The Potentials for the Ecological Management of Landscape Connectivity Including Aquatic Ecosystems in Northeast Albania
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
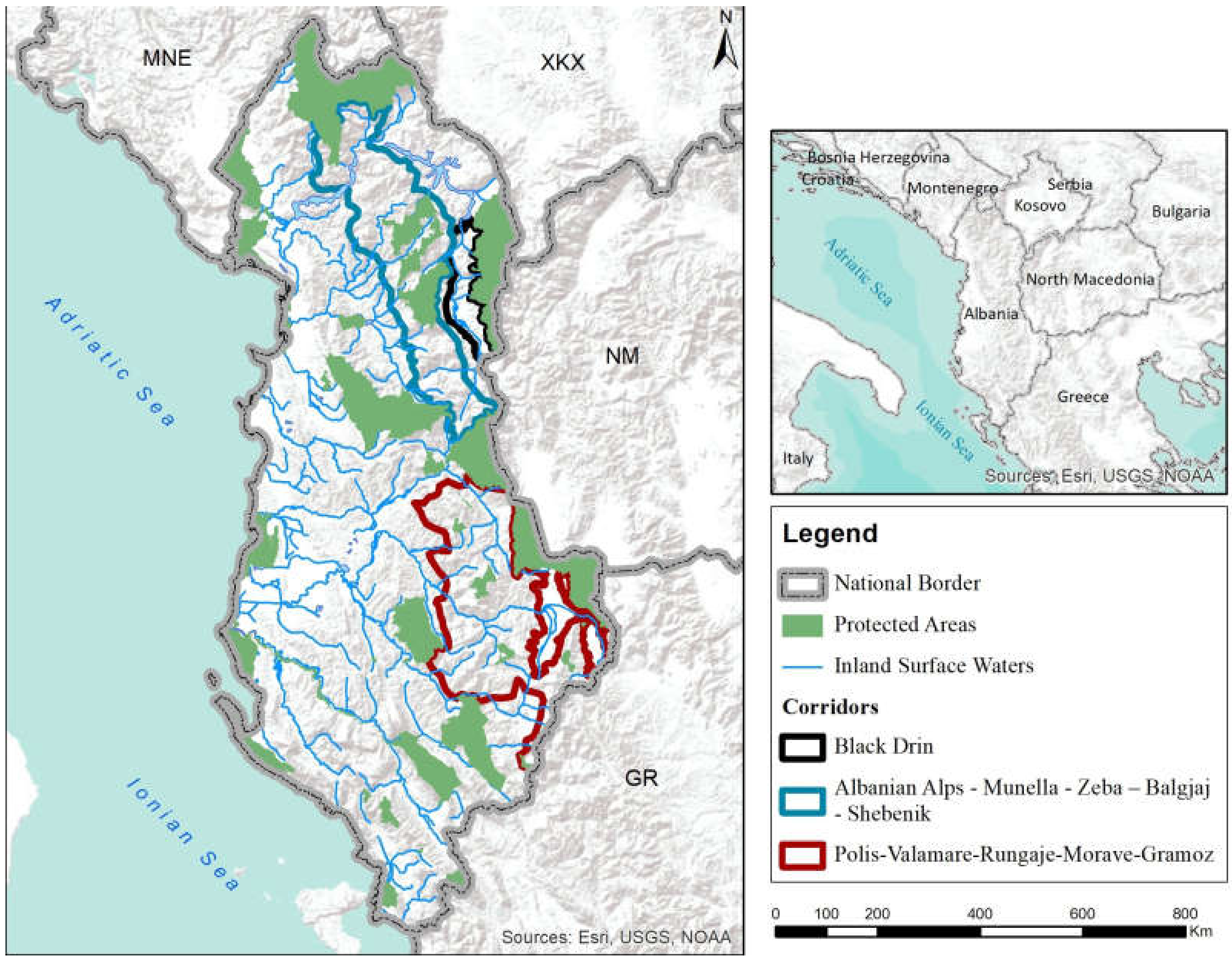
4.1. Corridors Biota Connect Aquatic and Terrestrial Habitats throughout Freshwater Ecosystem
4.2. Aquatic Connectivity in Landscape Settings
- Water pollution caused mostly due to a lack of the wastewater treatment facilities as well as a lack of integrated management approaches;
- Relatively unregulated fishery practices and illegal fishing, use of destructive methods of fishing;
- Non-native fish species, accelerated abundance with unpredicted sequences to native endemic species; impacts on specific spawning grounds for specific species particularly due to serious impacts caused by water use in the agriculture sector with a constant presence of run-offs and no abatement plans;
- Poor integration of fishery management practices into the entire management of the area (including protected one as Nature Park Korrab-Koritnik, etc.), which is recognized internationally for its rich biodiversity and abundance of species, proclaimed as an important area for the conservation of European species and habitat., and IBA;
- Low rate of local awareness for the fish biodiversity, conservation threats. The awareness and knowledge are limited to a couple of commercial fish species.
