Diversity and Seasonality of Aquatic Beetles (Coleoptera) in Three Localities of the State of Tlaxcala, Central Mexico
Abstract
:1. Introduction
2. Materials and Methods
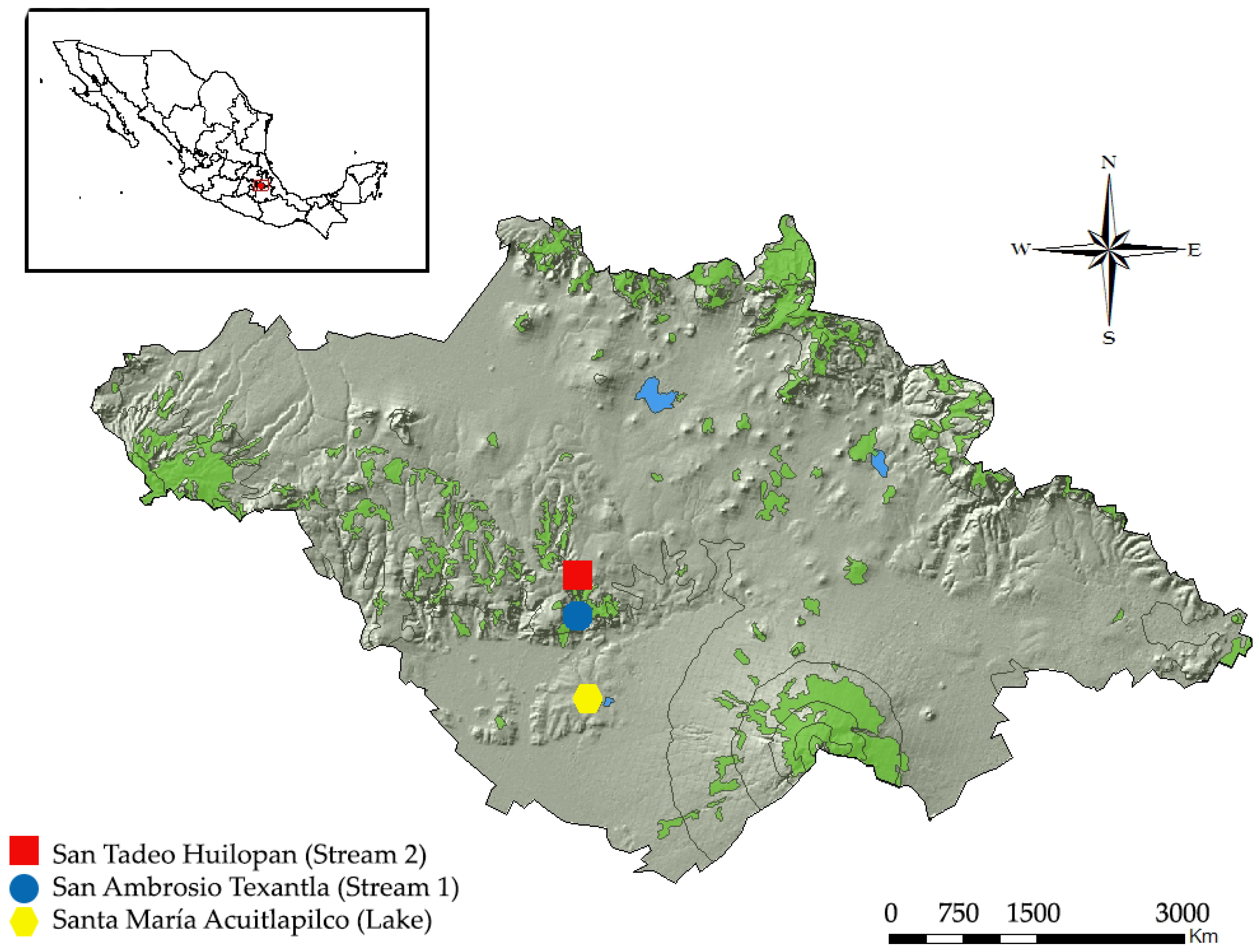
2.1. Study Sites
2.2. Sampling
2.3. Dissecting and Curating
2.4. Data Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McCafferty, W.P. Aquatic Entomology: The Fishermen’s and Ecologists’ Illustrated Guide to Insects and Their Relatives, 1st ed.; Jones and Bartlett Publishers: Boston, MA, USA, 1981; pp. 202–236. [Google Scholar]
- White, D.S.; Roughley, R.E. Aquatic Coleoptera. In An Introduction to the Aquatic Insects of North America, 4th ed.; Merritt, R.W., Cummins, K.W., Berg, M.B., Eds.; Kendall Hunt Publishing Company: Dubuque, IA, USA, 2008; pp. 571–671. [Google Scholar]
- Miserendino, M.L.; Archangelsky, M. Aquatic Coleoptera distribution and environmental relationships in a large Patagonian river. Int. Rev. Hydrobiol. 2006, 91, 423–437. [Google Scholar] [CrossRef]
- Jäch, M.A.; Balke, M. Global diversity of water beetles (Coleoptera) in freshwater. Hydrobiologia 2008, 595, 419–442. [Google Scholar] [CrossRef]
- Archangelsky, M.; Manzo, V.; Michat, M.C.; Torres, P.L.M. Coleoptera. In Macroinvertebrados Bentónicos Sudamericanos: (Sistemática y Biología); Domínguez, E., Fernández, H.R., Eds.; Fundación Miguel Lillo: Tucumán, Argentina, 2009; pp. 411–468. [Google Scholar]
- Bilton, D.T.; Ribera, I.; Short, A.E.Z. Water beetles as models in ecology and evolution. Annu. Rev. Entomol. 2019, 64, 359–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pakulnicka, J. The formation of water beetle fauna in anthropogenic. Oceanol. Hydrobiol. Stud. 2008, 37, 31–42. [Google Scholar] [CrossRef] [Green Version]
- Pakulnicka, J.; Nowakowski, J.J. The effect of hydrological connectivity on water beetles fauna in water bodies within the floodplain of a lowland river (Neman river, Belarus). Oceanol. Hydrobiol. Stud. 2012, 41, 7–17. [Google Scholar] [CrossRef] [Green Version]
- Buczyńska, E.; Buczyński, P. Aquatic insects of man-made habitats: Environmental factors determining the distribution of Caddisflies (Trichoptera), Dragonflies (Odonata), and Beetles (Coleoptera) in Acidic Peat Pools. J. Insect Sci. 2019, 19, 17. [Google Scholar] [CrossRef] [Green Version]
