Small Indigenous Fish: A Potential Source of Valuable Nutrients in the Context of Bangladesh
Abstract
:1. Introduction
2. Methodology
2.1. Search Strategy
2.2. Selection Process and Data Extraction
3. Results and Discussion
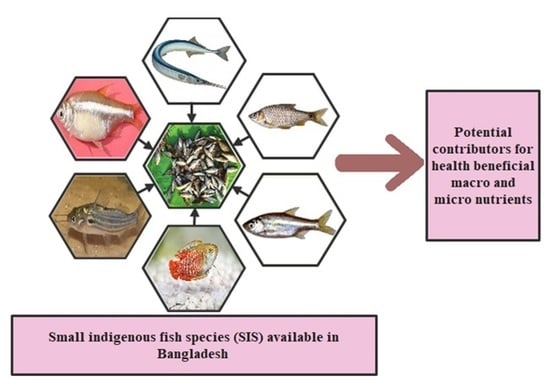
3.1. Small Indigenous Fish Species (SIS)
3.2. SIS Resources and Their Micronutrients
| Fish Species | Amino Acids | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Asp | Thr | Ser | Glu | Pro | Gly | Ala | Val | Cys | Met | Iso | Leu | Tyr | His | Lys | Arg | Try | Phe | |
| A. mola | 9.82 | 5.72 | 6.68 | 16.31 | 0.38 | 13.74 | 10.50 | 0.84 | 3.15 | 1.72 | 5.45 | 9.62 | 1.39 | 4.41 | 5.17 | 1.87 | 1.73 | 1.5 |
| P. sarana | 9.63 | 4.79 | 3.48 | 20.31 | 4.61 | 4.47 | 6.47 | 5.21 | 0.80 | 1.83 | 3.07 | 8.05 | 2.58 | 1.21 | 11.17 | 5.66 | 1.13 | - |
| H. chela | 6.33 | 4.29 | 2.41 | 10.79 | 3.86 | 4.74 | 4.47 | 4.07 | 0.50 | 1.34 | 4.56 | 6.92 | 1.84 | 4.86 | 10.98 | 2.78 | 1.38 | 3.84 |
| C. phulo | 3.78 | 1.87 | 1.40 | 6.96 | 2.25 | 2.99 | 2.93 | 2.50 | 0.31 | 1.46 | 2.35 | 3.51 | 1.84 | 1.03 | 4.13 | 3.20 | - | 2.07 |
| Ambassis spp. | 9.52 | 3.23 | 2.34 | 14.88 | 3.29 | 3.31 | 4.39 | 4.48 | 0.74 | 2.05 | 4.22 | 7.05 | 4.81 | 3.30 | 11.30 | 6.21 | 1.12 | - |
| P. stigma | 2.80 | 1.68 | 1.30 | 5.76 | 2.31 | 3.22 | 2.88 | 2.24 | 0.24 | 1.22 | 2.02 | 3.00 | 1.60 | 1.11 | 3.36 | 2.71 | - | 1.85 |
| C. striatus | 10.74 | 4.24 | 3.60 | 21.6 | 4.0 | 3.75 | 5.49 | 5.54 | 2.40 | 2.47 | 4.50 | 8.76 | 1.90 | 3.16 | 13.26 | 4.87 | - | 2.91 |
| G. chapra | 3.53 | 1.93 | 1.43 | 6.72 | 2.30 | 3.22 | 3.03 | 2.64 | 0.26 | 1.49 | 2.31 | 3.48 | 1.81 | 1.08 | 4.10 | 3.17 | - | 2.13 |
| O. niloticus | 12.91 | 5.32 | 4.05 | 17.05 | 4.07 | 6.68 | 7.36 | 5.81 | 0.84 | 2.97 | 6.58 | 9.83 | 1.47 | 2.53 | 15.76 | 5.62 | - | 3.10 |
3.3. Vitamins
3.3.1. Vitamin A
3.3.2. Vitamin B12
3.3.3. Vitamin D
3.3.4. Vitamin E
3.3.5. Vitamin B9 (Folate)
3.4. Minerals
3.4.1. Iron
3.4.2. Zinc
3.4.3. Calcium
3.4.4. Iodine
3.4.5. Selenium
3.4.6. Other Minerals
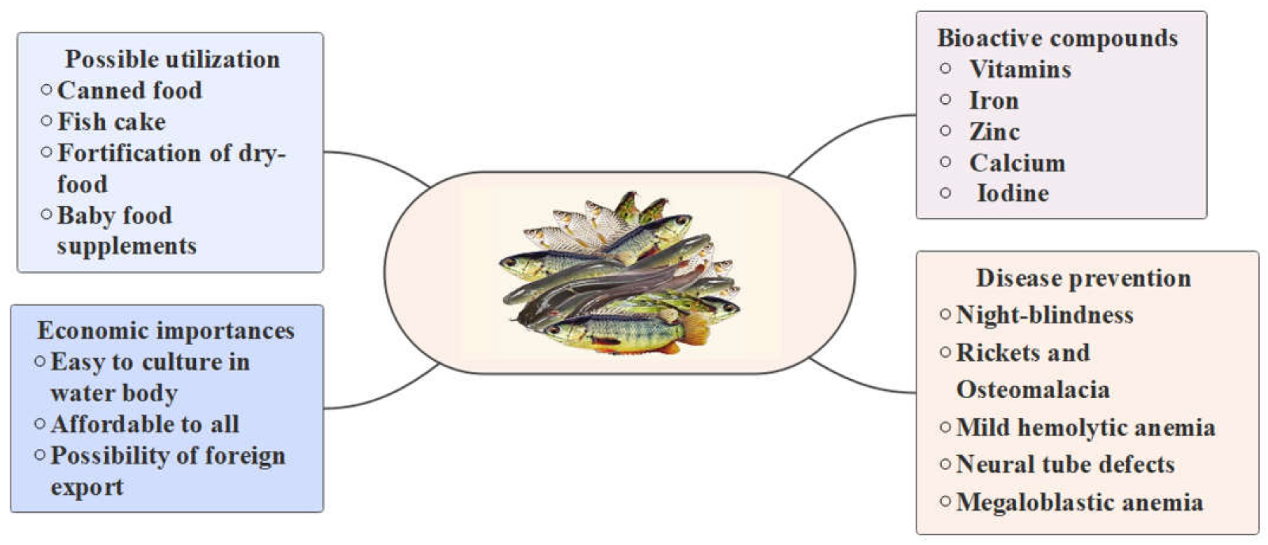
4. Possible Utilization of SIS in Future Foods
5. Implication of This Review Work
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aziz, M.S.B.; Hasan, N.A.; Mondol, M.M.R.; Alam, M.M.; Haque, M.M. Decline in fish species diversity due to climatic and anthropogenic factors in Hakaluki Haor, an ecologically critical wetland in northeast Bangladesh. Heliyon 2021, 7, e05861. [Google Scholar] [CrossRef] [PubMed]
- Kostori, F.A.; Parween, S.; Islam, M.N. Availability of small indigenous species (SIS) of fish in the Chalan Beel-the largest wetland of Bangladesh. Univ. J. Zool. Rajshahi Univ. 2011, 30, 67–72. [Google Scholar] [CrossRef]
- Roos, N.; Leth, T.; Jakobsen, J.; Thilsted, S.H. High vitamin A content in some small indigenous fish species in Bangladesh: Perspectives for food-based strategies to reduce vitamin A deficiency. Int. J. Food Sci. Nutr. 2002, 53, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Roos, N.; Mazharul Islam, M.; Thilsted, S.H. Small fish is an important dietary source of vitamin A and calcium in rural Bangladesh. Int. J. Food Sci. Nutr. 2003, 54, 329–339. [Google Scholar] [CrossRef]
- Roos, N.; Islam, M.M.; Thilsted, S.H. Small indigenous fish species in Bangladesh: Contribution to vitamin A, calcium and iron intakes. J. Nutr. 2003, 133, 4021S–4026S. [Google Scholar] [CrossRef]
- Bogard, J. The Contribution of Fish to Nutrition and Food Security: Informing the Evidence Base for Agricultural Policy in Bangladesh. Ph.D. Thesis, The University of Queensland, Brisbane, Australia, 3 November 2017. [Google Scholar]
- Belton, B.; van Asseldonk, I.J.M.; Thilsted, S.H. Faltering fisheries and ascendant aquaculture: Implications for food and nutrition security in Bangladesh. Food Policy 2014, 44, 77–87. [Google Scholar] [CrossRef]
- Thilsted, S. The potential of nutrient-rich small fish species in aquaculture to improve human nutrition and health. In Proceedings of the Global Conference on Aquaculture 2010, Phuket, Thailand, 22–25 September 2010; pp. 57–73. [Google Scholar]