4.3. Legal Aspects of Fish and Ecosystem in Black Drini River Catchment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pulsford, I.; Lindenmayer, D.; Wyborn, C.; Lausche, B.; Worboys, G.L.; Vasilijević, M.; Lefroy, T. Connectivity conservation management. In Protected Area Governance and Management; Worboys, G.L., Lockwood, M., Kothari, A., et al., Eds.; ANU Press: Canberra, Australia, 2015; pp. 851–888. [Google Scholar]
- Haddad, N.M.; Bowne, D.R.; Cunningham, A.; Danielson, B.J.; Levey, D.J.; Sargent, S.; Spira, T. Corridor use by diverse taxa. Ecology 2003, 84, 609–615. [Google Scholar] [CrossRef]
- Driscoll, D.A.; Banks, S.C.; Barton, P.S.; Ikin, K.; Lentini, P.; Lindenmayer, D.B.; Smith, A.L.; Berry, L.E.; Burns, E.L.; Edworthy, A.; et al. The trajectory of dispersal research in conservation biology. PLoS ONE 2014, 9, e95053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiens, J.A.; Schooley, R.L.; Weekes, R.D. Patchy landscapes and animal movements: Dobeetles percolate? Oikos 1997, 78, 257–264. [Google Scholar] [CrossRef]
- Trajçe, A.; Shumka, S.; Mersini, K.; Ivanov, G.; Melovski, D.; Stojanov, A.; von Arx, M. Conservation of the Critically Endangered Balkan Lynx—Achievements and Aspirations. In Proceedings of the International Conference on Biological and Environmental Sciences, Tirana, Albania, 26–28 September 2008; pp. 34–43. [Google Scholar]
- APA. Review of the Current Status of Protected Areas in Albania; Association for the Protected Areas of Albanian: Tirana, Albania, 2019; p. 419. [Google Scholar]
- Lindenmayer, D.B.; Fischer, J. Tackling the habitat fragmentation panchreston. Trends Ecol. Evol. 2007, 22, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Saura, S.; Bertzky, B.; Bastin, L.; Battistella, L.; Mandrici, A.; Dubois, A. Protected area connectivity: Shortfalls in global targets and country-level Priorities. Biol. Conserv. 2018, 219, 53–67. [Google Scholar] [CrossRef]
- Hilty, J.; Worboys, G.L.; Keeley, A.; Woodley, S.; Lausche, B.; Locke, H.; Carr, M.; Pulsford, I.; Pittock, J.; White, J.W.; et al. Guidelines for Conserving Connectivity through Ecological Networks and Corridors; Best Practice Protected Area Guidelines Series No. 30; IUCN: Gland, Switzerland, 2020. [Google Scholar]
- Beale, C.M.; Baker, N.E.; Brewer, M.J.; Lennon, J.J. Protected area networks and savannah bird biodiversity in the face of climate change and land degradation. Ecol. Lett. 2013, 16, 1061–1068. [Google Scholar] [CrossRef]
- Juffe-Bignoli, D.; Burgess, N.D.; Bingham, H.; Belle, E.M.S.; de Lima, M.G. Protected Planet Report 2014; UNEP-WCMC: Cambridge, UK, 2014. [Google Scholar]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Weiss, S.; Apostolou, A.; Đug, S.; Marčić, Z.; Mušović, M.; Oikonomou, A.; Shumka, S.; Škrijelj, R.; Simonović, P.; Vesnić, A.; et al. Endangered Fish Species in Balkan Rivers: Their distributions and threats from hydropower development. Riverwatch EuroNatur 2018, 162. [Google Scholar]