- Arce-Pérez, R. Lista preliminar de coleópteros acuáticos del Estado de Morelos, México. Acta Zool. Mex. 1995, 65, 43–53. [Google Scholar] [CrossRef]
- Arce-Pérez, R.; Roughley, R.E. Lista anotada y claves para los Hydradephaga (Coleoptera: Adephaga: Dytiscidae, Noteridae, Haliplidae, Gyrinidae) de México. Dugesiana 1999, 6, 69–104. [Google Scholar] [CrossRef]
- Santiago-Fragoso, S.; Spangler, P.J. Elmidae (Coleoptera). In Biodiversidad, Taxonomía y Biogeografía de Artrópodos de México: Hacia una Síntesis de su Conocimiento, Volumen II, 1st ed.; Llorente Bousquets, J., González Soriano, E., Papavero, N., Eds.; Universidad Nacional Autónoma de México: Mexico City, México, 2000; Volume 2, pp. 421–438. [Google Scholar]
- Bueno-Soria, J.; Santiago-Fragoso, S.; Barba-Álvarez, R. Insectos acuáticos. In Biodiversidad del Estado de Tabasco, 1st ed.; Bueno, J., Álvarez, F., Santiago, S., Eds.; Instituto de Biología, UNAM-CONABIO: Mexico City, México, 2005; pp. 195–224. [Google Scholar]
- Arce-Pérez, R.; Moron, M.A. Sinopsis de los Hydrophiloidea de México (Coleoptera: Hydrophilidae, Helophoridae, Epimetopidae, Georissidae e Hydrochidae), con una clave para la identificación de los géneros. Rev. Mex. Biodivers. 2011, 82, 491–514. [Google Scholar] [CrossRef] [Green Version]
- Arce-Pérez, R.; Novelo-Gutiérrez, R. Coleópteros acuáticos de la reserva de la biosfera de la Michilia, Durango, México. Folia Entomol. Mex. 1991, 81, 341–344. [Google Scholar]
- Santiago-Fragoso, S.; Sandoval-Manrique, J.C. Coleópteros acuáticos y su relación con la dinámica fisicoquímica del río Cuautla (tramo Teltencingo-Anenecuilco), Morelos México. Hidrobiológica 2001, 11, 19–30. [Google Scholar]
- Campbell, W.R.; Arce-Pérez, R.; Gómez-Anaya, J.A. Taxonomic distinctness and aquatic Coleoptera: Comparing a perennial and intermittent stream with differing geomorphologies in Hidalgo, México. Aquat. Ecol. 2008, 42, 103–113. [Google Scholar] [CrossRef]
- Arce-Pérez, R.; Novelo-Gutiérrez, R.; Gómez-Anaya, J.A. Coleópteros acuáticos de la zona de influencia de la central hidroeléctrica “Ing. Fernando Hiriart Balderrama” (C. H. Zimapán), Hidalgo, México. Coleoptera: Polyphaga y Myxophaga. Acta Zool. Mex. 2010, 26, 639–667. [Google Scholar] [CrossRef] [Green Version]