- Belton, B.; Thilsted, S.H. Fisheries in transition: Food and nutrition security implications for the global South. Glob. Food Secur. 2014, 3, 59–66. [Google Scholar] [CrossRef]
- Kongsbak, K.; Thilsted, S.H.; Wahed, M.A. Effect of consumption of the nutrient-dense, freshwater small fish Amblypharyngodon mola on biochemical indicators of vitamin A status in Bangladeshi children: A randomised, controlled study of efficacy. Br. J. Nutr. 2008, 99, 581–597. [Google Scholar] [CrossRef]
- Thilsted, S.H.; Roos, N.; Hassan, N. The role of small indigenous fish species in food and nutrition security in Bangladesh. Naga ICLARM Q. 1997, 20, 82–84. [Google Scholar]
- Mohanty, B.; Pati, M.K.; Bhattacharjee, S.; Hajra, A.; Sharma, A. Small Indigenous Fishes and Their Importance in Human Health. In Advances in Fish Research; Narendra Publishing House: Delhi, India, 2013; pp. 257–278. [Google Scholar]
- Tacon, A.G.; Metian, M. Fish matters: Importance of aquatic foods in human nutrition and global food supply. Rev. Fish. Sci. 2013, 21, 22–38. [Google Scholar] [CrossRef]
- Faridullah, M.; Roy, V.C.; Begam, H.; Nushy, N.H.; Ashrafullah, M.; Talha, M.A.; Rana, M.M. Assessment of Physicochemical, Nutritional and Biochemical Properties of “Shidal” Processed from Two Different Species of SIS Available in Bangladesh. Middle East J. Sci. Res. 2022, 30, 40–48. [Google Scholar]
- Mazumder, D.; Lorenzen, K. Developing aquaculture of small native species (SNS) in Bangladesh: Village level agroecological change and the availability of SNS. Naga ICLARM Q. 1999, 22, 20–23. [Google Scholar]
- Bayen, S.; Sinha, A.; Aftabuddin, M.; Roy, A.; Parida, P.; Ghosh, A.; Das, B. Developing mola (Amblypharyngodon mola) based fish culture practices for addressing livelihood and nutritional security of rural populace of Indian Sundarban. J. Entomol. Zool. Stud. 2020, 8, 7880. [Google Scholar]
- Roos, N.; Wahab, M.A.; Chamnan, C.; Thilsted, S.H. The role of fish in food-based strategies to combat vitamin A and mineral deficiencies in developing countries. J. Nutr. 2007, 137, 1106–1109. [Google Scholar] [CrossRef]
- Siekmann, J.H.; Allen, L.H.; Bwibo, N.O.; Demment, M.W.; Murphy, S.P.; Neumann, C.G. Animal source foods to improve micronutrient nutrition and human function in developing countries. J. Nutr. 2003, 133, 3972S–3980S. [Google Scholar] [CrossRef]
- Dey, M.M.; Surathkal, P. Value chains in aquaculture and fisheries in Bangladesh. In Transforming Agriculture in South Asia; Routledge: Abingdon-on-Thames, UK, 2020; pp. 195–210. [Google Scholar]
- Banna, M.H.A.; Al Zaber, A.; Rahman, N.; Siddique, M.A.M.; Siddique, M.A.B.; Hagan, J.E., Jr.; Rifat, M.; Nsiah-Asamoah, C.N.A.; Seidu, A.-A.; Ahinkorah, B.O. Nutritional Value of Dry Fish in Bangladesh and Its Potential Contribution to Addressing Malnutrition: A Narrative Review. Fishes 2022, 7, 240. [Google Scholar] [CrossRef]
- Bogard, J.R.; Thilsted, S.H.; Marks, G.C.; Wahab, M.A.; Hossain, M.A.; Jakobsen, J.; Stangoulis, J. Nutrient composition of important fish species in Bangladesh and potential contribution to recommended nutrient intakes. J. Food Compos. Anal. 2015, 42, 120–133. [Google Scholar] [CrossRef]
- Khalili Tilami, S.; Sampels, S. Nutritional value of fish: Lipids, proteins, vitamins, and minerals. Rev. Fish. Sci. Aquac. 2018, 26, 243–253. [Google Scholar] [CrossRef]
- Hossain, M.; Ahmed, Z.; Leunda, P.; Roksanul Islam, A.; Jasmine, S.; Oscoz, J.; Miranda, R.; Ohtomi, J. Length–weight and length–length relationships of some small indigenous fish species from the Mathabhanga River, southwestern Bangladesh. J. Appl. Ichthyol. 2006, 22, 301–303. [Google Scholar] [CrossRef]
- Saha, M.K.; Barman, B.K. A Strategy on increase production and marketing of Mola and other Small Indigenous Species of Fish (SIS) in Bangladesh. In The WorldFish Center Working Papers; WorldFish: Dhaka, Bangladesh, 2020. [Google Scholar]
