Abstract
The use of seaweed as a source of bioactive extracts has received increased attention from the market in recent years—particularly for nutraceutical applications. In this context, this study evaluated the nutraceutical application of seaweed biomass and extracts from three seaweeds from Portugal: Ulva sp., Laminaria ochroleuca, and Chondrus crispus. For each of the said seaweeds, four different extracts were obtained using GRAS solvents—acetone (A), ethanol (E), ethanol–water (1:1) (EW), and one polysaccharide-rich extract (P) using water and further precipitation with ethanol. The bioactive potential of the extracts was assessed in terms of antioxidant capacity (ABTS•+, DPPH•, •NO, O2•− scavenging, and ORAC-FL assay) and anti-inflammatory capacity (COX inhibition and human red blood cell membrane stabilisation). Furthermore, the biochemical profile was determined for the raw biomass and extracts to better comprehend their possible applications as nutraceuticals. The results show that all extracts have antioxidant potential. Five extracts (L. ochroleuca E, EW, and P and Ulva sp. E and P) showed anti-inflammatory capacity. Overall, L. ochroleuca EW extract exhibited the most promising potential as both an antioxidant and anti-inflammatory and is an interesting candidate nutraceutical ingredient.
1. Introduction
In 2019, the seaweed biomass market was estimated at USD 5.9 billion, with the human consumption application segment accounting for about 80% [1]. In terms of consumption, seaweeds have been a part of the Asian diet for a long time, but in Western countries, they are still limited and mainly linked to sushi, other Asian cuisines, and functional foods [1].
The concept of “functional foods” was coined in response to a widespread interest in certain foods that may benefit one’s health. Food and nutrition research has progressed beyond detecting and fixing nutritional deficiencies to developing foods that promote optimal health and lower illness risk [1]. Seaweeds are known for their high contents of polysaccharides, minerals, and vitamins. However, more recently, bioactive compounds, such as polyphenols, peptides, lipids, and pigments, have drawn attention due to several bioactivities, such as antioxidant, anti-inflammatory, anti-tumour, anti-obesity and anti-hypertensive effects [1,2]. Therefore, seaweeds now appear to be a great potential source of bioactive compounds as ingredients in functional foods [2].
Antioxidant compounds are gaining attention due to their potential to prevent several oxidative-stress-associated diseases, such as inflammation—which occurs when the balance between antioxidants and reactive oxygen species (ROS) is disrupted because of either the depletion of antioxidants or the accumulation of ROS [3].
Furthermore, there is a growing interest in natural antioxidants as replacements for synthetic ones due to increased safety concerns [4]. Many synthetic antioxidants (e.g., butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT)) used as food preservatives are considered to have carcinogenic and/or toxic effects on animal models [4]. Thus, algal antioxidant compounds have a great potential for improving the oxidative stability of food products [5].
In fact, crude seaweed extracts (acetonic, aqueous, methanolic, and ethanolic) have already been shown to have interesting antioxidant properties [6,7], and the presence of phenolic compounds, pigments, polysaccharides, fatty acids, and peptides in seaweed extracts is linked to these activities [8]. For the extraction of bioactive compounds to be used in the food industry, food GRAS (Generally Recognised as Safe) solvents with lower environmental impact and toxicity (such as water, ethanol, and acetone) are required.
Seaweeds are very diverse organisms and are commonly organised into three groups according to their pigments: green, red, and brown, which have potentially different biochemical characteristics. Seaweed communities on the Portuguese coast are also remarkably diverse in terms of species, since this region is a biogeographical transition zone (between the Mediterranean and the Atlantic regions) due to the presence of a latitudinal temperature gradient, with overlapping cold- and warm-water seaweed species [9]. Since at least the 14th century, seaweeds have been harvested for use as fertilizers [10], although they are a natural and significant resource that is yet underexplored and investigated, particularly in terms of their economic potential. Within the three groups of green, red, and brown seaweeds, Ulva sp., Chondrus crispus and Laminaria ochroleuca, respectively, represent the most frequent species (>25% coverage) on the rocky shores of Northern Portugal [10].
Therefore, due to the worldwide trend towards the use of natural compounds in the food/nutraceutical market [1], this work aimed to preliminarily assess the potential of the use of seaweeds from the Northern Portugal coast in the food market as either (i) a nutrient-rich ingredient, (ii) a source of nutraceutical ingredients, namely, those with antioxidant and anti-inflammatory potential, and (iii) a source of antioxidant compounds to be used as food preservatives.
2. Materials and Methods
2.1. Seaweed Biomass Source
Biomass from three seaweed species, Ulva sp. (Chlorophyta), Chondrus crispus (Rhodophyta), and Laminaria ochroleuca (Phaeophyceae), were produced in enclosed systems for this study. Ulva sp. and C. crispus were produced in a pilot land-based system at CIIMAR (Portugal) in 400 L tanks receiving water from fish tanks to increase the nutrient concentration and yield. L. ochroleuca was produced in a pilot open-water installation and settled off Sagres (Portugal) in an oyster production farm. Seaweed biomass was dried at 60 °C for 48 h and stored in dry conditions.
2.2. Nutritional Value of Seaweed Biomass
Protein, fat, ash, moisture, and carbohydrate contents were evaluated using AOAC-based methods: the Kjeldahl assay based on 990.03 AOAC for proteins, the Soxhlet extraction method based on 945.16 AOAC for fat, and the 942.05 AOAC method for ash, and for carbohydrates, the content was estimated by subtracting the other components (Carbohydrates = 100% − Proteins % − Fat % − Water % − Ash %); finally, for moisture content, the 2000 AOAC method was used.
2.3. Bioactive Compound Extraction
In order to obtain extracts rich in different groups of potential bioactive compounds, for each seaweed, four different extracts were obtained with organic GRAS solvents—acetone (A), ethanol (E), a mixture of ethanol: water (1:1, v/v) (EW), and a fourth aqueous extract that was produced to obtain a polysaccharide-rich extract (P).
The extractions were performed in triplicate using 250 mg of dry biomass with a final solvent volume of 15 mL. In order to improve the extraction rate, cells were crushed using a bead beater (Precellys Homogenizer, Bertin, France) using 3 cycles with 5 mL of solvent each (6 series of 8000 rpm for 30 s with a 45 s pause) and 670 mg of ceramic beads to maximise cell disruption. Extracts were centrifuged at 2000× g for 10 min, and the supernatant was dried in a rotavapor system. EW extract was further freeze-dried to remove the water.
Polysaccharides were precipitated using water extract and cold ethanol (−20 °C) in a 1:3 ratio [11]. The solution was centrifuged at 2000× g for 10 min, and the polysaccharide phase was dried in a dry oven (60 °C). All extracts were stored in low humidity (desiccator) in the dark until further analyses.
2.4. Antioxidant Capacity Assessment
The antioxidant capacity of each extract was assessed by five different assays. Four scavenging assays were performed: two that measure total activity against synthetic radicals (ABTS•+ and DPPH•) and another two that measure activity against biological reactive species (O2•-, and •NO). Additionally, the oxygen radical absorbance capacity (ORAC-FL) assay was also determined. All extracts were resuspended in 20% DMSO at a concentration of 4 mg mL−1 and further diluted for each assay. The antioxidant capacity was calculated from Trolox equivalent curves and expressed as mgTE g−1. The assays were performed in triplicate.
2.4.1. ABTS•+ Radical Scavenging
The ABTS•+ scavenging assay was performed as described by Granados-Guzman et al. [12] for all of the extracts at a final concentration of 500 μg mL−1. In summary, 63 μL of each sample was transferred to a 96-well plate and mixed with 180 μL of ABTS•+. The reaction was incubated for 6 min in darkness, and the absorbance was read at 734 nm. The extract absorbance interference was reduced by measuring a blank using distilled water instead of ABTS•+.
