.; Nascentes, C.C.; Cecon, P.R.; Fontes, R.L.F.; Pereira, J.L. Heavy metal availability in soil amended with composted urban solid wastes. Environ. Monit. Assess. 2006, 112, 309–326. [Google Scholar] [CrossRef] [PubMed]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef]
- Bhattacharyya, P.; Chakrabarti, K.; Chakraborty, A.; Tripathy, S.; Powell, M.A. Fractionation and bioavailability of Pb in municipal solid waste compost and Pb uptake by rice straw and grain under submerged condition in amended soil. J. Geosci. 2008, 12, 41–45. [Google Scholar] [CrossRef]
- Zulfiqar, U.; Farooq, M.; Hussain, S.; Maqsood, M.; Hussaind, M.; Ishfaqa, M.; Ahmada, M.; Anjumf, M.Z. Lead toxicity in plants: Impacts and remediation. J. Environ. Manag. 2019, 250, 109557. [Google Scholar] [CrossRef]
- Kohli, S.K.; Handa, N.; Bali, S.; Khanna, K.; Arora, S.; Sharma, A.; Bhardwaj, R. Current Scenario of Pb Toxicity in Plants: Unraveling Plethora of Physiological Responses. Rev. Environ. Contam. Toxicol. 2020, 249, 153–197. [Google Scholar]
- Giannakoula, A.; Therios, I.; Chatzissavvidis, C. Effect of Lead and Copper on Photosynthetic Apparatus in Citrus (Citrus aurantium L.) Plants. The Role of Antioxidants in Oxidative Damage as a Response to Heavy Metal Stress. Plants 2021, 10, 155. [Google Scholar] [CrossRef]
- Sharma, R.K.; Agrawal, M.; Marshall, F. Heavy metal contamination of soil and vegetables in suburban areas of Varanasi, India. Ecotoxicol. Environ. Saf. 2007, 66, 258–266. [Google Scholar] [CrossRef]
- Schützendübel, A.; Nikolova, P.; Rudolf, C.; Polle, A. Cadmium and H2O2 -induced oxidative stress in Populus × canescens roots. Plant Physiol. Biochem. 2002, 40, 577–584. [Google Scholar] [CrossRef]
- Woo, S.; Yum, S.; Park, H.S.; Lee, T.K.; Ryu, J.C. Effects of heavy metals on antioxidants and stress-responsive gene expression in Javanese medaka (Oryzias javanicus). CBP 2009, 149, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Jais, N.M.; Mohamed, R.; Al-Gheethi, A.; Hashim, M.A. The dual roles of phycoremediation of wet market wastewater for nutrients and heavy metals removal and microalgae biomass production. Clean Technol. Environ. Policy 2017, 19, 37–52. [Google Scholar] [CrossRef]
- Kumar, V.; Nanda, M. Microalgae: A Promising Tool for Remediation of Heavy Metals. In Biostimulation Remediation Technologies for Groundwater Contaminants; IGI Global: Hershey, PA, USA, 2018; pp. 141–153. [Google Scholar]
- Anbuchezhian, R.; Karuppiah, V.; Li, Z. Prospect of marine algae for production of industrially important chemicals. In Algal Biorefinery: An Integrated Approach; Springer: Cham, Switzerland, 2015; pp. 195–217. [Google Scholar]
- Koul, B.; Sharma, K.; Shah, M.P. Phycoremediation: A sustainable alternative in wastewater treatment (WWT) regime. Environ. Technol. Innov. 2022, 25, 102040. [Google Scholar] [CrossRef]
- Ahmad, S.; Pandey, A.; Pathak, V.V.; Tyagi, V.V.; Kothari, R. Phycoremediation: Algae as eco-friendly tools for the removal of heavy metals from wastewaters. In Bioremediation of Industrial Waste for Environmental Safety; Springer: Berlin/Heidelberg, Germany, 2020; pp. 53–76. [Google Scholar]
- Poo, K.M.; Son, E.B.; Chang, J.S.; Ren, X.; Choi, Y.J.; Chae, K.J. Biochars derived from wasted marine macro-algae (Saccharina japonica and Sargassum fusiforme) and their potential for heavy metal removal in aqueous solution. J. Environ. Manag. 2018, 206, 364–372. [Google Scholar] [CrossRef]
- Yan, A.; Wang, Y.; Tan, S.N.; Mohd Yusof, M.L.; Ghosh, S.; Chen, Z. Phytoremediation: A promising approach for revegetation of heavy metal-polluted land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef]
- Tiwari, J.; Ankit; Shweta; Kumar, S.; Korstad, J.; Bauddh, K. Ecorestoration of polluted aquatic ecosystems through rhizofiltration. In Phytomanagement of Polluted Sites; Elsevier: Amsterdam, The Netherlands, 2019; pp. 179–201. [Google Scholar]
- Aken, B.V.; Correa, P.A.; Schnoor, J.L. Phytoremediation of polychlorinated biphenyls: New trends and promises. Environ. Sci. Technol. 2009, 44, 2767–2776. [Google Scholar] [CrossRef] [Green Version]
- Wuana, R.A.; Okieimen, F.E. Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. Int. Sch. Res. Not. 2011, 2011, 402647. [Google Scholar] [CrossRef] [Green Version]
- Jacob, J.M.; Karthik, C.; Saratale, R.G.; Kumar, S.S.; Prabakar, D.; Kadirvelu, K. Biological approaches to tackle heavy metal pollution: A survey of literature. J. Environ. Manag. 2018, 217, 56–70. [Google Scholar] [CrossRef]