- Dey, M.M.; Garcia, Y.T.; Praduman, K.; Piumsombun, S.; Haque, M.S.; Li, L.; Radam, A.; Senaratne, A.; Khiem, N.T.; Koeshendrajana, S. Demand for fish in Asia: A cross-country analysis. Aust. J. Agric. Resour. Econ. 2008, 52, 321–338. [Google Scholar] [CrossRef]
- Nurhasan, M.; Maehre, H.K.; Malde, M.K.; Stormo, S.K.; Halwart, M.; James, D.; Elvevoll, E.O. Nutritional composition of aquatic species in Laotian rice field ecosystems. J. Food Compos. Anal. 2010, 23, 205–213. [Google Scholar] [CrossRef]
- Burke, T.J.; Segrin, C. Examining diet- and exercise-related communication in romantic relationships: Associations with health behaviors. Health Commun. 2014, 29, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Thilsted, S.H.; Thorne-Lyman, A.; Webb, P.; Bogard, J.R.; Subasinghe, R.; Phillips, M.J.; Allison, E.H. Sustaining healthy diets: The role of capture fisheries and aquaculture for improving nutrition in the post-2015 era. Food Policy 2016, 61, 126–131. [Google Scholar] [CrossRef]
- Aditya, G.; Pal, S.; Saha, G. An assessment of fish species assemblages in rice fields in West Bengal, India: Implications for management. J. Appl. Ichthyol. 2010, 26, 535–539. [Google Scholar] [CrossRef]
- Manna, R.; Bhattacharjya, B. Incorporation of new construction material into indigenous technological knowledge—A case study of V shaped fish trap of eastern India. Indian J. Tradit. Knowl. 2009, 8, 548–550. [Google Scholar]
- Samajdar, I.; Saikia, S.K. Traditional fishing gears of Birbhum district, West Bengal, India. Indian J. Tradit. Knowl. 2014, 13, 187–194. [Google Scholar]
- Hossain, M.Y. Morphometric relationships of length-weight and length-length of four Cyprinid small indigenous fish species from the Padma River (NW Bangladesh). Turk. J. Fish. Aquat. Sci. 2010, 10, 131–134. [Google Scholar] [CrossRef]
- Kohinoor, A.; Wahab, M.; Islam, M.; Thilsted, S. Culture potentials of mola (Amblypharyngodon mola), chela (Chela cachius) and punti (Puntius sophore) under monoculture system. Bangladesh J. Fish Res. 2001, 5, 123–134. [Google Scholar]
- Wahab, M.A.; Kunda, M.; Azim, M.E.; Dewan, S.; Thilsted, S.H. Evaluation of freshwater prawn-small fish culture concurrently with rice in Bangladesh. Aquac. Res. 2008, 39, 1524–1532. [Google Scholar] [CrossRef]
- Kunda, M.; Azim, M.E.; Wahab, M.A.; Dewan, S.; Roos, N.; Thilsted, S.H. Potential of mixed culture of freshwater prawn (Macrobrachium rosenbergii) and self-recruiting small species mola (Amblypharyngodon mola) in rotational rice–fish/prawn culture systems in Bangladesh. Aquac. Res. 2008, 39, 506–517. [Google Scholar] [CrossRef]
- Sinha, A.; Gogoi, P.; Dam Roy, S. Small Indigenous Fish (SIF): Status and Contributions in Nutrition and Livelihood Security of India: A Review. Agric. Rev. 2022. [Google Scholar] [CrossRef]
- Gross, R.; Schoeneberger, H.; Pfeifer, H.; Preuss, H.-J. The four dimensions of food and nutrition security: Definitions and concepts. SCN News 2000, 20, 20–25. [Google Scholar]
- Gopakumar, K. Biochemical Composition of Indian Food Fish; Central Institute of Fisheries Technology: Cochin, India, 1997. [Google Scholar]
- Vilain, C.; Baran, E.; Gallego, G.; Samadee, S. Fish and the nutrition of rural Cambodians. Asian J. Agric. Food Sci. 2016, 4, 26–34. [Google Scholar]
- Negesse, T.; Tera, A. Effects of feeding different levels of cooked and sun dried fish offal on carcass traits of growing Rhode Island Red chicks. Trop. Anim. Health Prod. 2010, 42, 45–54. [Google Scholar] [CrossRef]
- Shantz, E.M.; Brinkman, J.H. Biological activity of pure vitamin A2. J. Biol. Chem. 1950, 183, 467–471. [Google Scholar] [CrossRef]
- Grases, F.; Simonet, B.M.; Prieto, R.M.; March, J.G. Dietary phytate and mineral bioavailability. J. Trace Elem. Med. Biol. 2001, 15, 221–228. [Google Scholar] [CrossRef]
- Larsen, T.; Thilsted, S.H.; Kongsbak, K.; Hansen, M. Whole small fish as a rich calcium source. Br. J. Nutr. 2000, 83, 191–196. [Google Scholar] [CrossRef]