2.4.2. DPPH• Radical Scavenging
The DPPH• scavenging assay was performed as described by Bobo-García et al. [13] for all of the extracts at a final concentration of 500 μg mL−1. Briefly, 63 μL of each sample was transferred to a 96-well plate and mixed with 180 μL of DPPH•. Samples were incubated for 30 min in darkness, and the absorbance was read at 515 nm. The extract absorbance interference was reduced by measuring a blank using methanol instead of DPPH•.
2.4.3. O2•− Radical Scavenging
The O2•− scavenging assay was performed as described by Pinho et al. [14] for all of the extracts at a final concentration of 500 μg mL−1. The reaction was performed using 50 μL of each sample transferred to a 96-well plate together with 50 μL of NADH (166 μM), 150 μL of NBT (43 μM), and 50 μL of PMS (2.7 μM). Samples were incubated for 5 min in darkness, and the absorbance was read at 560 nm. The extract interference was reduced by measuring a blank using phosphate buffer (19 μM, pH 7.4) instead of PMS.
2.4.4. NO Radical Scavenging
The •NO scavenging assay was performed as described by Pinho et al. [14] for all of the extracts at a final concentration of 500 μg mL−1. Briefly, 100 μL of each dilution was transferred to a 96-well plate together with 100 μL of SNP (20 mM) and incubated for 60 min with 20 μmolphotons m−2 s−1 of fluorescent light. Then, 100 μL of Griess reagent (1.0% sulphanilamide and 0.1% N-(1)-naphthylethylenediamine in 2% phosphoric acid) was added to the solution and incubated for 10 min in darkness, and the absorbance was read at 562 nm. The extract interference was reduced by measuring a blank using 2% phosphoric acid instead of the Griess reagent.
2.4.5. Oxygen Radical Absorbance Capacity (ORAC-FL) Assay
The ORAC-FL assay was performed as described by Davalos et al. [15] for all of the extracts in a range of concentrations from 1.5 to 80 μg mL−1. First, 20 μL of each dilution was transferred to a 96-well plate together with 120 μL of fluorescein (70 nM) and incubated for 15 min at 37 °C. After, 60 μL of AAPH solution (12 mM) was added. The fluorescence was then recorded every minute for 80 min. A blank was prepared using phosphate buffer instead of the extract solution, and eight calibration solutions using Trolox (1–8 μM) were used for the standard curve. ORAC-FL values were obtained by regression equations between the net area under the fluorescence decay curve (AUC) and antioxidant concentration. ORAC-FL values were expressed as Trolox equivalents using the standard curve calculated for each assay.
2.5. Anti-Inflammatory Capacity Potential Assessment
2.5.1. Human Red Blood Cell (HRBC) Membrane Stabilisation by Heat Induction
The HRBC assay was performed according to Moualek et al. [16]. Fresh blood was collected intravenously from a healthy human volunteer into heparinised tubes to prevent coagulation. Blood was centrifuged at 1250× g for 5 min and washed three times with an equal volume of sodium phosphate buffer (PBS, 10 mM, pH 7.4). HRBC was reconstituted in a 40% (v/v) suspension with isotonic PBS. Salicylic acid (3.6 mM) was used as a positive control, and 20% DMSO (in PBS) was used as a negative control. The assay solution consisted of 2% HRBC mixed with the same volume of extract diluted in 20% DMSO at a final concentration of 500 μg mL−1 and incubated for 20 min at 54 °C. Then, the solution was cooled in a tap water bath and centrifuged at 1250× g for 5 min. The absorbance of the supernatant was measured spectrophotometrically at 540 nm using a microplate reader (Thermofisher GO, Waltham, MA, USA). The inhibition of HRBC degradation was calculated for each extract and expressed as a percentage of inhibition (%).
2.5.2. Cyclooxygenase (COX-2) Enzymatic Activity
The inhibition of COX-2 enzymatic activity was tested in triplicate using the COX inhibitor screening assay kit (Cayman Chemical, Ann Arbor, MI, USA) according to the manufacturer’s instructions. Dried extracts were resuspended in DMSO and tested at a concentration of 500 μg mL−1.
2.6. Biochemical Characterisation of Extracts
2.6.1. Soluble Proteins
The soluble protein content was determined by the Bradford method [17] following the manufacturer’s protocol (Biorad, Hercules, CA, USA). The quantification was performed using 10 mg of extract solubilised in 1 mL of phosphate buffer (50 mM, pH 7), and bovine serum albumin (BSA) (Alfa Aesar, Ward Hill, MA, USA) was used to obtain a standard curve. The protein content was expressed as a percentage of extract weight (%). The assay was performed in triplicate.
2.6.2. Lipids
The content of lipids was quantified gravimetrically using a chloroform/methanol (2:1, v/v) extraction [18] using 100 mg of extract in 5 mL of solvent. Afterward, the non-polar fraction was collected, dried, and weighed to determine the lipid content of the extract. The content of lipids was expressed as a percentage of extract weight (%). The assay was performed in triplicate.
2.6.3. Carbohydrates
The carbohydrate content was quantified spectrophotometrically through the phenol–sulphuric acid method [19]. The quantification was performed using 10 mg of extract resuspended in sulphuric acid (1 M). Glucose (Alfa Aesar, Ward Hill, MA, USA) was used to obtain a standard curve. The carbohydrate content was expressed as a percentage of extract weight (%). The assay was performed in triplicate.
2.6.4. Phenolic Compounds
The total phenolic compound content was determined by the Folin–Ciocalteu method [20]. The quantification was performed using 10 mg of extract resuspended in methanol–water (4:1, v/v). Gallic Acid (Sigma-Aldrich, St. Louis, MO, USA) was used to obtain a standard curve, and thus, the phenolic compound content was expressed as a percentage of extract weight (%). The assay was performed in triplicate.
2.7. Statistical Analysis
Experimental data were analysed using GraphPad Prism V. 7.0. A Shapiro-Wilk test of normality was performed before the analysis of variance (ANOVA) to confirm the normal distribution of the residuals. A two-way ANOVA with Tukey’s multi-comparison test was used to find differences between the antioxidant capacity, anti-inflammatory capacity, and biochemical composition between extracts. A principal component analysis was performed using GraphPad Prism V. 9.0 using the data from bioactive screening and biochemical profiles from all evaluated extracts.
3. Results
3.1. Nutritional Characterisation of Seaweed Biomass
The dried biomass of the three studied seaweeds was evaluated in terms of nutritional value (Table 1). In terms of moisture, Ulva sp. held the highest content (11.7 ± 0.5%DW), while L. ochroleuca presented the lowest value, which was 2-fold lower than Ulva sp. In addition, L. ochroleuca biomass contained a low protein content (8.9 ± 0.2%DW) compared to the other two seaweeds with ca. 25%DW (2.7-fold higher).

Table 1.
Nutritional characterisation of dry seaweed biomass (average ± standard deviation, n = 3). Lowercase letters indicate statistical differences between species for the same parameter (p < 0.05).
In terms of fat, all three seaweeds had a low content, no more than 0.6%, as usually found in these organisms [21]. As expected, the main component of the biomass from all three species was carbohydrates, with C. crispus and L. ochroleuca being the ones with the highest amount (ca. 47%). L. ochroleuca showed a high ash content (38.2 ± 0.4), which was two times higher than C. crispus.