- Kotrba, P. Microbial biosorption of metals—general introduction. In Microbial Biosorption of Metals; Kotrba, P., Mackova, M., Macek, T., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 1–6. [Google Scholar]
- Darda, S.; Papalas, T.; Zabaniotou, A. Biofuels journey in Europe: Currently the way to low carbon economy sustainability is still a challenge. J. Clean. Prod. 2019, 208, 575–588. [Google Scholar] [CrossRef]
- Ajayan, K.V.; Selvaraju, M.; Thirugnanamoorthy, K. Growth and heavy metals accumulation potential of microalgae grown in sewage wastewater and petrochemical effluents. Pak. J. Biol. Sci. 2011, 14, 805–811. [Google Scholar] [CrossRef] [Green Version]
- Yu, Q.; Matheickal, J.T.; Yin, P.; Kaewsarn, P. Heavy metal uptake capacities of common marine macro algal biomass. Water Res. 1999, 33, 1534–1537. [Google Scholar] [CrossRef]
- Salama, E.S.; Kurade, M.B.; Abou-Shanab, R.A.; El-Dalatony, M.M.; Yang, I.-S.; Min, B.; Jeon, B.-H. Recent progress in microalgal biomass production coupled with wastewater treatment for biofuel generation. Renew. Sustain. Energy Rev. 2017, 79, 1189–1211. [Google Scholar] [CrossRef]
- Xia, A.; Herrmann, C.; Murphy, J.D. How do we optimize third-generation algal biofuels? Biofuels Bioprod. Biorefin. 2015, 9, 358–367. [Google Scholar] [CrossRef]
- Villar-Argaiz, M.; Medina-Sánchez, J.M.; Biddanda, B.A.; Carrillo, P. Predominant non-additive effects of multiple stressors on autotroph C: N: P ratios propagate in freshwater and marine food webs. Front. Microbiol. 2018, 9, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsikoti, C.; Genitsaris, S. Review of Harmful Algal Blooms in the Coastal Mediterranean Sea, with a Focus on Greek Waters. Diversity 2021, 13, 396. [Google Scholar] [CrossRef]
- Stevenson, J. Ecological assessments with algae: A review and synthesis. J. Phycol. 2014, 50, 437–461. [Google Scholar] [CrossRef]
- Singh, G.; Patidar, S.K. Development and applications of attached growth system for microalgae biomass production. BioEnergy Res. 2021, 14, 709–722. [Google Scholar] [CrossRef]
- Ahmad, A.; Banat, F.; Alsafar, H.; Hasan, S.W. Algae biotechnology for industrial wastewater treatment, bioenergy production, and high-value bioproducts. Sci. Total Environ. 2022, 806, 150585. [Google Scholar] [CrossRef]
- Mustafa, S.; Bhatti, H.N.; Maqbool, M.; Iqbal, M. Microalgae biosorption, bioaccumulation and biodegradation efficiency for the remediation of wastewater and carbon dioxide mitigation: Prospects, challenges and opportunities. J. Water Process. Eng. 2021, 41, 102009. [Google Scholar] [CrossRef]
- Malyan, S.K.; Bhatia, A.; Tomer, R.; Harit, R.C.; Jain, N.; Bhowmik, A.; Kaushik, R. Mitigation of yield-scaled greenhouse gas emissions from irrigated rice through Azolla, Blue-green algae, and plant growth–promoting bacteria. Environ. Sci. Pollut. Res. 2021, 28, 51425–51439. [Google Scholar] [CrossRef]
- Ramanan, R.; Kim, B.H.; Cho, D.H.; Oh, H.M.; Kim, H.S. Algae–bacteria interactions: Evolution, ecology and emerging applications. Biotechnol. Adv. 2016, 34, 14–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rai, L.C.; Har, D.K.; Frieder, H.M.; Carl, J.S. Services of algae to the environment. J. Microbiol. Biotechnol. 2000, 10, 119–136. [Google Scholar]
- Alam, F.; Mobin, S.; Chowdhury, H. Third generation biofuel from algae. Procedia Eng. 2015, 105, 763–768. [Google Scholar] [CrossRef]
- Ullah, K.; Ahmad, M.; Sharma, V.K.; Lu, P.; Harvey, A.; Zafar, M.; Sultana, S. Assessing the potential of algal biomass opportunities for bioenergy industry: A review. Fuel 2015, 143, 414–423. [Google Scholar] [CrossRef]
- Energy, J.O.R. Retracted: Microalgae as a Renewable Source of Energy: A Niche Opportunity. J. Renew. Energy 2021, 2021, 9813285. [Google Scholar] [CrossRef]
- Gheorghe, S.; Stoica, C.; Vasile, G.G.; Nita-Lazar, M.; Stanescu, E.; Lucaciu, I.E. Metals toxic effects in aquatic ecosystems: Modulators of water quality. In Water Quality; Tutu, H., Ed.; InTech: Rijeka, Croatia, 2017; pp. 60–89. [Google Scholar]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. Exp. Suppl. 2012, 101, 133–164. [Google Scholar]
- Sarwar, N.; Imran, M.; Shaheen, M.R.; Ishaque, W.; Kamran, M.A.; Matloob, A.; Rehim, A.; Hussain, S. Phytoremediation strategies for soils contaminated with heavy metals: Modifications and future perspectives. Chemosphere 2017, 171, 710–721. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, 04691. [Google Scholar] [CrossRef]