- Park, J.S.; Kim, S.Y.; Lee, S.C.; Jeong, Y.R.; Roy, V.C.; Rizkyana, A.D.; Chun, B.S. Edible oil extracted from anchovies using supercritical CO2: Availability of fat-soluble vitamins and comparison with commercial oils. J. Food Process. Preserv. 2021, 45, e15441. [Google Scholar] [CrossRef]
- West, K.P., Jr. Extent of vitamin A deficiency among preschool children and women of reproductive age. J. Nutr. 2002, 132, 2857S–2866S. [Google Scholar] [CrossRef]
- Rice, A.L.; West, K.P., Jr.; Black, R.E. Vitamin A deficiency. In Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attributable to Selected Major Risk Factors; WHO: Geneva, Switzerland, 2004. [Google Scholar]
- Roos, N. Fish Consumption and Aquaculture in Rural Bangladesh: Nutritional Contribution and Production Potential of Culturing Small Indigenous Fish Species (SIS) in Pond Polyculture with Commonly Cultured Carps. Ph.D. Thesis, The Royal Veterinary and Agricultural University, Frederiksberg, Denmark, 2001. [Google Scholar]
- Suo, L.; VanBuren, C.; Hovland, E.D.; Kedishvili, N.Y.; Sundberg, J.P.; Everts, H.B. Dietary Vitamin A Impacts Refractory Telogen. Front. Cell Dev. Biol. 2021, 9, 571474. [Google Scholar] [CrossRef]
- De Benoist, B. Conclusions of a WHO Technical Consultation on folate and vitamin B12 deficiencies. Food Nutr. Bull. 2008, 29, S238–S244. [Google Scholar] [CrossRef] [PubMed]
- Huskisson, E.; Maggini, S.; Ruf, M. The role of vitamins and minerals in energy metabolism and well-being. J. Int. Med. Res. 2007, 35, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Lock, E.J.; Waagbø, R.; Wendelaar Bonga, S.; Flik, G. The significance of vitamin D for fish: A review. Aquac. Nutr. 2010, 16, 100–116. [Google Scholar] [CrossRef]
- Holick, M. A Millenium Perspective Vitamin D. J. Cell Biochem. 2003, 88, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Moneo, E.; Stigliano, S.; Hedström, A.; Kaczka, A.; Malvik, M.; Waldthaler, A.; Maisonneuve, P.; Simon, P.; Capurso, G. Deficiency of fat-soluble vitamins in chronic pancreatitis: A systematic review and meta-analysis. Pancreatology 2016, 16, 988–994. [Google Scholar] [CrossRef]
- Cavalier, L.; Ouahchi, K.; Kayden, H.J.; Di Donato, S.; Reutenauer, L.; Mandel, J.-L.; Koenig, M. Ataxia with isolated vitamin E deficiency: Heterogeneity of mutations and phenotypic variability in a large number of families. Am. J. Hum. Genet. 1998, 62, 301–310. [Google Scholar] [CrossRef]
- Roos, N.; Wahab, M.A.; Hossain, M.A.R.; Thilsted, S.H. Linking human nutrition and fisheries: Incorporating micronutrient-dense, small indigenous fish species in carp polyculture production in Bangladesh. Food Nutr. Bull. 2007, 28, S280–S293. [Google Scholar] [CrossRef]
- Shaalan, A.; El-Sherbiny, M.; El-Abaseri, T.; Shoaeir, M.; Abdel-Aziz, T.M.; Mohamed, M.I.; Zaitone, S.; Mohammad, H. Supplement with Calcium or Alendronate Suppresses Osteopenia Due to Long Term Rabeprazole Treatment in Female Mice: Influence on Bone TRAP and Osteopontin Levels. Front. Pharmacol. 2020, 11, 00583. [Google Scholar] [CrossRef]
- Wu, W.; He, L.; Liang, Y.; Yue, L.; Peng, W.; Jin, G.; Ma, M. Preparation process optimization of pig bone collagen peptide-calcium chelate using response surface methodology and its structural characterization and stability analysis. Food Chem. 2019, 284, 80–89. [Google Scholar] [CrossRef]
- Oatley, K. Fiction: Simulation of social worlds. Trends Cogn. Sci. 2016, 20, 618–628. [Google Scholar] [CrossRef]
- World Health Organization. Vitamin and Mineral Requirements in Human Nutrition; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Lall, S.P.; Kaushik, S.J. Nutrition and metabolism of minerals in fish. Animals 2021, 11, 2711. [Google Scholar] [CrossRef]
- Bilandžić, N.; Sedak, M.; Đokić, M.; Varenina, I.; Kolanović, B.S.; Božić, Đ.; Brstilo, M.; Šimić, B. Determination of zinc concentrations in foods of animal origin, fish and shellfish from Croatia and assessment of their contribution to dietary intake. J. Food Compos. Anal. 2014, 35, 61–66. [Google Scholar] [CrossRef]