- Torres-García, U.; Pérez-Valladares, C.X.; Herrería-Diego, Y.; Pineda-López, R.F. Efecto de los factores ambientales sobre la diversidad de insectos hemimetábolos y coleópteros acuáticos en la cuenca del Río Xichú, Guanajuato, México. Rev. Biol. Trop. 2014, 62, 69–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinton, H.E. A monographic revision of the Mexican water beetles of the family Elmidae. Novit. Zool. 1940, 42, 217–396. [Google Scholar]
- Pérez-Rodríguez, R.; Saldaña-Arias, A.; Badillo-Solis, A.; Vicente-Velázquez, V. Datos ecológicos sobre Dytiscidae e Hidrophilidae (Insecta: Coleoptera) de tres embalses de Tlaxcala, México. Rev. Soc. Mex. Hist. Nat. 2003, 1, 57–67. [Google Scholar]
- INEGI. Síntesis Geográfica de Tlaxcala; Secretaría de Programación y Presupuesto: Mexico City, México, 2021; pp. 1–41. [Google Scholar]
- Fonseca, J.; Pérez-Crespo, M.J.; Cruz, M.; Porras, B.; Hernández-Rodríguez, E.; Martínez y Pérez, J.L.; Lara, C. Aves acuáticas de la Laguna de Acuitlapilco, Tlaxcala, México. Huitzil 2012, 13, 104–109. [Google Scholar] [CrossRef]
- Magurran, A.E. Ecological Diversity and its Measurement; Princeton University Press: Princeton, NJ, USA, 1988; pp. 7–45. [Google Scholar] [CrossRef]
- Colwell, R.K.; Coddington, J.A. Estimating terrestrial biodiversity through extrapolation. Philos. Trans. R. Soc. B Biol. Sci. 1994, 345, 101–118. [Google Scholar] [CrossRef] [Green Version]
- Colwell, R.K.; Mao, C.X.; Chang, J. Interpolating, extrapolating, and comparing incidence-based species accumulation curves. Ecology 2004, 85, 2717–2727. [Google Scholar] [CrossRef] [Green Version]
- Chao, A.; Chiu, C.-H. Species richness: Estimation and comparison. In Wiley StatsRef: Statistics Reference Online; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 1–26. [Google Scholar] [CrossRef]
- Colwell, R.K. EstimateS: Statistical Estimation of Species Richness and Shared Species from Samples. Version 9.1. 2022. User’s Guide and Application. Available online: https://osf.io/su57f/ (accessed on 3 November 2022).