- Heckathorn, S.A.; Mueller, J.K.; LaGuidice, S.; Zhu, B.; Barrett, T.; Blair, B.; Dong, Y. Chloroplast small heat-shock proteins protect photosynthesis during heavy metal stress. Am. J. Bot. 2004, 91, 1312–1318. [Google Scholar] [CrossRef]
- Le Gall, H.; Philippe, F.; Domon, J.M.; Gillet, F.; Pelloux, J.; Rayon, C. Cell wall metabolism in response to abiotic stress. Plants 2015, 4, 112–166. [Google Scholar] [CrossRef]
- Wang, S.; Shi, X. Molecular mechanisms of metal toxicity and carcinogenesis. Mol. Cell. Biochem. 2001, 222, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak, K.S. Oxidative DNA and protein damage in metal induced toxicity and carcinogenesis. Free Radic. Biol. Med. 2002, 32, 958–967. [Google Scholar] [CrossRef]
- Beyersmann, D.; Hartwig, A. Carcinogenic metal compounds: Recent insight into molecular and cellular mechanisms. Arch. Toxicol. 2008, 82, 493–512. [Google Scholar] [CrossRef] [PubMed]
- Tchounwou, P.B.; Ishaque, A.B.; Schneider, J. Cytotoxicity and transcriptional activation of stress genes in human liver carcinoma cells (HepG2) exposed to cadmium chloride. Mol. Cell. Biochem. 2001, 222, 21–28. [Google Scholar] [CrossRef]
- Patlolla, A.; Barnes, C.; Yedjou, C.; Velma, V.; Tchounwou, P.B. Oxidative stress, DNA damage and antioxidant enzyme activity induced by hexavalent chromium in Sprague Dawley rats. Environ. Toxicol. 2009, 24, 66–73. [Google Scholar] [CrossRef] [Green Version]
- Tchounwou, P.B.; Centeno, J.A.; Patlolla, A.K. Arsenic toxicity, mutagenesis and carcinogenesis—A health risk assessment and management approach. Mol. Cell. Biochem. 2004, 255, 47–55. [Google Scholar] [CrossRef]
- Yedjou, C.G.; Tchounwou, P.B. Oxidative stress in human leukemia cells (HL-60), human liver carcinoma cells (HepG2) and human Jerkat-T cells exposed to arsenic trioxide. Met. Ions Biol. Med. 2006, 9, 298–303. [Google Scholar]
- Sutton, D.J.; Tchounwou, P.B. Mercury induces the externalization of phosphatidylserine in human proximal tubule (HK-2) cells. Int. J. Environ. Res. Public Health 2007, 4, 138–144. [Google Scholar] [CrossRef] [Green Version]
- Tchounwou, P.B.; Yedjou, C.G.; Foxx, D.; Ishaque, A.; Shen, E. Lead induced cytotoxicity and transcriptional activation of stress genes in human liver carcinoma cells (HepG2). Mol. Cell. Biochem. 2004, 255, 161–170. [Google Scholar] [CrossRef]
- Gautam, R.K.; Sharma, S.K.; Mahiya, S.; Chattopadhyaya, M.C. Contamination of heavy metals in aquatic media: Transport, toxicity and technologies for remediation. In Heavy Metals in Water: Presence, Removal and Safety; RSC Publishing: London, UK, 2014; pp. 1–24. [Google Scholar]
- Chowdhary, P.; Hare, V.; Raj, A. Book review: Environmental pollutants and their bioremediation approaches. Front. Bioeng. Biotechnol. 2018, 6, 193. [Google Scholar] [CrossRef]
- Singh, R.L.; Singh, P.K. Global environmental problems. In Principles and Applications of Environmental Biotechnology for a Sustainable Future; Springer: Singapore, 2017; pp. 13–41. [Google Scholar]
- Khan, I.; Ali, M.; Aftab, M.; Shakir, S.; Qayyum, S.; Haleem, K.S.; Tauseef, I. Mycoremediation: A treatment for heavy metal-polluted soil using indigenous metallotolerant fungi. Environ. Monit. Assess. 2019, 191, 622. [Google Scholar] [CrossRef] [PubMed]
- Asati, A.; Pichhode, M.; Nikhil, K. Effect of heavy metals on plants: An overview. Int. J. Appl. Innov. Eng. Manag. 2016, 5, 56–66. [Google Scholar]
- Shukla, V.; Shukla, P.; Tiwari, A. Lead poisoning. India J. Med. Spec. 2018, 9, 146–149. [Google Scholar] [CrossRef]
- Fatima, G.; Raza, A.M.; Hadi, N.; Nigam, N.; Mahdi, A.A. Cadmium in human diseases: It’s more than just a mere metal. Indian J. Clin. Biochem. 2019, 34, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Obasi, P.N.; Akudinobi, B.B. Potential health risk and levels of heavy metals in water resources of lead–zinc mining communities of Abakaliki, southeast Nigeria. Appl. Water Sci. 2020, 10, 184. [Google Scholar] [CrossRef]
- Zahra, N.; Kalim, I. Perilous effects of heavy metals contamination on human health. Pak. J. Anal. Environ. Chem. 2017, 18, 1–17. [Google Scholar] [CrossRef]
- Priyadarshini, E.; Priyadarshini, S.S.; Pradhan, N. Heavy metal resistance in algae and its application for metal nanoparticle synthesis. Appl. Microbiol. Biotechnol. 2019, 103, 3297–3316. [Google Scholar] [CrossRef]