- Rahman, S.; Ahmed, T.; Rahman, A.S.; Alam, N.; Ahmed, A.S.; Ireen, S.; Chowdhury, I.A.; Chowdhury, F.P.; Rahman, S.M. Status of zinc nutrition in Bangladesh: The underlying associations. J. Nutr. Sci. 2016, 5, e25. [Google Scholar] [CrossRef]
- Heard, M.; Chamberlain, A.; Sherlock, J. Uptake of lead by humans and effect of minerals and food. Sci. Total Environ. 1983, 30, 245–253. [Google Scholar] [CrossRef]
- Clase, C.M.; Ki, V.; Holden, R.M. Water-Soluble Vitamins in People with Low Glomerular Filtration Rate or On Dialysis: A Review. Semin. Dial. 2013, 26, 546–567. [Google Scholar] [CrossRef]
- Craviari, T.; Pettifor, J.M.; Thacher, T.D.; Meisner, C.; Arnaud, J.; Fischer, P.R.; Group, R.C. Rickets: An overview and future directions, with special reference to Bangladesh: A summary of the Rickets Convergence Group Meeting, Dhaka, 26–27 January 2006. J. Health Popul. Nutr. 2008, 26, 112. [Google Scholar]
- Fischer, P.; Rahman, A.; Cimma, J.-P.; Kyaw-Myint, T.; Kabir, A.; Talukder, K.; Hassan, N.; Manaster, B.; Staab, D.; Duxbury, J. Nutritional rickets without vitamin D deficiency in Bangladesh. J. Trop. Pediatr. 1999, 45, 291–293. [Google Scholar] [CrossRef]
- Chen, W.; Feng, Q.; Zhang, G.; Li, L.; Jin, S. Effect of energy input on flocculation process and flotation performance of fine scheelite using sodium oleate. Miner. Eng. 2017, 112, 27–35. [Google Scholar] [CrossRef]
- Singh, R.; Parihar, P.; Prasad, S.M. Simultaneous exposure of sulphur and calcium hinder As toxicity: Up-regulation of growth, mineral nutrients uptake and antioxidants system. Ecotoxicol. Environ. Saf. 2018, 161, 318–331. [Google Scholar] [CrossRef]
- Moestue, H.; De Pee, S.; Hall, A.; Hye, A.; Sultana, N.; Ishtiaque, M.; Huq, N.; Bloem, M. Conclusions about differences in linear growth between Bangladeshi boys and girls depend on the growth reference used. Eur. J. Clin. Nutr. 2004, 58, 725–731. [Google Scholar] [CrossRef]
- Roy, V.C.; Kamal, M.; Faridullah, M.; Haque, S.A.; Reza, M.S. Influence of salt and herbal substance on the drying and reconstitution performance of Bombay duck, Harpodon nehereus. J. Fish. 2014, 2, 59–63. [Google Scholar] [CrossRef]
- Lithi, U.J.; Faridullah, M.; Roy, V.C.; Roy, K.C.; Alam, A.N. Efficiency of organic pesticides, turmeric (Curcuma longa) and neem (Azadirachta indica) against dry fish beetle (Dermestes sp.) during storage condition: Efficiency of organic pesticides against beetle. J. Bangladesh Agric. Univ. 2019, 17, 110–116. [Google Scholar] [CrossRef]
- Nielsen, H.; Roos, N.; Thilsted, S.H. The impact of semi-scavenging poultry production on the consumption of animal source foods by women and girls in Bangladesh. J. Nutr. 2003, 133, 4027S–4030S. [Google Scholar] [CrossRef] [PubMed]





| Species | Order | Family | Local Name |
|---|---|---|---|
| 1Aborichthys elongates (Hora, 1921) | Cypriniformes | Nemacheilidae | Rimum, Ribb |
| 1Acanthocobitis botia (F. Hamilton, 1822) | Cypriniformes | Nemacheilidae | Gadera, Chikli |
| 1Ailia coila (F. Hamilton, 1822) | Siluriformes | Ailiidae | Patasi, Kajuli |
| 1Ailiichthys punctate (Day, 1872) | Siluriformes | Ailiidae | Jamuna ailia |
| 1Amblyceps laticeps (McClelland, 1842) | Siluriformes | Amblycipitidae | Amblyceps |
| 1Amblyceps mangois (Blyth, 1858) | Siluriformes | Amblycipitidae | Tayek, Chikka |
| 1Amblypharyngodon microlepis (Bleeker, 1854) | Cypriniformes | Cyprinidae | Indian carplet |
| 1Amblypharyngodon mola (Hamilton, 1822) | Cypriniformes | Cyprinidae | Mola carplet, |
| 1Anabas cobojius (F. Hamilton, 1822) | Anabantiformes | Anabantidae | Ganjetic koi |
| 1Anabas testudineus (Bloch, 1792) | Anabantiformes | Anabantidae | Koi, Kawai |
| 1Aplocheilus parvus (Sundara Raj, 1916) | Cyprinodontiformes | Aplocheilidae | Dwarf panchax |
| 1Aplocheilus panchax (F. Hamilton, 1822) | Cyprinodontiformes | Aplocheilidae | Charbeki |
| 1Aspidoparia jaya (Hamilton, 1822) | Cypriniformes | Cyprinidae | Chola, Jaya |