- Abell, R.; Thieme, M.L.; Revenga, C.; Bryer, M.; Kottelat, M.; Bogutskaya, N.; Coad, B.; Mandrak, N.; Balderas, S.C.; Bussing, W.; et al. Freshwater Ecoregions of the World: A New Map of BiogeographicUnits for Freshwater Biodiversity Conservation. BioScience 2008, 58, 403–414. [Google Scholar] [CrossRef] [Green Version]
- Dill, W.A. Inland fisheries of Europe. In EIFAC Technical Paper; No. 52 Suppl; FAO: Rome, Italy, 1993; p. 36. [Google Scholar]
- Frasheri, A.; Bushati, S.; Bare, V. Geophysical outlook on structure of the Albanides. J. Balk. Geophys. Soc. 2009, 12, 9–30. [Google Scholar]
- Glasnović, P.; Krystufek, B.; Sovinc, A.; Bojović, M. Protected Areas for a Living Planet, Dinaric Arc Ecoregion Project; WWF Mediterranean: Koper, Slovenia, 2009; p. 327. [Google Scholar]
- Stolton, S.; Dudley, N. METT Handbook: A Guide to Using the Management Effectiveness Tracking Tool (METT); WWF: London, UK, 2016; p. 175. [Google Scholar]
- Shumka, S.; Berberi, E.; Kulici, M.; Mucaj, S.; Vladi, F. Assessing the relationship between biodiversity conservation and slow food cultures in selected protected areas in Albania. BiodiversitasJ. Biol. Divers. 2022, 23, 1319–1326. [Google Scholar] [CrossRef]
- BirdLife International. Monitoring Important Bird Areas: A Global Framework, Version 1.2.; BirdLife International: Cambridge, UK, 2016; p. 37. [Google Scholar]
- Bonte, D.; Van Dyck, H.; Bullock, J.M.; Coulon, A.; Delgado, M.; Gibbs, M.; Lehouck, V.; Matthysen, E.; Mustin, K.; Saastamoinen, M.; et al. Costs of dispersal. Biol. Rev. 2012, 87, 290–312. [Google Scholar] [CrossRef]
- Melovski, D.; Shumka, S.; Jovanovska, D.; Brajanoska, R.; Velevski, M.; Nakev, S.; Avukatov, V.; Hoxha, B.; Melovska, N.; Custerevska, R.; et al. Feasibility Study on Enhancing Connectivity Conservation in the PONT Focus Region: Albania and North Macedonia Description of the Selected Connectivity Conservation Areas; PONT Survey Report; PONT: Tirana, Albania, 2022; p. 65. [Google Scholar]
- Wainwright, J.; Turnbull, L.; Ibrahim, T.G.; Lexartza-Artza, I.; Thornton, S.F.; Brazier, R.E. Linking Environmental Regimes, Space and Time: Interpretations of Structural and Functional Connectivity. Geomorphology 2011, 126, 387–404. [Google Scholar] [CrossRef]
- Karr, J.R. Protecting Aquatic Ecosystems: Clean Water Is Not Enough Pages 7–13 in Biological Assessment and Criteria: Tools for Water Resource Planning and Decision Making; Davis, W.S., Simon, T.P., Eds.; Lewis Publishers: Boca Raton, FL, USA, 1995. [Google Scholar]
- Karr, J.R.; Dudley, D.R. Ecological Perspective on Water Quality Goals. Environ. Manag. 1981, 5, 55–68. [Google Scholar] [CrossRef]
- Economou, A.N.; Giakoumi, S.; Vardakas, L.; Barbieri, R.; Stoumboudi, M.; Zogaris, S. The freshwater ichthyofauna of Greece—An update based on a hydrographic basin survey. Mediterr. Mar. Sci. 2007, 8, 91–166. [Google Scholar] [CrossRef] [Green Version]
- Šanda, R.; Vukić, J.; Choleva, L.; Křížek, J.; Šedivá, A.; Shumka, S.; Wilson, I.F. Distribution of loach fishes (Cobitidae, Nemacheilidae) in Albania, with genetic analysis of populations of Cobitisohridana. Folia Zool. 2008, 57, 42–50. [Google Scholar]