- Majumder, S.; Gupta, S.; Raghuvanshi, S. Removal of dissolved metals by bioremediation. In Heavy Metals in Water: Presence, Removal and Safety; Sharma, S.K., Ed.; The Royal Society of Chemistry: Cambridge, UK, 2015; pp. 44–56. [Google Scholar]
- Kumar, A.; Yadav, A.N.; Mondal, R.; Kour, D.; Subrahmanyam, G.; Shabnam, A.A.; Khan, S.A.; Yadav, K.K.; Sharma, G.K.; Cabral-Pinto, M.; et al. Myco-remediation: A mechanistic understanding of contaminants alleviation from natural environment and future prospect. Chemosphere 2021, 284, 131325. [Google Scholar] [CrossRef]
- Peng, W.; Li, X.; Xiao, S.; Fan, W. Review of remediation technologies for sediments contaminated by heavy metals. J. Soils Sediments 2018, 18, 1701–1719. [Google Scholar] [CrossRef]
- Matagi, S.; Swaiand, D.; Mugabe, R. A review of heavy metal removal mechanisms in wetlands. Afr. J. Trop. Hydrobiol. Fish. 1998, 8, 23. [Google Scholar] [CrossRef] [Green Version]
- Mitra, N.; Rezvan, Z.; Seyed Ahmad, M.; Gharaie, M.; Hosein, M. Studies of water arsenic and boron pollutants and algae phytoremediation in three springs, Iran. Int. J. Ecosys. 2012, 2, 32–37. [Google Scholar] [CrossRef] [Green Version]
- Saini, S.; Dhania, G. Cadmium as an environmental pollutant: Ecotoxicological effects, health hazards, and bioremediation approaches for its detoxification from contaminated sites. In Bioremediation of Industrial Waste for Environmental Safety; Springer: Singapore, 2020; pp. 357–387. [Google Scholar]
- Al-Homaidan, A.A.; Al-Ghanayem, A.A.; Areej, A.H. Green algae as bioindicators of heavy metal pollution in Wadi Hanifah Stream, Riyadh, Saudi Arabia. Int. J. Water Resour. Arid. Environ. 2011, 1, 10. [Google Scholar]
- Bonanno, G.; Orlando-Bonaca, M. Trace elements in Mediterranean seagrasses and macroalgae. A review. Sci. Total Environ. 2018, 618, 1152–1159. [Google Scholar] [CrossRef] [PubMed]
- Gosavi, K.; Sammut, J.; Gifford, S.; Jankowski, J. Macroalgal biomonitors of trace metal contamination in acid sulfate soil aquaculture ponds. Sci. Total Environ. 2004, 324, 25–39. [Google Scholar] [CrossRef]
- Rainbow, P.S. Biomonitoring of heavy metal availability in the marine environment. Mar. Pollut. Bull. 1995, 31, 183–192. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.I.; Mansour, M.S. Phytoremediation for the elimination of metals, pesticides, PAHs, and other pollutants from wastewater and soil. In Phytobiont and Ecosystem Restitution; Springer: Singapore, 2018; pp. 101–136. [Google Scholar]
- Davis, T.A.; Volesky, B.; Mucci, A. A review of the biochemistry of heavy metal biosorption by brown algae. Water Res. 2003, 37, 4311. [Google Scholar] [CrossRef]
- Nielsen, H.D.; Burridge, T.R.; Brownlee, C.; Brown, M.T. Prior exposure to Cu contamination influences the outcome of toxicological testing of Fucus serratus embryos. Mar. Pollut. Bull. 2005, 50, 1675–1680. [Google Scholar] [CrossRef]
- Priyadarshani, I.; Sahu, D.; Rath, B. Microalgal bioremediation: Current practices and perspectives. J. Biochem. Technol. 2011, 3, 299–304. [Google Scholar]
- Perales-Vela, H.V.; Peña-Castro, J.M.; Cañizares Villanueva, R.O. Heavy metal detoxification in eukaryotic microalgae. Chemosphere 2006, 64, 1–10. [Google Scholar] [CrossRef]
- Monteiro, C.M.; Castro, P.M.L.; Malcata, F.X. Metal uptake by microalgae: Underlying mechanisms and practical applications. Biotechnol. Prog. 2012, 28, 299–311. [Google Scholar] [CrossRef]
- Figueira, M.M.F.; Volesky, B.; Azarian, K.; Ciminelli, V.S.T. Multi-metal bio-sorption in a column using Sargassum biomass. In Biohydrometallurgy and the Environment Toward the Mining of the 21st Century (Part B): International Biohydrometallurgy Symposium-Proceedings; Amils, R., Ballester, A., Eds.; Elsevier Science: Amsterdam, The Netherlands, 1999; pp. 503–512. [Google Scholar]
- Rajamani, S.; Siripornadulsil, S.; Falcao, V.; Torres, M.A.; Colepicolo, P.; Sayre, R. Phycorremediation of heavy metals Using Transgenic Microalgae. In Transgenic Microalgae as Green Cell Factories; León, R., Galván, A., Fernández, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 99–107. [Google Scholar]
- de-Bashan, L.E.; Bashan, Y. Immobilized micro algae for removing pollutants: Review of practical aspects. Bioresour. Technol. 2010, 101, 1611–1627. [Google Scholar] [CrossRef] [PubMed]