3.2. Antioxidant Capacity of Seaweed Extracts
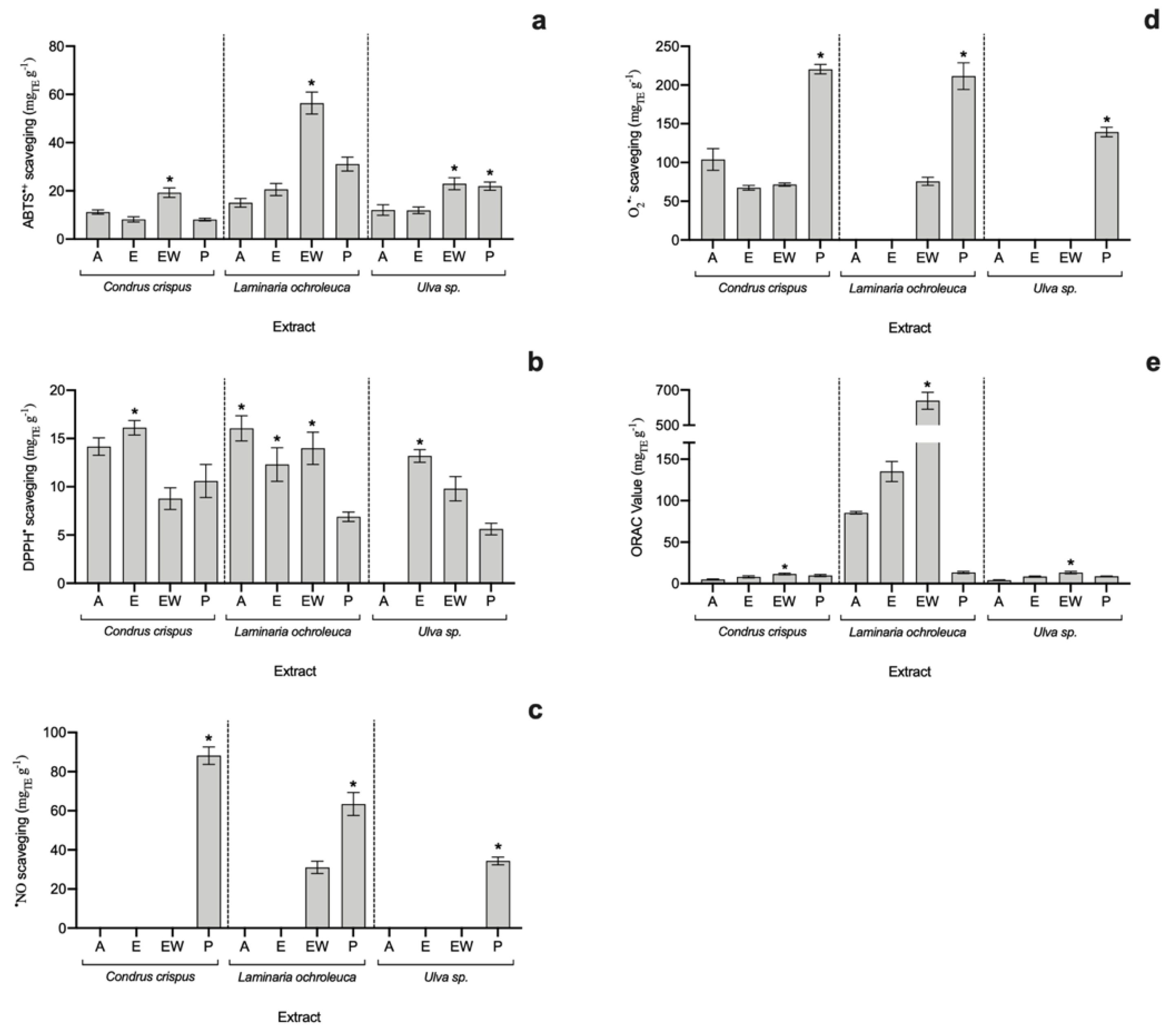
It is known that some radicals, such as •NO and O2•−, are responsible for triggering non-desirable metabolic pathways, some of them resulting in inflammation and, in the worst scenario, tumourigenesis [22]. In addition, it was found that synthetic antioxidants used in the food industry to act as food preservatives may cause tumourigenesis [23]. Thus, it is crucial to find antioxidant compounds from natural sources to avoid the use of synthetic ones that are also able to block the action of said •NO and O2•− radicals. For that reason, the antioxidant capacity of seaweed extracts was measured using five methods, allowing a comprehensive evaluation of the bioactive potential of these organisms (Figure 1). While total radical scavenging capacity evaluated by ABTS•+ and DPPH• assays may indicate the potential of extracts to be used in food conservation, the specific antioxidant assays (•NO and O2•−) reveal their nutraceutical potential. Moreover, the ORAC-FL assay has been recommended for the total antioxidant evaluation of foods and beverages [24].
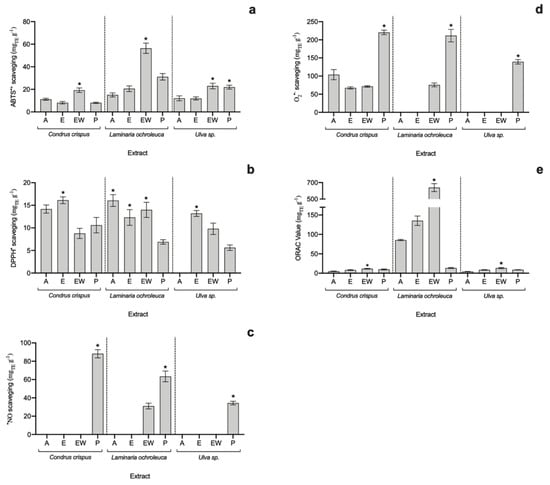
Figure 1.
Antioxidant capacity of seaweed extracts in terms of (a) ABTS•+, (b) DPPH•, (c) •NO, and (d) O2•− scavenging assays and (e) ORAC-FL assay (average ± standard deviation, n = 3). Extracts: acetone (A), ethanol (E), ethanol–water (EW) and polysaccharides (P). The asterisk symbol (*) indicates the highest value per species (p < 0.05).
When it comes to total radical scavenging capacity, the EW and E extracts of all analysed algae exhibited the highest potential, particularly the EW of L. ochroleuca (56.41 ± 4.56 mgTE g−1 for ABTS•+ and 13.99 ± 1.68 mgTE g−1 for DPPH•), which was 1.8-fold higher than the second-best extract, L. ochroleuca P (in ABTS assay), but not statistically different (p > 0.05) from L. ochroleuca A in the DPPH• assay.
Polysaccharide-rich extracts (P) from all three species attained the highest antioxidant values against •NO and O•− radicals, revealing their nutraceutical potential. Additionally, looking at specific activity against •NO, apart from P extracts, only L. ochroleuca EW showed scavenging capacity (31.06 ± 3.16 mgTE g−1), while the highest value was achieved by C. crispus P extract (88.21 ± 4.46 mgTE g−1). Regarding the O2•− scavenging assay, the highest value was found in C. crispus P (220.38 ± 6.04 mgTE g−1) and L. ochroleuca P (211.44 ± 17.20 mgTE g−1) extracts, with no statistical differences (p > 0.05).
Finally, concerning the oxygen radical absorbance capacity (ORAC-FL), although all extracts seem to have an antioxidant effect, L. ochroleuca showed much higher potential than the other species, with a special highlight for L. ochroleuca EW extract (638.90 ± 48.30 mgTE g−1), which was 4.7-fold higher than the second-best extract (L. ochroleuca E).