- Snoj, A.; Marić, S.; Berrebi, P.; Crivelli, A.J.; Shumka, S.; Sušnik, S. Genetic architecture of trout from Albania as revealed by mtDNA control region variation. Genet. Sel. Evol. 2009, 41, 22. [Google Scholar] [CrossRef] [Green Version]
- Marková, S.; Šanda, R.; Crivelli, A.; Shumka, S.; Wilson, I.F.; Vukić, J.; Berrebi, P.; Kotlík, P. Nuclear and mitochondrial DNA sequence data reveal the evolutionary history of Barbus (Cyprinidae) in the ancient lake systems of the Balkans. Mol. Phylogenetics Evol. 2010, 55, 488–500. [Google Scholar] [CrossRef]
- Poljakov, G.D.; Filipi, N.; Basho, K.; Hysenaj, A. Pesquit e Shqiperise (Fishes of Albania); Mihal Duri: Tirana, Albania, 1958; p. 210. [Google Scholar]
- Rakaj, N. Iktiofauna e Shqiperise; Shtepia Botuese e Librit Universitar: Tirana, Albania, 1995; p. 700. [Google Scholar]
- Bianco, P.G.; Kottelat, M. Scardiniusknezevici, a new species of rudd from Lake Skadar, Montenegro (Teleostei: Cyprinidae). Ichthyol. Explor. Freshw. 2005, 16, 231–238. [Google Scholar]
- Bogutskaya, N.G.; Zupančič, P.; Naseka, A.M. Two new species of freshwater fishes of the genus Alburnoides, A. fangfangae and A. Devolli (Actinopterygii: Cyprinidae), from the Adriatic Sea basin in Albania. Proc. Zool. Inst. RAS 2010, 314, 448–468. [Google Scholar] [CrossRef]
- Schiemer, F.; Giti, G.; Keckeis, H.; Staras, M. Ecological status and problems of the Danube River and its fish fauna: A Review. In Ecological Status and Problems of the Danube; FAO: Bangkok, Thailand, 2004; pp. 273–298. [Google Scholar]
- Freyhof, J.; Brooks, E. European Red List of Freshwater Fishes; Publications Office of the European Union: Luxembourg, 2011; p. 61. [Google Scholar]
- Shumka, S.; Paparisto, A.; Grazhdani, S. Identification of non-native freshwater fishes in Albania and assessment of their potential threats to the national biological freshëater diversity. In Proceedings of the BALWOIS Conference, Ohrid, North Macedonia, 21–31 May 2008; pp. 34–40. [Google Scholar]
- Shumka, S.; Apostolou, A. Current Knowledge on the Status of the Most Common Non-indigenous Fish Species in the Transboundary Greater Prespa Lake (Albanian Side). Acta ZoolBulg. 2018, 70, 203–209. [Google Scholar]
- Schiemer, F.; Spindler, T.; Wintersberger, H.; Schneider, A.; Chovanec, A. Fish fry associations: Important indicators for the ecological status of large rivers. Int. Ver. Theor. Angew. Limnol. Verh. 1991, 24, 2497–2500. [Google Scholar] [CrossRef]
- Shumka, L.; Peri, L.; Lato, E. The Needs for Determining Degradation Risks from Temperature and Relative Humidity of Post-Byzantine Church Indoor Environment. J. Environ. Manag. Tour. 2020, 11, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Shumka, L. Comparison of Indoor Climate Features Following Different Climate Guidelines in Conservation Examples of Selected Churches in Albania. Int. J. Conserv. Sci. 2019, 10, 623–630. [Google Scholar]




| Species | English Name | IUCN Global | Albanian Red List | Habitat Directive | Bern Convention | Albanian/Balkan Endemics |
|---|---|---|---|---|---|---|
| Alburnoidesohridanus (Karaman, 1928) | Ohridspirlin | VU (D2) | Ohrid Lake/Balkan endemic | |||