- Brinza, L.; Dring, M.J.; Gavrilescu, M. Marine micro and macroalgal species as biosorbents for heavy metals. Environ. Eng. Manag. J. 2007, 6, 237–251. [Google Scholar] [CrossRef]
- Mondal, M.; Halder, G.; Oinam, G.; Indrama, T.; Tiwari, O.N. Bioremediation of organic and inorganic pollutants using microalgae. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 223–235. [Google Scholar]
- Suresh, B.; Ravishankar, G.A. Phytoremediation—A novel and promising approach for environmental clean-up. Crit. Rev. Biotechnol. 2004, 24, 97–124. [Google Scholar] [CrossRef]
- Tsuji, N.; Hirayanagi, N.; Iwabe, O.; Namba, T.; Tagawa, M.; Miyamoto, S.; Miyasaka, H.; Takagi, M.; Hirata, K.; Miyamoto, K. Regulation of phytochelatin synthesis by zinc and cadmium in marine green alga Dunaliella tertiolecta. Phytochemistry 2003, 62, 453–459. [Google Scholar] [CrossRef]
- Pflugmacher, S.; Sandermann, H. Cytochrome P450 monooxygenases for fatty acids and xenobiotics in marine microalgae. Plant Physiol. 1998, 117, 123–128. [Google Scholar] [CrossRef] [Green Version]
- French, C.E.; Rosser, S.J.; Davies, G.J.; Nicklin, S.; Bruce, N.C. Biodegradation of explosives by transgenic plants expressing pentaerythritol tetranitrate reductase. Nat. Biotechnol. 1999, 17, 491–494. [Google Scholar] [CrossRef]
- Hannink, N.; Rosser, S.J.; French, C.E.; Basran, A.; Murray, J.A.H.; Nicklin, A.; Bruce, N.C. Phyto-detoxification of TNT by transgenic plants expressing a bacterial nitroreductase. Nat. Biotechnol. 2001, 19, 1168–1172. [Google Scholar] [CrossRef]
- De Filippis, L.F.; Pallaghy, C.K. Heavy Metals: Sources and Biological Effects. In Advances in Limnology Series: Algae and Water Pollution, E; Rai, L.C., Gaur, J.P., Soeder, C.J., Eds.; Scheizerbartsche Press: Stuttgart, Germany, 1994; pp. 31–77. [Google Scholar]
- Wang, T.C.; Weissman, J.C.; Ramesh, G.; Varadarajan, R.; Benemann, J.R. Bioremoval of Toxic Elements with Aquatic Plants and Algae. In Bioremediation of Recalcitrant Organics; Hinchee, R.E., Anderson, D.B., Hoeppel, R.E., Eds.; Battelle Press: Columbus, OH, USA, 1995; p. 65. [Google Scholar]
- Upadhyay, A.K.; Singh, R.; Singh, D.P. Phycotechnological approaches toward wastewater management. In Emerging and Eco-Friendly Approaches for Waste Management; Springer: Singapore, 2019; pp. 423–435. [Google Scholar]
- Bursali, E.A.; Cavas, L.; Seki, Y.; Bozkurt, S.S.; Yurdakoc, M. Sorption of boron by invasive marine seaweed: Caulerpa racemosa var. cylindracea. Chem. Eng. J. 2009, 150, 385–390. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, S.; Tan, S.; Lin, Y.; Tay, J.H. Aerobic granules: A novel zinc biosorbent. Lett. Appl. Microbiol. 2002, 35, 548–551. [Google Scholar] [CrossRef]
- Jin-Fen, P.; Rong-Gen, L.; Li, M. A review of heavy metal adsorption by marine algae. Chin. J. Oceanol. Limnol. 2000, 18, 260–264. [Google Scholar] [CrossRef]
- Oukarroum, A. Alleviation of metal-induced toxicity in aquatic plants by exogenous compounds: A mini-review. Water Air Soil Pollut. 2016, 227, 204. [Google Scholar] [CrossRef]
- Abd El-Hameed, M.M.; Abuarab, M.; Al-Ansari, N.; Mottaleb, S.A.; Bakeer, G.A.; Gyasi-Agyei, Y.; Mokhtar, A. Phytoremediation of Contaminated Water by Cadmium (Cd) Using Two Cyanobacteria Species (Anabaena Variabilis and Nostoc Muscorum). Environ. Sci. Eur. 2021, 33, 1–16. [Google Scholar]
- Dönmez, G.C.; Aksu, Z.; Ozturk, A.; Kutsal, T. A comparative study on heavy metal biosorption characteristics of some algae. Process Biochem. 1999, 34, 885–892. [Google Scholar] [CrossRef]
- Ahad, R.I.A.; Goswami, S.; Syiem, M.B. Biosorption and equilibrium isotherms study of cadmium removal by Nostoc muscorum Meg 1: Morphological, physiological and biochemical alterations. 3 Biotech 2017, 7, 104. [Google Scholar] [CrossRef] [Green Version]
- Manzoor, F.; Karbassi, A.; Golzary, A. Removal of heavy metal contaminants from wastewater by using Chlorella vulgaris beijerinck: A review. Curr. Environ. Manag. (Former. Curr. Environ. Eng.) 2019, 6, 174–187. [Google Scholar] [CrossRef]
- Zhai, J.; Li, X.; Li, W.; Rahaman, M.H.; Zhao, Y.; Wei, B.; Wei, H. Optimization of biomass production and nutrients removal by Spirulina platensis from municipal wastewater. Ecol. Eng. 2017, 108, 83–92. [Google Scholar] [CrossRef]
- Shukla, R.; Pandey, A.K.; Mishra, K.N. The efficacy of modified cyanobacterial biomass to remove Cr (VI) ions from aqueous solution. Indian J. Sci. Res. 2017, 16, 31–34. [Google Scholar]