3.3. Anti-Inflammatory Potential of Seaweed Extracts
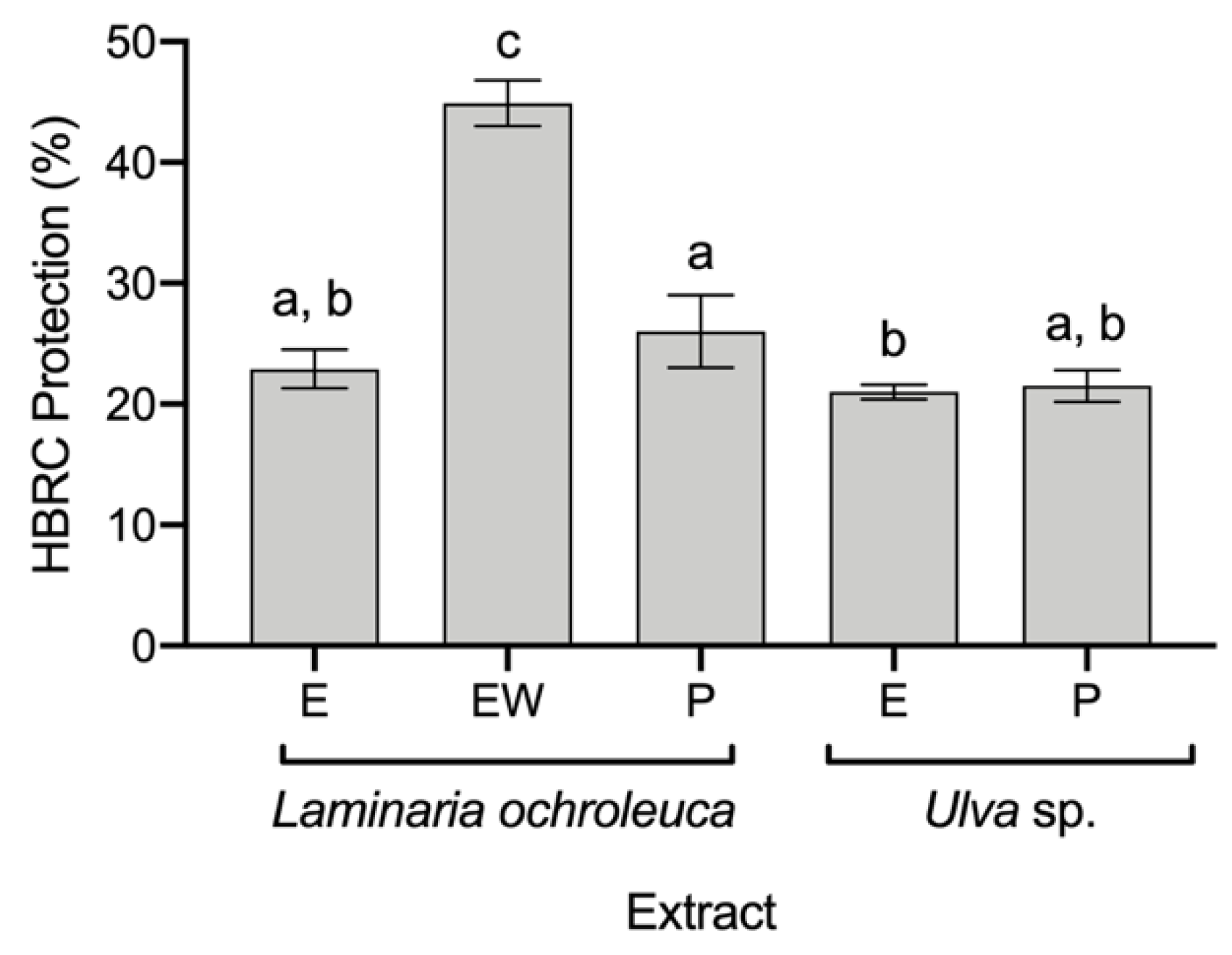
Although all extracts were evaluated for HRBC protection against thermal degradation, only five extracts had protective effects (Figure 2): L. ochroleuca E, EW, and P extracts and Ulva sp. E and P extracts. The highest protective effect was found in L. ochroleuca EW extract, with 44.9 ± 1.6% of protection, almost twice the protection afforded by the other extracts, with ca. of 23% of protection. Furthermore, for the COX-2 inhibition assay, only L. ochroleuca EW extract was evaluated, resulting in an inhibition of 32.8 ± 2.42% of the enzymatic activity at a concentration of 500 μg mL−1.
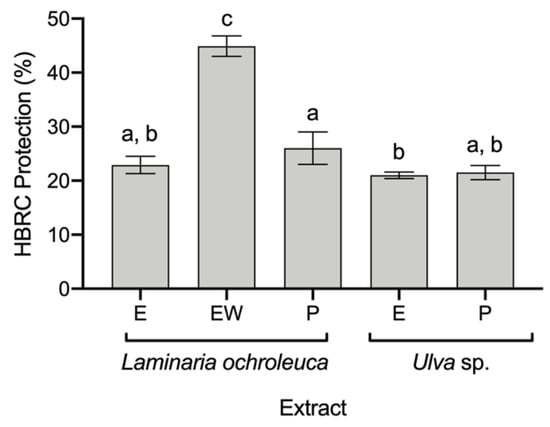
Figure 2.
Anti-inflammatory capacity of seaweed extracts in terms of their protection of human red blood cells (HRBCs) from thermal degradation (average ± standard deviation, n = 3). Extracts: ethanol (E), ethanol: water (EW), and polysaccharides (P). a–c Different letters above bars mean statistical differences (p < 0.05).
3.4. Biochemical Composition of Seaweed Extracts
To better understand the potential of the seaweed extracts, their biochemical composition was determined (Table 2). For all of the different algal species, the extracts’ biochemical compositions followed a clear trend according to the solvent used in the extraction and its polarity: i.e., proteins and carbohydrates were better extracted with polar solvents, particularly with an aqueous counterpart, and lipids were better extracted with non-polar solvents, such as acetone and ethanol.

Table 2.
Biochemical composition of seaweed extracts (average ± standard deviation, n = 3). Extracts: acetone (A), ethanol (E), ethanol–water (EW), and polysaccharides (P). Bold letters indicate the highest value in each biochemical group in each species. a–i Lowercase letters indicate statistical differences between species for the same parameter (p < 0.05).
In terms of proteins, the EW system allowed the extraction of the highest content in all three species (ca. 13%DryExtract), while the carbohydrate content was higher in the polysaccharide-rich extract (P) at ca. 70%DryExtract. Although the total amount of lipids in the raw biomass was extremely low, the highest value was found in acetonic extracts.