| 1Cabdio morar (Hamilton, 1822) | Cypriniformes | Cyprinidae | Olahalale |
| 1Badis badis (F. Hamilton, 1822) | Cypriniformes | Badidae | Badis |
| 1Barilius bendelisis (F. Hamilton, 1807) | Cypriniformes | Cyprinidae | Bhareli, Zhorya, Korang |
| 1Barilius vagra (F. Hamilton, 1822) | Cypriniformes | Cyprinidae | Korang |
| 1Barilius shacra (F. Hamilton, 1822) | Cypriniformes | Cyprinidae | Bola, Shacra baril |
| 1Batasio batasio (Blyth, 1860) | Siluriformes | Bagridae | Tista batasio |
| 1Botia dario (F. Hamilton, 1822) | Cypriniformes | Botiidae | Botuk mach, loach |
| 1Botia rostrata (Günther, 1868) | Cypriniformes | Botiidae | Gangetic loach |
| 1Danio rerio (F. Hamilton, 1822) | Cypriniformes | Cyprinidae | Poncha geraldi |
| 1Chanda nama (F. Hamilton, 1822) | Cypriniformes | Ambassidae | Chanda, Kachki |
| 1Parambassis ranga (F. Hamilton, 1822) | Cypriniformes | Ambassidae | Chanari, Ranga chanda |
| 1Channa gachua (F. Hamilton, 1822) | Anabantiformes | Channidae | Dokrya, Bothua |
| 1Channa orientalis (Bloch and J. G. Schneider, 1801) | Anabantiformes | Channidae | Cheinga, Cheng |
| 1Channa punctata (Bloch, 1793) | Anabantiformes | Channidae | Lata, Spotted snake head, Gadisha |
| 1Channa stewartii (Playfair, 1867) | Anabantiformes | Channidae | Sengalee, Assamese snake head |
| 1Clarias batrachus (Linnaeus, 1758) | Siluriformes | Clariidae | Magur |
| 1Trichogaster chuna (F. Hamilton, 1822) | Anabantiformes | Osphronemidae | Sunset gourami |
| 1Trichogaster lalius (F. Hamilton, 1822) | Anabantiformes | Osphronemidae | Khosti, Kunggee |
| 1Crossocheilus latius (Hamilton, 1822) | Cypriniformes | Cyprinidae | Gangetic latia |
| 1Danio dangila (F. Hamilton, 1822) | Cypriniformes | Cyprinidae | Laupati, Nipati |
| 1Danio rerio (F. Hamilton, 1822) | Cypriniformes | Cyprinidae | Zebra fish, Anju, Pocha-geraidi |
| 1Devario aequipinnatus (McClelland, 1839) | Cypriniformes | Cyprinidae | Balooki, vannathipodi |
| 1Esomus danrica (F. Hamilton, 1822) | Cypriniformes | Cyprinidae | Darikana, jongia, Dendu |
| 1Eutropiichthys vacha (Hamilton, 1822) | Siluriformes | Schilbeidae | Bacha, Neemuch |
| 1Gagata cenia (Hamilton, 1822) | Siluriformes | Sisoridae | Indian gagata |
| 1Garra annandalei (Hora, 1921) | Cypriniformes | Cyprinidae | Nungnga |
| 1Garra lamta (F. Hamilton, 1822) | Cypriniformes | Cyprinidae | Pathorchata, Dohjei |
| 1Glossogobius giuris (F. Hamilton, 1822) | Gobiiformes | Gobiidae | Tank goby, Gulah |
| 1Glyptothorax chindwinica (Vishwanath and Linthoingambi, 2007) | Siluriformes | Sisoridae | Nau, Pattarchatta |
| 1Gudusia chapra (F. Hamilton, 1822) | Clupeiformes | Clupeidae | Khoira, Karati, Chapra |
| 1Heteropneustes fossilis (Bloch, 1794) | Siluriformes | Siluriformes | Singhi |
| 1Laubuka laubuca (F. Hamilton, 1822) | Cypriniformes | Cyprinidae | Dankena, Dorikana |
| 1Mystus bleekeri (F. Day, 1877) | Siluriformes | Bagridae | Palwa, Tengara |
| 1Mystus gulio (Hamilton, 1822) | Siluriformes | Bagridae | Nona tangra, Gule tangra |
| 1Mystus malabaricus (Jerdon, 1849) | Siluriformes | Bagridae | Shingeti |
| 1Mystus tengara (Hamilton, 1822) | Siluriformes | Bagridae | Striped dwarf catfish |
| 1Mystus vittatus (Bloch, 1794) | Siluriformes | Bagridae | Tangra |
| 1Nandus nandus (Hamilton, 1822) | Siluriformes | Nandidae | Gangetic leaffish |
| 1Ompok siluroides (Lacépède, 1803) | Siluriformes | Siluridae | Pabda, Khababia |
| 1Oreichthys cosuatis (F. Hamilton, 1822) | Cypriniformes | Cyprinidae | Khavli |
| 1Osteobrama cotio (Hamilton, 1822) | Cypriniformes | Cyprinidae | Maura |
| 1Pellona sp. (Valenciennes, 1847) | Clupeiformes | Pristigasteridae | Pellona |
| 1Chagunius chagunio (Hamilton, 1822) | Cypriniformes | Cyprinidae | Chaguni |
| 1Puntius chola (F. Hamilton, 1822) | Cypriniformes | Cyprinidae | Swamp barb |
| 1Pethia gelius (F. Hamilton, 1822) | Cypriniformes | Cyprinidae | Glass barb |
| 1Pethia phutunio (F. Hamilton, 1822) | Cypriniformes | Cyprinidae | Dwarf barb |