- Sood, A.; Renuka, N.; Prasanna, R.; Ahluwalia, A.S. Cyanobacteria as potential options for wastewater treatment. In Phytoremediation; Springer: Cham, Switzerland, 2015; pp. 83–93. [Google Scholar]
- Tran, H.T.; Vu, N.D.; Matsukawa, M.; Okajima, M.; Kaneko, T.; Ohki, K.; Yoshikawa, S. Heavy metal biosorption from aqueous solutions by algae inhabiting rice paddies in Vietnam. J. Environ. Chem. Eng. 2016, 4, 2529–2535. [Google Scholar] [CrossRef]
- Al-Homaidan, A.A.; Alabdullatif, J.A.; Al-Hazzani, A.A.; Al-Ghanayem, A.A.; Alabbad, A.F. Adsorptive removal of cadmium ions by Spirulina platensis dry biomass. Saudi J. Biol. Sci. 2015, 22, 795–800. [Google Scholar] [CrossRef] [Green Version]
- Anjana, K.; Kaushik, A.; Kiran, B.; Nisha, R. Biosorption of Cr (VI) by immobilized biomass of two indigenous strains of cyanobacteria isolated from metal contaminated soil. J. Hazard. Mater. 2007, 148, 383–386. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V. Mercury detoxification by absorption, mercuric ion reductase, and exopolysaccharides: A comprehensive study. Environ. Sci. Pollut. Res. 2020, 27, 27181–27201. [Google Scholar] [CrossRef] [PubMed]
- Apiratikul, R.; Pavasant, P. Batch and column studies of biosorption of heavy metals by Caulerpa lentillifera. Biores. Technol. 2008, 99, 2766–2777. [Google Scholar] [CrossRef] [PubMed]
- Lodeiro, P.; Herrero, R.; de Sastre Vicente, M.E. The use of protonated Sargassum muticum as biosorbent for cadmium removal in a fixed-bed column. J. Hazard. Mater. 2006, 137, 244–253. [Google Scholar] [CrossRef]
- Vilar, V.J.P.; Botelho, C.M.S.; Boaventura, R.A.R. Lead uptake by algae Gelidium and composite material particles in a packed bed column. Chem. Eng. J. 2008, 144, 420–430. [Google Scholar] [CrossRef]
- Senthilkumar, R.; Vijayaraghavan, K.; Thilakavathi, M.; Iyer, P.V.R.; Velan, M. Seaweeds for the remediation of wastewaters contaminated with Zinc (II) ions. J. Hazard. Mater. B 2006, 136, 791–799. [Google Scholar] [CrossRef]
- Izquierdo, M.; Gabaldon, C.; Marzal, P.; Alvarez Hornos, F.J. Modeling of copper fixed-bed biosorption from wastewater by Posidonia oceanica. Biores. Technol. 2010, 101, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.H.; Hashim, M.A. Copper biosorption on immobilized seaweed biomass: Column breakthrough characteristics. J. Environ. Sci. 2007, 19, 928–932. [Google Scholar] [CrossRef]
- Arco, M.M.; Hanela, S.; Duran, J.; Dos Santos Afonso, M. Biosorption of Cu(II), Zn(II), Cd(II) and Pb(II) by dead biomasses of green alga Ulva lactuca and the development of a sustainable matrix for adsorption implementation. J. Hazard. Mater. 2012, 213–214, 123–132. [Google Scholar] [CrossRef]
- Mei, L.; Xitao, X.; Renhao, X.; Zhili, L. Effects of strontium-induced stress on marine microalgae Platymonas subcordiformis (Chlorophyta: Volvocales). Chin. J. Oceanol. Limnol. 2006, 24, 154. [Google Scholar] [CrossRef]
- De Filippis, L.F. The effect of sublethal concentration of mercury and Zinc on Chlorella IV characteristics of a general enzyme system for metallic ions. Zeit Schr. Panzenphysiologie. 1978, 86, 339. [Google Scholar] [CrossRef]
- De Filippis, L.F.; Pallaghy, C.K. The effect of sub-lethal concentrations of mercury and zinc on chlorella: III. Development and possible mechanisms of resistance to metals. Zeit Schr. Panzenphysiologie. 1976, 79, 323–335. [Google Scholar] [CrossRef]
- Wang, B.; Wang, J.; Zhang, W.; Meldrum, D.R. Application of synthetic biology in cyanobacteria and algae. Front. Microbiol. 2012, 3, 344. [Google Scholar] [CrossRef] [Green Version]
- Verma, S.; Kuila, A. Bioremediation of heavy metals by microbial process. Environ. Technol. Innov. 2019, 14, 100369. [Google Scholar] [CrossRef]
- Singh, A.; Prasad, S.M. Remediation of heavy metal contaminated ecosystem: An overview on technology advancement. Int. J. Environ. Sci. Technol. 2015, 12, 353–366. [Google Scholar] [CrossRef]
- Chekroun, K.B.; Baghour, M. The role of algae in phytoremediation of heavy metals: A review. J. Mater. Environ. Sci. 2013, 4, 873–880. [Google Scholar]
- Javanbakht, V.; Alavi, S.A.; Zilouei, H. Mechanisms of heavy metal removal using microorganisms as biosorbent. Water Sci. Technol. 2014, 69, 1775–1787. [Google Scholar] [CrossRef] [PubMed]
- Jan, S.; Parray, J.A. Approaches to Heavy Metal Tolerance in Plants. Springer: Singapore, 2016.