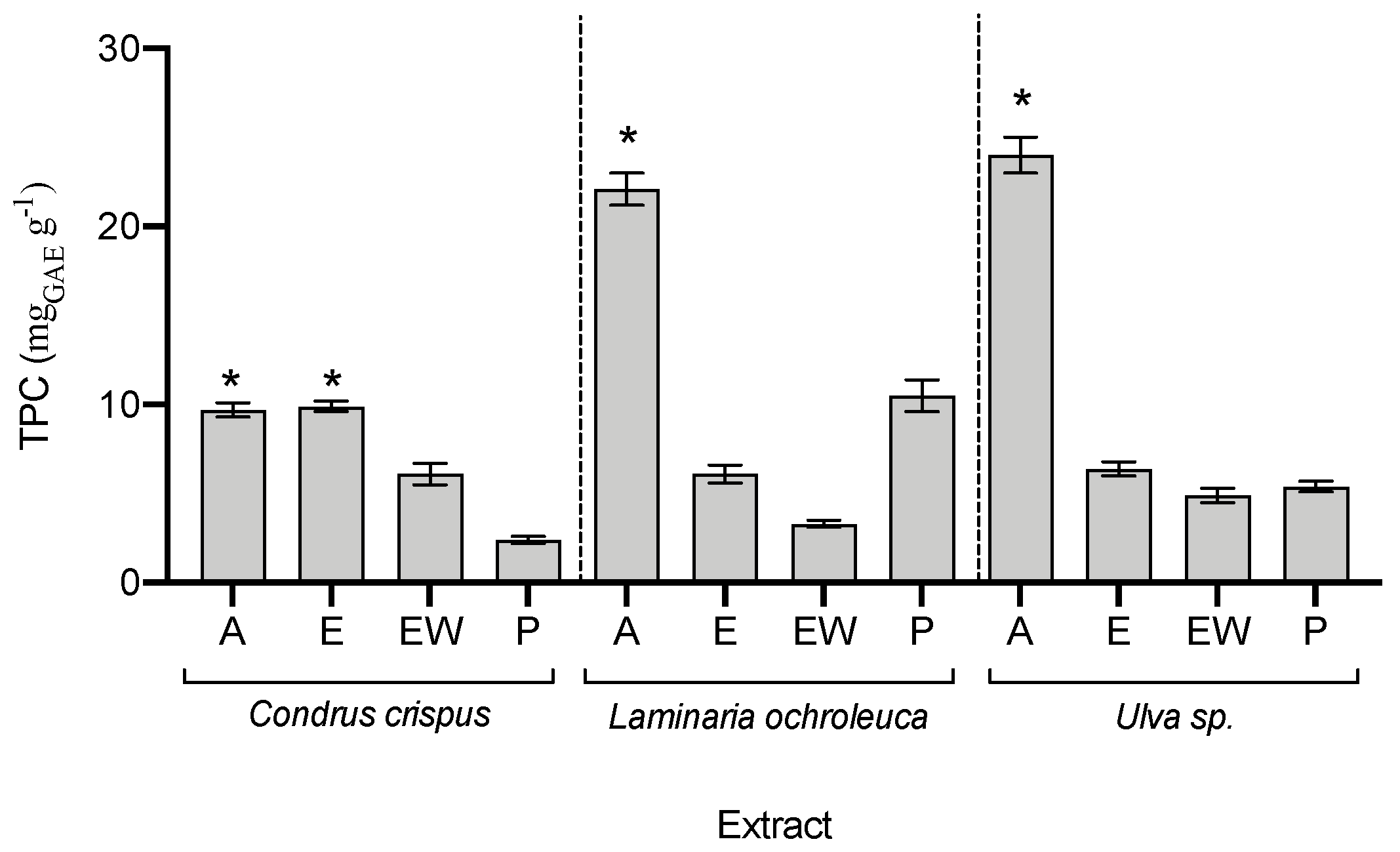
Regarding the phenolic compound content (Figure 3), acetonic extracts had the highest phenolic values in the three species, being two-fold higher than the content found in the other extracts. As phenolic compounds are usually associated with a high antioxidant capacity, a correlation was attempted with the five antioxidant assays; however, no correlation was found (p > 0.05).
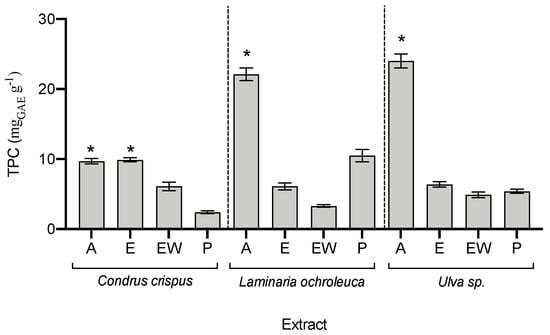
Figure 3.
Total phenolic compounds (TPC) in seaweed extracts (average ± standard deviation, n = 3). Extracts: acetone (A), ethanol (E), ethanol–water (EW), and polysaccharides (P). The asterisk symbol (*) indicates the highest value per species (p < 0.05).
3.5. Principal Component Analysis
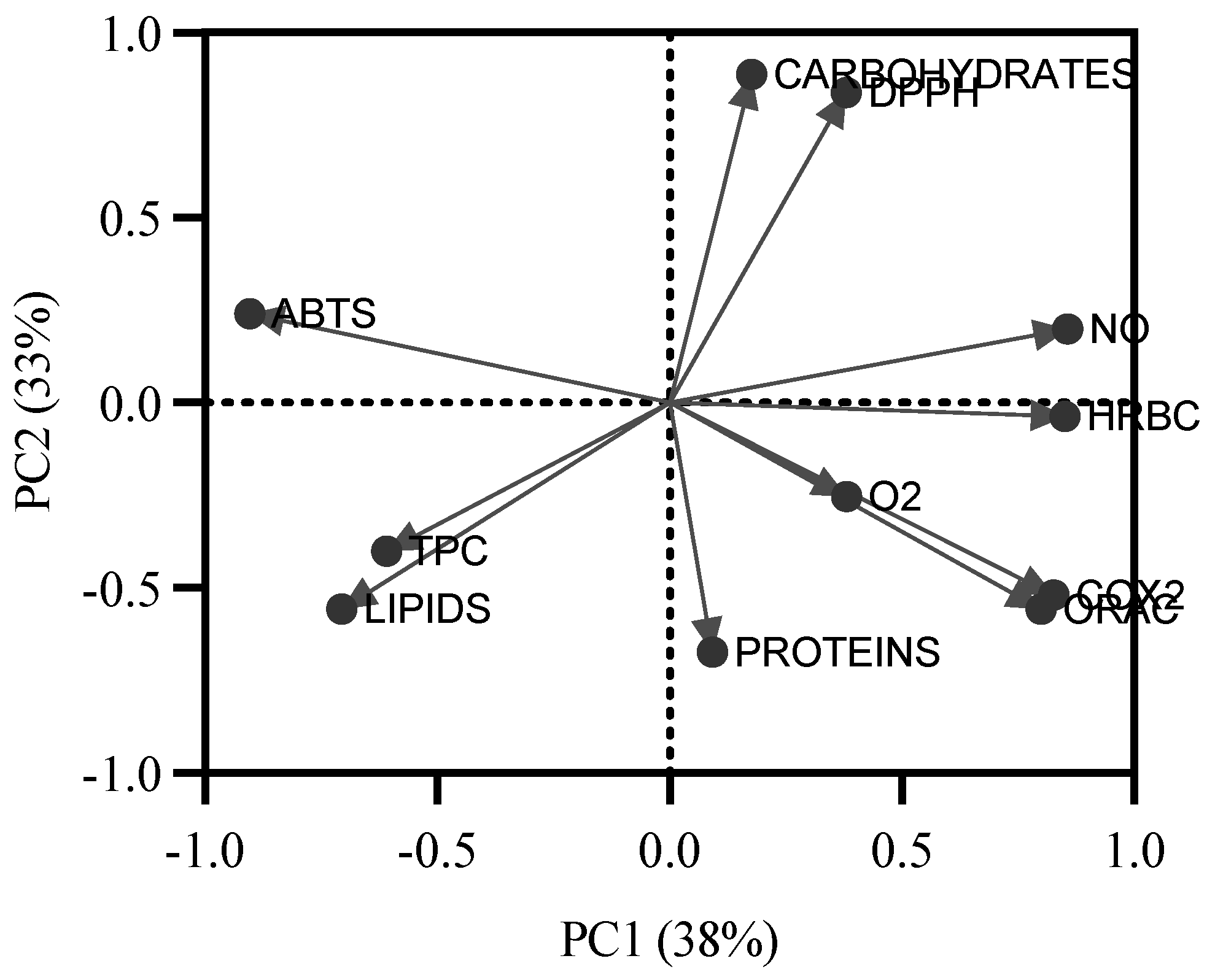
As no direct correlation was found between the biochemical profile and the bioactive potential, a principal component analysis was performed to gain a better understanding of the influence of biochemical groups on the bioactive capacity of the extracts (Figure 4). Two principal components explain 71% of the variation in the variables. The first principal component (PC1) explains 38% of the total variation, while the second (PC2) explains 33%. Based on the polarity of compounds, PC1 splits the data into lipophilic and hydrophilic compounds. Notably, most of the bioactive potential is related to hydrophilic groups, while ABTS•+ antioxidant potential would be more related to lipophilic compounds.