- Jost, L. Entropy and diversity. Oikos 2006, 113, 363–375. [Google Scholar] [CrossRef]
- Chao, A.; Chiu, C.H.; Jost, L. Unifying species diversity, phylogenetic diversity, functional diversity, and related similarity/differentiation measures through Hill numbers. Annu. Rev. Ecol. Evol. Syst. 2014, 45, 45–67. [Google Scholar] [CrossRef] [Green Version]
- Chao, A.; Gotelli, N.G.; Hsieh, T.C.; Sander, E.L.; Ma, K.H.; Colwell, R.K.; Ellison, A.M. Rarefaction and extrapolation with Hill numbers: A framework for sampling and estimation in species diversity studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef] [Green Version]
- Chao, A.; Jost, L. Estimating diversity and entropy profiles via discovery rates of new species. Methods Ecol. Evol. 2015, 6, 873–882. [Google Scholar] [CrossRef]
- Carvalho, J.C.; Cardoso, P.; Gomes, P. Determining the relative roles of species replacement and species richness differences in generating beta-diversity patterns. Glob. Ecol. Biogeogr. 2012, 21, 760–771. [Google Scholar] [CrossRef]
- Carvalho, J.C.; Cardoso, P.; Borges, P.A.V.; Schmera, D.; Podani, J. Measuring fractions of beta diversity and their relationships to nestedness: A theoretical and empirical comparison of novel approaches. Oikos 2013, 122, 825–834. [Google Scholar] [CrossRef] [Green Version]
- Cummins, K.W.; Merritt, R.W.; Berg, M.B. Ecology and distribution of aquatic insects. In An introduction of Aquatic Insects of North America, 4th ed.; Merritt, R.W., Cummins, K.W., Berg, M.B., Eds.; Kendall Hunt Publishing Company: Dubuque, IA, USA, 2008; pp. 105–122. [Google Scholar]
- Hammond, P.M.; Hine, S.J. Coleoptera: The beetles. In Identifying British Insects and Arachnids, 1st ed.; Barnard, P.C., Ed.; Cambridge University Press: London, UK, 1999; pp. 80–138. [Google Scholar]
- Pakulnicka, J.; Górski, A.; Bielecki, A. Enviromental factors associated with biodiversity and the occurrence of rare, threatened, thermophilous species of aquatic beetles in the anthropogenic pounds of the Masurian Lake District. Biodivers. Conserv. 2015, 24, 429–1445. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, A.N.; Holmen, M. The Aquatic Adephaga (Coleoptera) of Fennoscandia and Denmark. II. Dytisicidae, 1st ed.; EJ Brill: Leiden, The Netherlands; New York, NY, USA, 1995; pp. 79–81. [Google Scholar]
- Elliot, J.M. The ecology of riffle beetles (Coleoptera: Elmidae). Freshw Rev. 2008, 1, 189–203. [Google Scholar] [CrossRef]
- Manzo, V. Los élmidos de la región Neotropical (Coleoptera: Byrrhoidea: Elmidae): Diversidad y distribución. Rev. Soc. Entomol. Arg. 2013, 72, 199–212. [Google Scholar]
- Brown, H.P. Aquatic Dryopoid Beetles (Coleoptera) of the United States, 1st ed.; United States Environmental Protection Agency: Cincinnati, OH, USA, 1976; pp. 25–54.