| Alburnusscoranza (Heckel et Kner, 1858) | Scadar Bleak | LC | Balkan endemic | |||
| Barbatulasturanyi (Steindachner, 1892) | Brook loach | LC | Ohrid Lake/Balkan endemic | |||
| Barbus rebeli (Köller, 1925) | Ohrid barbell | LC | LR | Balkan edemic | ||
| Carassius gibelio (Bloch, 1782) | Prussian carp | NE | ||||
| Chondrostomaohridanus (Karaman, 1924) | Ohridnasse | DD | Balkan endemic | |||
| Cobitisohridana (Karaman, 1928) | Ohrid spined loach | LC | LR | Balkan endemic | ||
| Cypriniscarpio (Linnaeus, 1758) | Carp | VU (A2ce) | ||||
| Eudentomyzonstankokaramani (Karaman, 1974) | Drini brook lamprej | LC | II | Drini River | ||
| Gobio scadarensis (Karaman, 1924) | Scadar gudgeon | VU (D2) | LR/VU (D2) | Balkan endemic | ||
| Hypophthalmichthys molitrix (Valenciennes 1844) | Silver carp | Alien | ||||
| Oncorhynchus mykiss (Walbaum, 1792) | Rainbow trout | Alien | ||||
| Pachychilonpictum (Heckel et Kner, 1858) | Ohrid roach | LC | III | Balkan endemic | ||
| Pelasgus minutes (Karaman, 1924) | Ohridminow | DD | Ohrid Lake/Black Drini | |||
| Percafluviatilis (Linnaeus, 1758) | European perch | LC | ||||
| Phoxinus Lum/ohridanus? | Italian Minow spp | LC | ||||
| Pseudorasbora parva (Temmini&Schlegel, 1846) | Stone moroke | Alien | ||||
| Rhodeusamarus (Bloch, 1782) | Bitterling | LC | II | III | ||
| Rutilus ohridanus (Karaman, 1924) | Roach | DD | Balkan endemic | |||
| Salariafluviatilis (Asso, 1801) | Freshwater blenny | LC | III | |||
| Salmo farioides (Karaman, 1937) | Brown trout | LC | VU | |||
| Squaliusplatyceps (Bonaparte, 1837) | Chub | LC | Balkan endemic | |||
| Sander lucioperca (Linnaeus, 1758) | Pike-perch | LC |
| Threat Status | IUCN Red List | Albanian Red List |
|---|---|---|
| Critically Endangered (CR) | 1 | - |
| Endangered (EN) | - | - |
| Vulnerable (VU) | 4 | 2 |
| Near Threatened (NT) | - | - |
| Least Concern (LC) | 12 | 2 |
| Data Deficient (DD) | 3 | - |
| Not Evaluated (NE) | 3 | 19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shumka, L.; Papastefani, A.; Shumka, S.; Mali, S. The Potentials for the Ecological Management of Landscape Connectivity Including Aquatic Ecosystems in Northeast Albania. Hydrobiology 2023, 2, 44-54. https://doi.org/10.3390/hydrobiology2010004
Shumka L, Papastefani A, Shumka S, Mali S. The Potentials for the Ecological Management of Landscape Connectivity Including Aquatic Ecosystems in Northeast Albania. Hydrobiology. 2023; 2(1):44-54. https://doi.org/10.3390/hydrobiology2010004
Chicago/Turabian StyleShumka, Laura, Andi Papastefani, Spase Shumka, and Sotir Mali. 2023. "The Potentials for the Ecological Management of Landscape Connectivity Including Aquatic Ecosystems in Northeast Albania" Hydrobiology 2, no. 1: 44-54. https://doi.org/10.3390/hydrobiology2010004
APA StyleShumka, L., Papastefani, A., Shumka, S., & Mali, S. (2023). The Potentials for the Ecological Management of Landscape Connectivity Including Aquatic Ecosystems in Northeast Albania. Hydrobiology, 2(1), 44-54. https://doi.org/10.3390/hydrobiology2010004