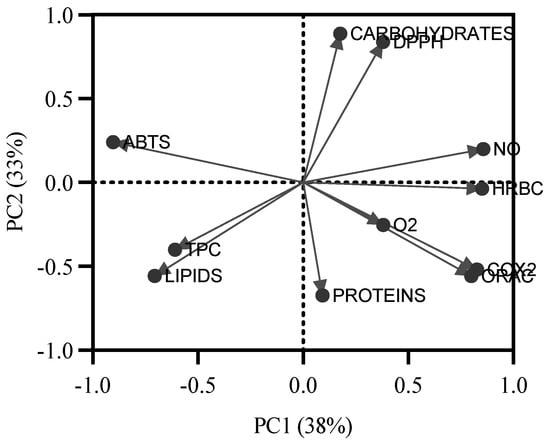
Figure 4.
Results of the principal component analysis applied to all values of antioxidant capacity of seaweed extracts (ABTS•+, DPPH•, •NO, O2•−, and ORAC-FL), anti-inflammatory capacity (COX-2 and HRBC), and the biochemical profile (proteins, carbohydrates, lipids, and total phenolic compounds (TPC)).
4. Discussion
As functional ingredients, extracts can be used in two different ways: as a source of a specific bioactive capacity or as a source of a specific compound (or a group of compounds). Here, both evaluations were considered: first, the antioxidant and anti-inflammatory capacity of the seaweed extracts, and second, the biochemical composition of the extracts.
The antioxidant potential of seaweeds has been proposed for a long time; for example, Yan et al. [25] screened the potential of 27 seaweeds from China in terms of DPPH• scavenging potential. The authors used successive solvent extraction (chloroform > ethyl acetate > acetone > methanol > water) to use the whole biomass. Similar to the present study, the potential of different extracts depended not only on the species but also on the solvent choice. Desmarestia viridis (Ochrophyta) was highlighted as holding a high antioxidant potential in methanol, acetone, and ethyl acetate extracts, although it showed almost no activity in the chloroform fraction.
As mentioned before, the evaluation of antioxidant capacity should consider different mechanisms of action and groups of compounds. As extracts are complex matrices, the synergetic effect of molecules also dramatically impacts the final bioactive capacity. While ABTS•+, DPPH•, O2•−, and •NO assays are based on scavenging capacity, ORAC-FL is based on the inhibition of peroxyl-radical-induced oxidation and the protection of the degraded compound.
Special attention must be given to L. ochroleuca EW extract. Its great potential was revealed in total antioxidant assays, namely, the ABTS•+, DPPH•, and ORAC-FL assays, which revealed an antioxidant capacity of up to 5-fold higher than the second-best extract. This may indicate its great potential to be used to replace synthetic food preservatives, particularly in the beverage segment [24]. In addition, the interesting results of L. ochroleuca EW extract in O2•− and •NO radical tests indicate that its application as a component in food or beverage compositions could be polyvalent.
The bioactive potential of L. ochroleuca has been exploited before, as it contains a diverse composition of phenolic compounds, amino acids, pigments, and polysaccharides in a special fucoidan extract [26]. L. ochroleuca extracts have already been associated with depressant and analgesic effects on the central nervous system [27], hypoglycaemic capacity [28], and anti-inflammatory [29], antioxidant, and anti-tumour properties [26].
The EW extract may have an extra advantage in extracting both hydrophilic and lipophilic compounds. In comparison to other extracts of Laminaria species, the results of antioxidant capacity obtained in this study with EW extracts of L. ochroleuca seem to be higher than (for ABTS•+ and ORAC assays) or comparable to (DPPH• assay) others reported before, as observed in Table 3. It also must be noted that the anti-inflammatory values obtained for both assays are not very different from other specific extracts (Table 3). Hence, as in this study, a potential synergetic effect of these compounds may have contributed to the increased antioxidant potential of EW extract.

Table 3.
Comparison of the obtained antioxidant (mgTE g−1) and anti-inflammatory (% of inhibition) results of EW extracts of L. ochroleuca, marked in shade, with other reported values of Laminaria species or brown algae.
On the other hand, when it comes to specific radicals, such as O2•− and •NO, polysaccharide extracts showed the highest potential, regardless of the species. In particular, C. crispus P revealed its high potential; it was the best in scavenging •NO and, together with L. ochroleuca P, the best in scavenging O2•−. In addition, polysaccharides from seaweeds have been associated with anticoagulant, antithrombotic capacity, antimicrobial, anti-inflammatory, and antioxidant potential [26].
Regarding the anti-inflammatory capacity of extracts, only a few extracts had a protective effect in the HRBC assay. L. ochroleuca EW was shown to be the best extract, and its potential was evaluated for a more specific mechanism of action: the inhibition of the COX-2 enzyme. COX enzymes are involved in the production of prostaglandins and the induction of inflammatory reactions, and inhibiting these enzymes can alleviate symptoms such as pain and fever [34]. L. ochroleuca EW inhibited the enzyme’s activity by 30%, which indicates a potential that should be explored in more detail.
Inflammation is a protective reaction to an injury caused by physical trauma, toxic substances, or pathogens. Anti-inflammatory mechanisms comprise enzymes and signalling transcription factors. COX-1 and COX-2 enzymes have been extensively studied in inflammatory disorders and have been associated with illness incidence [35]. Moreover, lysosomal enzymes are released during the inflammatory process, causing tissue damage. These enzymes’ extracellular activity is thought to be linked to acute or chronic inflammation. Lysosomal membrane stabilisation is vital in regulating the inflammatory response by restricting the release of lysosomal components. The HRBC membrane is comparable to the lysosomal membrane, and the extract’s stability implies that lysosomal membranes will be stabilised [36].
The anti-inflammatory effect of a Laminaria species was described by Lee et al. [37] in a cell line study. The authors used Laminaria japonica aqueous extract to inhibit UVB-induced inflammation in the human keratinocyte (HaCat) cell line. The extract acted by maintaining the levels of nc 886 gene expression, subsequently inhibiting the production of the inflammatory response.
Regarding the nutritional composition of raw biomass, the species Ulva sp. and C. crispus were shown to be great sources of protein (25%), while L. ochroleuca could be used as a source of carbohydrates (47%) and minerals due to its ash content (38%), mainly containing twice the value reported before for this species [38]. However, the high ash content in Laminaria species has been reported before [39], where Laminaria digitata also showed 38%, suggesting the use of this seaweed as a food supplement of some mineral trace elements.