- Lahiri, S.; Ghosh, D.; Bhakta, J.N. Role of microbes in eco-remediation of perturbed aquatic ecosystem. In Handbook of Research on Inventive Bioremediation Techniques; Bhakta, J., Ed.; IGI Global: Hershey, PA, USA, 2017; pp. 70–107. [Google Scholar]
- Danouche, M.; El Ghachtouli, N.; El Baouchi, A.; El Arroussi, H. Heavy metals phycoremediation using tolerant green microalgae: Enzymatic and non-enzymatic antioxidant systems for the management of oxidative stress. J. Environ. Chem. Eng. 2020, 8, 104460. [Google Scholar] [CrossRef]
- Danouche, M.; El Ghachtouli, N.; El Arroussi, H. Phycoremediation mechanisms of heavy metals using living green microalgae: Physicochemical and molecular approaches for enhancing selectivity and removal capacity. Heliyon 2021, 7, 07609. [Google Scholar] [CrossRef]
- Kumar, K.S.; Dahms, H.U.; Won, E.J.; Lee, J.S.; Shin, K.H. Microalgae—A promising tool for heavy metal remediation. Ecotoxicol. Environ. Saf. 2015, 113, 329–352. [Google Scholar] [CrossRef]
- Park, D.M. Bioadsorption of rare earth elements through cell surface display of lanthanide binding tags. Environ. Sci. Technol. 2016, 50, 2735–2742. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Kyzas, G.Z. Progress in batch biosorption of heavy metals onto algae. J. Mol. Liq. 2015, 209, 77–86. [Google Scholar] [CrossRef]
- Zeraatkar, A.K.; Ahmadzadeh, H.; Talebi, A.F.; Moheimani, N.R.; McHenry, M.P. Potential use of algae for heavy metal bioremediation, a critical review. J. Environ. Manag. 2016, 181, 817–831. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Liu, Y.; Li, X.; Xuan, Z.; Ma, J. Biosorption of lead ion by chemically-modified biomass of marine brown algae Laminaria japonica. Chemosphere 2006, 64, 1122–1127. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Chen, J.P. A comprehensive review on biosorption of heavy metals by algal biomass: Materials, performances, chemistry, and modeling simulation tools. Bioresour. Technol. 2014, 160, 67–78. [Google Scholar] [CrossRef]
- Rijstenbil, J.W.; Gerringa, L.J.A. Interactions of algal ligands, metal complexation and availability, and cell responses of the diatom Ditylum brightwellii with a gradual increase in copper. Aquat. Toxicol. 2002, 56, 115–131. [Google Scholar] [CrossRef]
- Salama, E.S.; Roh, H.S.; Dev, S.; Khan, M.A.; Abou-Shanab, R.A.; Chang, S.W.; Jeon, B.H. Algae as a green technology for heavy metals removal from various wastewater. World J. Microbiol. Biotechnol. 2019, 35, 1–19. [Google Scholar] [CrossRef]
- Wang, S.; Vincent, T.; Faur, C.; Guibal, E. Alginate and algal-based beads for the sorption of metal cations: Cu (II) and Pb (II). Int. J. Mol. Sci. 2016, 17, 1453. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.; Shamshad, I.; Waqas, M.; Nawab, J.; Ming, L. Remediating industrial wastewater containing potentially toxic elements with four freshwater algae. Ecol. Eng. 2017, 102, 536–541. [Google Scholar] [CrossRef]
- Furey, P.C.; Deininger, A.; Liess, A. Substratum-associated microbiota. Water Environ. Res. 2016, 88, 1637–1671. [Google Scholar] [CrossRef]
- Kanchana, S.; Jeyanthi, J.; Kathiravan, R.; Suganya, K. Biosorption of heavy metals using algae: A review. Int. J. Pharma Med. Biol. Sci. 2014, 3, 1. [Google Scholar]
- Sweetly, J. Macroalgae as a potentially low-cost biosorbent for heavy metal removal: A review. Int. J. Pharm. Biol. Arch. 2014, 5, 17–26. [Google Scholar]
- Mustapha, M.U.; Halimoon, N. Microorganisms and biosorption of heavy metals in the environment: A review paper. J. Microb. Biochem. Technol. 2015, 7, 253–256. [Google Scholar] [CrossRef]
- Perpetuo, E.A.; Souza, C.B.; Nascimento, C.A.O. Engineering bacteria for bioremediation. In Progress in Molecular and Environmental Bioengineering from Analysis and Modeling to Technology Applications; Carpi, A., Ed.; Tech Publishers: Rijeka, Croatia, 2011; pp. 605–632. [Google Scholar]
- Mantzorou, A.; Navakoudis, E.; Paschalidis, K.; Ververidis, F. Microalgae: A potential tool for remediating aquatic environments from toxic metals. Int. J. Environ. Sci. Technol. 2018, 16, 1815–1830. [Google Scholar] [CrossRef]
- Tripathi, S.; Poluri, K.M. Adaptive and tolerance mechanism of microalgae in removal of cadmium from wastewater. In Algae; Springer: Singapore, 2021; pp. 63–88. [Google Scholar]
- Torres, M.A.; Barros, M.P.; Campos, S.C.G.; Pinto, E.; Rajamani, S.; Sayre, R.T.; Colepicolo, P. Biochemical biomarkers in algae and marine pollution: A review. Ecotoxicol. Environ. Saf. 2008, 71, 1–15. [Google Scholar] [CrossRef]
- Hayashi, Y.; Nakagawa, C.W.; Mutoh, N.; Isobe, M.; Goto, T. Two pathways in the biosynthesis of cadystins (gammaEC) nG in the cell-free system of the fission yeast. Biochem. Cell Biol. 1991, 69, 115–121. [Google Scholar] [CrossRef]
- Klapheck, S.; Schlunz, S.; Bergmann, L. Synthesis of phytochelatins and homo-phytochelatins in Pisum sativum L. Plant Physiol. 1995, 107, 515–521. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.J.; Zhou, J.M.; Goldsbrough, P.B. Characterization of phytochelatin synthase from tomato. Physiol. Planta 1997, 101, 165–172. [Google Scholar] [CrossRef]