| 1Puntius sarana (F. Hamilton, 1822) | Cypriniformes | Cyprinidae | Olive barb |
| 1Puntius sophore (F. Hamilton, 1822) | Cypriniformes | Cyprinidae | Sona punti/Pool barb |
| 1Puntius tirio (F. Hamilton, 1822) | Cypriniformes | Cyprinidae | Onespot barb |
| 1Puntius ticto (F. Hamilton, 1822) | Cypriniformes | Cyprinidae | Punti |
| 1Rasbora daniconius (F. Hamilton, 1822) | Cypriniformes | Cyprinidae | Dohni cona, Danikono |
| 1Salmostoma bacaila (F. Hamilton, 1822) | Cypriniformes | Cyprinidae | Chela, Kataria |
| 2Salmostoma phulo (F. Hamilton, 1822) | Cypriniformes | Cyprinidae | Orali, Finescale razorbelly minnow |
| 2Macrognathus aculeatus (Bloch, 1786) | Synbranchiformes | Mastacembelidae | Lesser spiny eel |
| 2Channa marulius (F. Hamilton, 1822) | Anabantiformes | Channidae | Great snakehead |
| 2Channa striata (Bloch, 1793) | Anabantiformes | Channidae | Striped snakehead |
| 2Xenentodon cancila (F. Hamilton, 1822) | Beloniformes | Belonidae | Freshwater needlefish |
| 2Macrognathus pancalus (F. Hamilton, 1822) | Synbranchiformes | Mastacembelidae | Striped spiny eel |
| 2Mystus cavasius (Hamilton, 1822) | Siluriformes | Bagridae | Gangetic mystus |
| 2Notopterus notopterus (Pallas, 1769) | Osteoglossiformes | Notopteridae | Bronze featherback |
| 2Mastacembelus armatus (Lacepède, 1800) | Synbranchiformes | Mastacembelidae | Zig-zag eel |
| 2Trichogaster fasciata (Bloch and J. G. Schneider, 1801) | Anabantiformes | Osphronemidae | Trichogaster fasciata |
| Small Indigenous Fish Species (SIS) | Nutrient Presence in 100 g Raw Fish (Different Edible Parts) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Vit- B12, D, E and B9 | Vit-A | ||||||||||
| V-B12 (µg) | V-D3 (µg) | V-D2 (µg) | V-E (µg) | B9 (µg) | β-Carotene (µg) | 13-Cis-Retinol (µg) | 13-Cis-dehyDroretinol (µg) | All-Trans-Retino (µg) | All-Trans-Dehydroretinol (µg) | Total Vitamin A (µg RAE) | |
| Tit Punti | 6.74 | 0.995 | nd | 0.16 | nd | 25 a | 4 a | 5 a | 11 a | 8 a | 21 |
| Rani, Bou | 6.4 | 0.12 | – | 0.63 | 3.2 | – | nd | nd | nd | 60 | 24 |
| Jat Punti | 4.01 | 1.29 | nd | 0.15 | nd | 13 a | 4 a | 9 a | 27 a | 49 a | 54 |
| Darkina | 12.5 | 6.31 | nd | 0.84 | nd | 100 a | 63 a | 48 a | 433 a | 381 a | 660 |
| Boro Kholisha | 5.55 | 3.13 | 2.1 | 0.12 | nd | 11 a | 5 a | 5 a | 34 a | 14 a | 46 |
| Guchi | 2.47 | 2.29 | nd | 0.11 | nd | 110 a | 1 a | 14 a | 9 a | 133 a | 78 |
| Meni, Bheda | 0.90 | 0.78 | – | 0.36 | 3.5 | – | nd | nd | 36 | 61 | 60 |
| Taki | 1.60 | nd | nd | 0.14 | nd | 22 a | 9 a | 13 a | 84 a | 104 a | 139 |
| Koi | 2.38 | 1.19 | nd | nd | 11.4 | 74 a | 61 a | 30 a | 163 a | 171 a | 295 |
| Chela | 5.64 | 4.00 | nd | 0.11 | nd | 21 a | 25 a | 9 a | 90 a | 45 a | 132 |
| Kajuli, Bashpata | 4.1 | 0.091 | – | 0.28 | 2.9 | – | nd | nd | 37 | nd | 37 |
| Tengra | 3.5 | 0.19 | – | 0.23 | 10 | – | nd | nd | nd | 29 | 12 |
| Foli | 2.0 | 0.70 | – | 0.64 | 18 | – | nd | nd | nd | nd | nd |
| Chapila | 6.99 | 4.92 | nd | nd | nd | nd a | 1 a | 21 a | 9 a | 136 a | 73 |
| Baim | 1.72 | 1.30 | 0.76 | nd | nd | 5 a | 1 a | 5 a | 1 a | 51 a | 27 |
| Mola (cultured) | 5.9 | 3.0 | – | 0.91 | 4.3 | – | 44 | 42 | 340 | 4590 | 2226 |
| Magur | 4.83 | nd | nd | 0.13 | 9.4 | 64 a | 4 a | 8 a | 7 a | 15 a | 25 |
| Tara Baim | 5.20 | nd | nd | 0.17 | nd | 135 a | 2 a | 15 a | 16 a | 120 a | 83 |
| Dhela | 4.7 | 0.14 | – | 0.24 | 6.6 | – | 15 | 68 | 28 | 2130 | 918 |
| Mola | 7.98 | 2.03 | 2.9 | 0.27 | nd | nd a | nd a | 460 a | 323 a | 4990 a | 2503 |
| Shing | 12.8 | nd | nd | 0.34 | nd | 45 a | 5 a | 11 a | 11 a | 22 a | 32 |
| Kachki | 3.55 | 1.5 | nd | 0.09 | nd | 15 a | 2 a | 30 a | 14 a | 122 a | 78 |
| Chanda | 6.42 | 11.9 | nd | 0.18 | nd | 43 a | 14 a | 51 a | 128 a | 433 a | 336 |
| Kuli, Bhut Bailla | 1.4 | 22 | – | 0.55 | 3.7 | – | nd | nd | 37 | nd | 37 |
| Gutum | 8.75 | nd | nd | 0.19 | nd | 25 a | 1 a | 9 a | 17 a | 131 a | 76 |
| Bele, Bailla | 2.1 | 1.6 | – | 0.17 | 6.7 | – | nd | nd | 18 | nd | 18 |
| Kakila | 2.89 | 1.4 | 0.66 | 0.40 | 9.2 | 56 a | 9 a | 12 a | 54 a | 53 a | 91 |