The biochemical profile of the extracts was aimed not only at providing insight into their nutritional values but also a correlating some groups of compounds and their bioactive potential, as many studies have reported a positive correlation between antioxidant capacity and total phenolic compounds. However, in this study, the extracts with higher phenolic contents (A extracts) did not show great potential, except for L. ochroleuca A in the DPPH• assay. This poor correlation was also found by Heo et al. [40], who evaluated different enzymatic extracts from seven brown seaweeds from Korea. The authors found that extracts from the same species with similar contents of phenolic compounds had completely different antioxidant capacities, and they proposed that other compounds, such as low-molecular-weight polysaccharides, proteins, or peptides, may influence the antioxidant potential, which can be associated with the results observed in the principal component analysis, which related hydrophilic compounds to most of the bioactive assays. Barbosa et al. [21] evaluated the fatty acid profile of L. ochroleuca from Northern Portugal, indicating a possible similarity to the one used in the present study. Although present at a low concentration, this seaweed was shown to have polyunsaturated fatty acids (PUFAs) with known antioxidant capacity, such as linoleic acid, γ-linolenic acid, and EPA (eicosapentaenoic acid), ranging from 70 to 160 mg kg DW−1.
Furthermore, in terms of application, seaweed extracts have been attracting the attention of the natural cosmetic industry, and many claims are already associated with extracts and raw biomass used in cosmetic formulations, including antioxidant, anti-ageing, photoprotective, and anti-inflammatory effects, among others [23]. Thus, in addition to nutraceutical applications, the extract evaluated here can also be used in other industries, such as cosmeceutical (cosmetic with nutraceutical potential).
5. Conclusions
Chondrus crispus, Laminaria ochroleuca, and Ulva sp. showed the potential to be exploited in the food and food supplement markets. While Ulva sp. and C. crispus showed great potential to be used as a source of protein, L. ochroleuca could be used as a source of carbohydrates and minerals.
In terms of extracts as functional ingredients, five extracts (L. ochroleuca E, EW, and P and Ulva sp. E and P) showed anti-inflammatory potential, thus revealing their potential for nutraceutical applications. L. ochroleuca EW extract is highlighted due to its great polyvalent potential. In addition to its great total antioxidant capacity, indicative of its high potential to replace synthetic food preservatives, particularly in the food and beverage segment, it was also revealed to have antioxidant potential against O2•− and •NO radicals and anti-inflammatory potential in the inhibition of COX-2. Hence, L. ochroleuca seems to be a promising seaweed to be exploited due to its polyvalent potential for application as (i) a nutrient-rich ingredient, particularly in minerals, (ii) a source of nutraceutical ingredients due to its antioxidant and anti-inflammatory potential, and (iii) a source of antioxidant compounds to be used as food preservatives.
Author Contributions
Conceptualisation, H.M.A., F.P., and A.C.G.; data curation, H.M.A., F.P., and I.C.; formal analysis, H.M.A. and T.G.T.; funding acquisition, I.S.-P. and A.C.G.; investigation, H.M.A., F.P., T.G.T., and I.C.; methodology, H.M.A. and F.P.; resources, I.S.-P. and A.C.G.; supervision, A.C.G.; writing—original draft, H.M.A. and F.P.; writing—review and editing, A.C.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was co-supported by a Ph.D. fellowship granted to author F.P. (SFRH/BD/136767/2018) funded by the Foundation for Science and Technology (FCT, Portugal) under the auspices of Operational Program Human Capital (POCH), supported by the European Social Fund and Portuguese funds (MECTES), as well as by national funds through FCT within the scope of ZEBRALGRE (PTDC/CVT-WEL/5207/2014), UIDB/04423/2020, and UIDP/04423/2020 for CIIMAR and UIDB/00511/2020 for LEPABE.
Institutional Review Board Statement
The study was conducted according to the guidelines of theDeclaration of Helsinki, and approved by the Institutional Ethics Committee of CIIMAR (protocolcode 001/2020 and date of approval 8 June 2020).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mohamed, S.; Hashim, S.N.; Rahman, H.A. Seaweeds: A Sustainable Functional Food for Complementary and Alternative Therapy. Trends Food Sci. Technol. 2012, 23, 83–96. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive Compounds in Seaweed: Functional Food Applications and Legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Bakir, S.; Catalkaya, G.; Ceylan, F.D.; Khan, H.; Guldiken, B.; Capanoglu, E.; Kamal, M.A. Role of Dietary Antioxidants in Neurodegenerative Diseases: Where Are We Standing? Curr. Pharm. Des. 2020, 26, 714–729. [Google Scholar] [CrossRef] [PubMed]
- Dellarosa, N.; Laghi, L.; Martinsdóttir, E.; Jónsdóttir, R.; Sveinsdóttir, K. Enrichment of Convenience Seafood with Omega-3 and Seaweed Extracts: Effect on Lipid Oxidation. LWT—Food Sci. Technol. 2015, 62, 746–752. [Google Scholar] [CrossRef]
- Corsetto, P.A.; Montorfano, G.; Zava, S.; Colombo, I.; Ingadottir, B.; Jonsdottir, R.; Sveinsdottir, K.; Rizzo, A.M. Characterization of Antioxidant Potential of Seaweed Extracts for Enrichment of Convenience Food. Antioxidants 2020, 9, 249. [Google Scholar] [CrossRef]
- Balboa, E.M.; Conde, E.; Moure, A.; Falqué, E.; Domínguez, H. In Vitro Antioxidant Properties of Crude Extracts and Compounds from Brown Algae. Food Chem. 2013, 138, 1764–1785. [Google Scholar] [CrossRef]
- Tenorio-Rodriguez, P.A.; Murillo-Álvarez, J.I.; Campa-Cordova, Á.I.; Angulo, C. Antioxidant Screening and Phenolic Content of Ethanol Extracts of Selected Baja California Peninsula Macroalgae. J. Food Sci. Technol. 2017, 54, 422–429. [Google Scholar] [CrossRef]
- Múzquiz de la Garza, A.R.; Tapia-Salazar, M.; Maldonado-Muñiz, M.; De La Rosa-Millán, J.; Gutiérrez-Uribe, J.A.; Santos-Zea, L.; Barba-Dávila, B.A.; Ricque-Marie, D.; Cruz-Suárez, L.E. Nutraceutical Potential of Five Mexican Brown Seaweeds. BioMed Res. Int. 2019, 2019, 3795160. [Google Scholar] [CrossRef]
- Melo, R.; Sousa-Pinto, I.; Antunes, S.C.; Costa, I.; Borges, D. Temporal and Spatial Variation of Seaweed Biomass and Assemblages in Northwest Portugal. J. Sea Res. 2021, 174, 102079. [Google Scholar] [CrossRef]
- Gaspar, R.; Pereira, L.; Sousa-Pinto, I. The Seaweed Resources of Portugal. Bot. Mar. 2019, 62, 499–525. [Google Scholar] [CrossRef]