- Ahner, B.A.; Kong, S.; Morel, F.M.M. Phytochelatin production in marine algae. An interspecific comparison. Limnol. Oceanogr. 1995, 40, 649–657. [Google Scholar] [CrossRef]
- Cobbett, C.; Goldsbrough, P. Phytochelatins and metallothioneins: Roles in heavy metal detoxification and homeostasis. Annu. Rev. Plant Biol. 2002, 53, 159–182. [Google Scholar] [CrossRef] [Green Version]
- Bačkor, M.; Pawlik-Skowrońska, B.; Bud’ová, J.; Skowroński, T. Response to copper and cadmium stress in wild type and copper tolerant strains of the lichen alga Trebouxi aerici: Metal accumulation, toxicity and non-protein thiols. Plant Growth Regul. 2007, 52, 17–27. [Google Scholar] [CrossRef]
- Chmielewská, E.; Medved, J. Bioaccumulation of heavy metals by green algae Cladophora glomerata in a refinery sewage lagoon. Croat. Chem. Acta 2001, 74, 135–145. [Google Scholar]
- Henriques, B. Bioaccumulation of Hg, Cd and Pb by Fucus vesiculosus in single and multi-metal contamination scenarios and its effect on growth rate. Chemosphere 2017, 171, 208–222. [Google Scholar] [CrossRef] [PubMed]
| Metal | Adverse Effects | Reference |
|---|---|---|
| As | Carcinogenic effects Hyperpigmentation, melanosis and keratosis in humans Genotoxic, as it leads to the generation of ROS and causes lipid peroxidation Immunotoxic Modulates co-receptor expression Causes Black foot disease | [64,65] |
| Hg | Mutagenic effects Minamata disease Hampers cholesterol | [66,67] |
| Cd | This leads to severe bone and kidney damage in humans Anemia, bronchitis, emphysema, Acute toxic effects in children | [68,69,70] |
| Zn | Causes anemia Phytotoxic Leads to a decrease in muscular coordination Causes pain in the abdomen | [71] |
| Cu | Phytotoxic Damages a range of aquatic fauna Corrosion and mucosal irritation Disturbs the central nervous system and can lead to depression | [65] |
| Cr | Irritates gastrointestinal mucosa Nephritis and death in humans at higher doses of Cr (VI) | [72] |
| Ni | High concentration may lead to DNA damage Negative effect on fauna Causes phytotoxicity | [65] |
| Pb | Phytotoxic High concentration may lead to metabolic poison Toxic to humans, aquatic fauna and livestock Hypertension leading to brain damage May lead to fatigue irritability, anemia and behavioral changes in children | [66,73,74] |
| Algae | Metal Removed | Description of Metal-Rich Surrounding | References |
|---|---|---|---|
| Anacystis nidulans | Cu | Solution of metal | [103] |
| Tolypothrix tenuis | Cd | Aqueous solution | [104] |
| Synechocystis sp., Scenedesmus obliquus, and Chlorella vulgaris | Cr (VI), Ni, Cu | Aqueous solution | [105] |
| Nostoc muscorum | Cd and Cu | Multi metal solution | [106] |
| N. rivularis and Nostoc linckia | Cd and Zn | Sewage water | [107] |
| Chlorella vulgaris | Ni and Cu | Single and binary metal solution | [108] |
| Spirulina sp. | Trace element | Copper smelter and refinery effluent | [109] |
| Aulosira fertilissima | Ni and Cr | Free-cell condition | [110] |
| Anabaena, subcylindrical, and Nostoc muscorum | Mn, Co, Pb and Cu | Industrial wastewater and sewage | [111] |
| Cladophora fascicularis | Pb and Cu | Aqueous solution | [112] |
| Spirulina platensis (Spi SORB) | Cu | Column reactor system | [113] |
| Chroococcus sp. and Nostoc calcicola | Cr | Metal-contaminated soil | [114] |
| Gloeocapsa and Lyngbya | Cr | Contaminated sites | [115] |
| Aphanothece flocculosa and Spirulina platensis | Hg | Wet biomass | [116] |
| Gloeothece sp. strain PCC 6909 | Cu | Wastewater | [117] |
| Hapalosiphon welwitschii Nägel | Cd | Metal solution | [118] |
| Phormidium sp. NTMS02 and Oscillatoria sp. NTMS01 | Cr (VI) | Aqueous solution | [119] |
| Caulerpa racemosa and Sargassum wightii | Cd, Pb, Cr (III and VI) | Aqueous solution | [120] |
| Caulerpa lentillifera | Cu (II), Pb (II), Cd (II), | Aqueous solution | [121] |
| Sargassum sp | Cu (II), Cd (II), Ni (II), Fe (III) | Aqueous solution | [122] |
| Gelidium sp. | Pb (II), Cu (II), Cr (III) | Aqueous solution | [123] |
| Ulva reticulat | Cu (II), Co (II), Ni (II), Zn (II) | Aqueous solution | [124] |
| Posidonia oceanica | Cu (II) | Aqueous solution | [125] |
| Sargassum baccularia | Cu (II) | Aqueous solution | [126] |
| Ulva lactuca | Cu (II), Zn (II), Cd (II), Pb (II) | Aqueous solution | [127] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ankit; Bauddh, K.; Korstad, J. Phycoremediation: Use of Algae to Sequester Heavy Metals. Hydrobiology 2022, 1, 288-303. https://doi.org/10.3390/hydrobiology1030021
Ankit, Bauddh K, Korstad J. Phycoremediation: Use of Algae to Sequester Heavy Metals. Hydrobiology. 2022; 1(3):288-303. https://doi.org/10.3390/hydrobiology1030021
Chicago/Turabian StyleAnkit, Kuldeep Bauddh, and John Korstad. 2022. "Phycoremediation: Use of Algae to Sequester Heavy Metals" Hydrobiology 1, no. 3: 288-303. https://doi.org/10.3390/hydrobiology1030021