| Ekthute | 3.0 | 2.4 | – | 0.65 | 11 | – | 18 | nd | 84 | nd | 98 |
| Small Indigenous Fish Species (SIS) | Nutrient Presence in 100 g Raw Fish | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fe (mg) | Zn (mg) | Ca (mg) | I (µg) | Se (µg) | P (mg) | Mg (mg) | Na (mg) | K (mg) | Mn (mg) | S (mg) | Cu (mg) | |
| Tit Punti | 3.4 a | 3.8 a | 1480 a | 19 | 10 a | – | 47 a | 61 a | 187 a | – | – | – |
| Rani, Bou | 2.5 | 4.0 | 1300 | 25 | 31 | 820 | 45 | 48 | 160 | 1.5 | 170 | 0.094 |
| Jat Punti | 2.2 a | 2.9 a | 1042 a | 20 | 9.5 a | – | 39 a | 53 a | 203 a | – | – | – |
| Darkina | 12 a | 4.0 a | 891 a | 81 | 12 a | – | 38 a | 110 a | 200 a | – | – | |
| Boro Kholisha | 4.1 | 2.3 | 1700 | 20 | 26 | 910 | 44 | 61 | 210 | 2.0 | 190 | 0.046 |
| Guchi | 2.7 a | 1.3 a | 491 a | 19 | 45 a | – | 34 a | 52 a | 294 a | – | – | – |
| Meni, Bheda | 0.84 | 1.6 | 1300 | 13 | 29 | 810 | 44 | 68 | 250 | 1.4 | 210 | 0.029 |
| Taki | 1.8 a | 1.5 a | 766 a | 18 | 15 a | – | 35 a | 47 a | 260 a | – | – | – |
| Koi | 0.87 | 0.60 | 85 | nd | 19 | 160 | 21 | 31 | 260 | 0.052 | 190 | 0.052 |
| Chela | 0.84 | 4.7 | 1000 | 19 | 32 | 590 | 39 | 28 | 85 | 0.60 | 170 | 0.052 |
| Kajuli, Bashpata | 0.82 | 1.2 | 110 | 7.1 | 27 | 140 | 22 | 26 | 130 | 0.17 | 200 | 0.059 |
| Tengra | 4.0 a | 3.1 a | 1093 a | 28 | 24 a | – | 36 a | 57 a | 203 a | – | – | – |
| Foli | 1.7 | 1.6 | 230 | nd | 22 | 270 | 34 | 53 | 280 | 0.078 | 260 | 0.058 |
| Chapila | 7.6 a | 2.1 a | 1063 a | 13 | 13.4 a | – | 41 a | 57 a | 281 a | – | – | – |
| Baim | 1.9 a | 1.1 a | 449 a | 13 | 12 a | – | 35 a | 47 a | 322 a | – | – | – |
| Mola (cultured) | 19 | 4.2 | 1400 | 33 | 19 | 700 | 49 | 31 | 58 | 1.9 | 160 | 0.047 |
| Magur | 1.2 | 0.74 | 59 | 22 | 22 | 210 | 26 | 61 | 350 | 0.021 | 180 | 0.050 |
| Tara Baim | 2.5 a | 1.2 a | 457 a | 13 | 15 a | – | 34 a | 46 a | 290 a | – | – | – |
| Dhela | 1.8 | 3.7 | 1200 | 9.5 | 29 | 660 | 39 | 37 | 110 | 0.60 | 170 | 0.046 |
| Mola | 5.7 a | 3.2 a | 853 a | 17 | 5 a | – | 35 a | 39 a | 152 a | – | – | – |
| Shing | 2.2 | 1.1 | 60 | nd | 31 | 220 | 37 | 54 | 300 | 0.038 | 230 | 0.057 |
| Kachki | 2.8 a | 3.1 a | 476 a | 6.0 | 7.5 a | – | 26 a | 38 a | 134 a | – | – | – |
| Chanda | 2.1 a | 2.6 a | 1153 a | 24 | 22 a | – | 45 a | 61 a | 206 a | – | – | – |
| Kuli, Bhut Bailla | 0.79 | 2.0 | 980 | 31 | 49 | 580 | 39 | 55 | 190 | 0.29 | 210 | 0.030 |
| Gutum | 3.3 | 2.5 | 950 | 16 | 36 | 650 | 57 | 45 | 240 | 0.46 | 190 | 0.054 |
| Bele, Bailla | 2.3 | 2.1 | 790 | 25 | 31 | 520 | 38 | 56 | 210 | 2.3 | 200 | 0.030 |
| Kakila | 0.65 | 1.9 | 610 | 37 | 29 | 450 | 35 | 49 | 190 | 0.47 | 240 | 0.046 |
| Estate | 1.5 | 3.6 | 1300 | 11 | 28 | 770 | 51 | 52 | 140 | 0.73 | 240 | 0.030 |
| Golsha | 1.8 | 1.3 | 120 | 13 | 41 | 180 | 26 | 33 | 210 | 0.22 | 220 | 0.039 |
| Modhu Pabda | 0.46 | 0.90 | 91 | 7.0 | 27 | 150 | 23 | 47 | 230 | 0.073 | 190 | 0.042 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, M.R.; Yeasmin, M.; Sadia, S.; Ali, M.S.; Haque, A.R.; Roy, V.C. Small Indigenous Fish: A Potential Source of Valuable Nutrients in the Context of Bangladesh. Hydrobiology 2023, 2, 212-234. https://doi.org/10.3390/hydrobiology2010014
Islam MR, Yeasmin M, Sadia S, Ali MS, Haque AR, Roy VC. Small Indigenous Fish: A Potential Source of Valuable Nutrients in the Context of Bangladesh. Hydrobiology. 2023; 2(1):212-234. https://doi.org/10.3390/hydrobiology2010014
Chicago/Turabian StyleIslam, Md Rakibul, Momota Yeasmin, Sultana Sadia, Md Sadek Ali, Ahmed Redwan Haque, and Vikash Chandra Roy. 2023. "Small Indigenous Fish: A Potential Source of Valuable Nutrients in the Context of Bangladesh" Hydrobiology 2, no. 1: 212-234. https://doi.org/10.3390/hydrobiology2010014
APA StyleIslam, M. R., Yeasmin, M., Sadia, S., Ali, M. S., Haque, A. R., & Roy, V. C. (2023). Small Indigenous Fish: A Potential Source of Valuable Nutrients in the Context of Bangladesh. Hydrobiology, 2(1), 212-234. https://doi.org/10.3390/hydrobiology2010014