- Hentati, F.; Delattre, C.; Ursu, A.V.; Desbrières, J.; Le Cerf, D.; Gardarin, C.; Abdelkafi, S.; Michaud, P.; Pierre, G. Structural Characterization and Antioxidant Activity of Water-Soluble Polysaccharides from the Tunisian Brown Seaweed Cystoseira Compressa. Carbohydr. Polym. 2018, 198, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Granados-Guzman, G.; Salazar-Aranda, R.; Garza-Tapia, M.; Castro-Rios, R.; Waksman de Torres, N. Optimization and Validation of Two High-Throughput Methods Indicating Antiradical Activity. Curr. Anal. Chem. 2017, 13, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Bobo-García, G.; Davidov-Pardo, G.; Arroqui, C.; Vírseda, P.; Marín-Arroyo, M.R.; Navarro, M. Intra-Laboratory Validation of Microplate Methods for Total Phenolic Content and Antioxidant Activity on Polyphenolic Extracts, and Comparison with Conventional Spectrophotometric Methods. J. Sci. Food Agric. 2015, 95, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Pinho, B.R.; Sousa, C.; Valentão, P.; Andrade, P.B. Is Nitric Oxide Decrease Observed with Naphthoquinones in LPS Stimulated RAW 264.7 Macrophages a Beneficial Property? PLoS ONE 2011, 6, e24098. [Google Scholar] [CrossRef]
- Dávalos, A.; Gómez-Cordovés, C.; Bartolomé, B. Extending Applicability of the Oxygen Radical Absorbance Capacity (ORAC-Fluorescein) Assay. J. Agric. Food Chem. 2004, 52, 48–54. [Google Scholar] [CrossRef]
- Moualek, I.; Iratni Aiche, G.; Mestar Guechaoui, N.; Lahcene, S.; Houali, K. Antioxidant and Anti-Inflammatory Activities of Arbutus Unedo Aqueous Extract. Asian Pac. J. Trop. Biomed. 2016, 6, 937–944. [Google Scholar] [CrossRef]
- Bradford, M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J Biol Chem 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Magalhães, L.M.; Santos, F.; Segundo, M.A.; Reis, S.; Lima, J.L.F.C. Rapid Microplate High-Throughput Methodology for Assessment of Folin-Ciocalteu Reducing Capacity. Talanta 2010, 83, 441–447. [Google Scholar] [CrossRef]
- Barbosa, M.; Fernandes, F.; Pereira, D.M.; Azevedo, I.C.; Sousa-Pinto, I.; Andrade, P.B.; Valentão, P. Fatty Acid Patterns of the Kelps Saccharina Latissima, Saccorhiza Polyschides and Laminaria Ochroleuca: Influence of Changing Environmental Conditions. Arab. J. Chem. 2020, 13, 45–58. [Google Scholar] [CrossRef]
- Pashkow, F.J.; Watumull, D.G.; Campbell, C.L. Astaxanthin: A novel potential treatment for oxidative stress and inflammation in cardiovascular disease. Am. J. Cardiol. 2008, 101, 58d–68d. [Google Scholar] [CrossRef] [PubMed]
- Morais, T.; Cotas, J.; Pacheco, D.; Pereira, L. Seaweeds Compounds: An Ecosustainable Source of Cosmetic Ingredients? Cosmetics 2021, 8, 8. [Google Scholar] [CrossRef]
- Bank, G.; Schauss, A. Antioxidant Testing: An ORAC Update. Nutraceuticals World 2004, 7, 2003–2004. [Google Scholar]
- Yan, X.; Nagata, T.; Fan, X. Antioxidative Activities in Some Common Seaweeds. Plant Foods Hum. Nutr. 1998, 52, 253–262. [Google Scholar] [CrossRef]
- Flórez-Fernández, N.; Torres, M.D.; González-Muñoz, M.J.; Domínguez, H. Recovery of Bioactive and Gelling Extracts from Edible Brown Seaweed Laminaria Ochroleuca by Non-Isothermal Autohydrolysis. Food Chem. 2019, 277, 353–361. [Google Scholar] [CrossRef]
- Vázquez-Freire, M.J.; Lamela, M.; Calleja, J.M. Laminaria Ochroleuca: A Preliminary Study of Its Effect on the Central Nervous System. Phytother. Res. 1994, 8, 422–425. [Google Scholar] [CrossRef]
- Lamela, M.; Anca, J.; Villar, R.; Otero, J.; Calleja, J.M. Hypoglycemic Activity Op Several Seaweed Extracts. J. Ethnopharmacol. 1989, 27, 35–43. [Google Scholar] [CrossRef]
- Bonneville, M.; Saint-Mezard, P.; Benetiere, J.; Hennino, A.; Pernet, I.; Denis, A.; Nicolas, N.F. Laminaria Ochroleuca Extract Reduces Skin Inflammation. J. Eur. Acad. Dermatol. Venereol. 2007, 21, 1124–1125. [Google Scholar] [CrossRef]
- Ummat, V.; Tiwari, B.K.; Jaiswal, A.K.; Condon, K.; Garcia-Vaquero, M.; O’Doherty, J.; O’Donnell, C.; Rajauria, G. Optimisation of Ultrasound Frequency, Extraction Time and Solvent for the Recovery of Polyphenols, Phlorotannins and Associated Antioxidant Activity from Brown Seaweeds. Mar. Drugs 2020, 18, 250. [Google Scholar] [CrossRef]
- Cui, C.; Lu, J.; Sun-Waterhouse, D.; Mu, L.; Sun, W.; Zhao, M.; Zhao, H. Polysaccharides from Laminaria japonica: Structural characteristics and antioxidant activity. LWT 2016, 73, 602–608. [Google Scholar] [CrossRef]
- Ahmad, T.; Eapen, M.S.; Ishaq, M.; Park, A.Y.; Karpiniec, S.S.; Stringer, D.N.; Sohal, S.S.; Fitton, J.H.; Guven, N.; Caruso, V.; et al. Anti-Inflammatory Activity of Fucoidan Extracts In Vitro. Mar. Drug 2021, 19, 702. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Dissanayake, A.A.; Xiao, C.; Gao, J.; Zhao, M.; Nair, M.G. The edible seaweed Laminaria japonica contains cholesterol analogues that inhibit lipid peroxidation and cyclooxygenase enzymes. PLoS ONE 2022, 17, e0258980. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.D.; Busquets-Cortés, C.; Capó, X.; Tejada, S.; Tur, J.A.; Pons, A.; Sureda, A. Cyclooxygenase-2 Inhibitors as a Ther-apeutic Target in Inflammatory Diseases. Curr. Med. Chem. 2018, 26, 3225–3241. [Google Scholar] [CrossRef]
- Adnan, A.Z.; Armin, F.; Sudji, I.R.; Novida, M.D.; Roesma, D.I.; Ali, H.A.; Fauzana, A. In Vitro Anti-Inflammatory Activity Test of Tinocrisposide and Freeze-Dried Aqueous Extract of Tinospora Crispa Stems on Human Red Blood Cell by Increasing Membrane Stability Experiment. Asian J. Pharm. Clin. Res. 2019, 12, 125–129. [Google Scholar] [CrossRef]
- Chippada, S.C.; Volluri, S.S.; Bammidi, S.R.; Vangalapati, M. In Vitro Anti Inflammatory Activity of Methanolic Extract of Centella Asiatica by HRBC Membrane Stabilisation. Rasayan J. Chem. 2011, 4, 457–460. [Google Scholar]
- Lee, K.S.; Cho, E.; Weon, J.B.; Park, D.; Fréchet, M.; Chajra, H.; Jung, E. Inhibition of UVB-Induced Inflammation by Laminaria Japonica Extract via Regulation of Nc886-PKR Pathway. Nutrients 2020, 12, 1958. [Google Scholar] [CrossRef]
- Pacheco, D.; Miranda, G.; Rocha, C.P.; Pato, R.L.; Cotas, J.; Gonçalves, A.M.M.; Dias Santos, S.M.; Bahcevandziev, K.; Pereira, L. Portuguese Kelps: Feedstock Assessment for the Food Industry. Appl. Sci. 2021, 11, 10681. [Google Scholar] [CrossRef]
- Rupérez, P. Mineral Content of Edible Marine Seaweeds. Food Chem. 2002, 79, 23–26. [Google Scholar] [CrossRef]
- Heo, S.J.; Park, E.J.; Lee, K.W.; Jeon, Y.J. Antioxidant Activities of Enzymatic Extracts from Brown Seaweeds. Bioresour. Technol. 2005, 96, 1613–1623. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).