- Moreno, S.E.; Barragán, F.; Pineda, E.; Pavón, N.P. Reanálisis de la diversidad alfa: Alternativas para interpretar y comparer información sobre comunidades ecológicas. Rev. Mex. Biodivers. 2011, 82, 1249–1261. [Google Scholar] [CrossRef] [Green Version]
- Peckarsky, B.L.; Fraissinet, P.R.; Penton, M.A.; Conklin, D.J., Jr. Freshwater Macroinvertebrates of Northeastern North America, 1st ed.; Cornell University Press: Ithaca, NY, USA, 1990; pp. 137–171. [Google Scholar]





| Family/Genus/Species | Study Sites | ||
|---|---|---|---|
| Stream 1 | Stream 2 | Lake | |
| Dytiscidae | |||
| Copelatus distinctus Aubé, 1838 1 | 2 | 14 | 0 |
| Hygrotus sp. 3 | 1 | 1 | 11 |
| Laccophilus mexicanus Aubé, 1838 3 | 7 | 12 | 22 |
| Liodessus affinis Say, 1823 3 | 9 | 18 | 23 |
| Platambus mexicanus (Larson, 2000) 3 | 16 | 17 | 1 |
| Rhantus gutticollis (Say, 1830) 3 | 56 | 34 | 9 |
| Rhantus sp. 1 | 2 | 0 | 0 |
| Clarkhydrus decemsignatus (Clark, 1862) 1 | 223 | 78 | 0 |
| Thermonectus basillaris (Harris, 1829) 3 | 3 | 0 | 20 |
| Thermonectus nigrofasciatus (Aubé, 1838) 3 | 8 | 24 | 2 |
| Gyrinidae | |||
| Gyrinus sp. 1 | 52 | 113 | 0 |
| Haliplidae | |||
| Peltodytes ovalis Zimmermann, 1924 3 | 9 | 98 | 4 |
| Dryopidae | |||
| Helichus productus LeConte, 1852 1 | 45 | 168 | 0 |
| Helichus suturalis LeConte, 1852 1 | 76 | 566 | 0 |
| Elmidae | |||
| Microcylloepus sp. 1 | 187 | 155 | 0 |
| Hydrophilidae | |||
| Berosus pugnax LeConte, 1863 2 | 0 | 0 | 147 |
| Paracymus regularis Wooldridge, 1969 2 | 0 | 0 | 1 |
| Paracymus sp. 1 | 4 | 100 | 0 |
| Tropisternus ellipticus (LeConte, 1855) 3 | 42 | 116 | 104 |
| Tropisternus lateralis (Fabricius, 1775) 3 | 2 | 1 | 321 |
| Total | 744 | 1515 | 665 |
| Study Sites | Diversity Index | |||||
|---|---|---|---|---|---|---|
| Observed Diversity | Estimated Diversity | |||||
| 0D | 1D | 2D | 0D | 1D | 2D | |
| Stream 1 | 18 | 7.71 | 5.5 | 18.5 | 7.813 | 5.5319 |
| Stream 2 | 16 | 8.08 | 5.37 | 17 | 8.1 | 5.53 |
| Lake | 13 | 4.54 | 3.2 | 14.4 | 4.604 | 3.2372 |
| Stream 2 | Lake | ||
|---|---|---|---|
| Stream 1 | βcc | 0.1111111 | 0.3684211 |
| β_3 | 0 | 0.1052632 | |
| βrich | 0.1111111 | 0.2631579 | |
| Stream 2 | βcc | X | 0.4736842 |
| β_3 | X | 0.3157895 | |
| βrich | X | 0.1578947 |
| Trophic Guild | Study Sites | ||
|---|---|---|---|
| Stream 1 | Stream 2 | Lake | |
| Predator | 379 | 311 | 88 |
| Herbivore/piercer | 4 | 100 | 148 |
| Herbivore/shredder | 130 | 832 | 4 |
| Decomposer/collector | 231 | 272 | 425 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luna-Luna, A.M.; Martins, C.C.; Lara, C.; Contreras-Ramos, A. Diversity and Seasonality of Aquatic Beetles (Coleoptera) in Three Localities of the State of Tlaxcala, Central Mexico. Hydrobiology 2023, 2, 244-259. https://doi.org/10.3390/hydrobiology2010016
Luna-Luna AM, Martins CC, Lara C, Contreras-Ramos A. Diversity and Seasonality of Aquatic Beetles (Coleoptera) in Three Localities of the State of Tlaxcala, Central Mexico. Hydrobiology. 2023; 2(1):244-259. https://doi.org/10.3390/hydrobiology2010016
Chicago/Turabian StyleLuna-Luna, Alba Magali, Caleb Califre Martins, Carlos Lara, and Atilano Contreras-Ramos. 2023. "Diversity and Seasonality of Aquatic Beetles (Coleoptera) in Three Localities of the State of Tlaxcala, Central Mexico" Hydrobiology 2, no. 1: 244-259. https://doi.org/10.3390/hydrobiology2010016
APA StyleLuna-Luna, A. M., Martins, C. C., Lara, C., & Contreras-Ramos, A. (2023). Diversity and Seasonality of Aquatic Beetles (Coleoptera) in Three Localities of the State of Tlaxcala, Central Mexico. Hydrobiology, 2(1), 244-259. https://doi.org/10.3390/hydrobiology2010016







