Abstract
Because animals threatened by visually oriented predators may respond in sun-lit daytime but not at night, invertebrate responses to predatory challenges may yield varying results based on the time period within the 24 h daily cycle. We predicted that in laboratory experiments aquatic isopods exposed to kairomones from predatory fish would spend more time immobilized in daylight to avoid detection than those not exposed to kairomones but that this difference would disappear under the cover of nighttime darkness. We further predicted that isopods in the absence of kairomones would move at elevated rates in the daytime compared with night, seeking a precautionary proximity to shelters. However, contrary to our predictions, Caecidotea communis isopods exhibited consistent activity (movement rate and proportion of time spent moving) when exposed to kairomones or in the absence of such cues, at all of the three diurnal cycle periods examined. Thus, Caecidotea communis displayed cathemerality (sometimes called metaturnality), the first documented case of this behavior in crustaceans.
1. Introduction
Aquatic isopods are prey for numerous species of vertebrate and invertebrate predators and can compose a large proportion of a predator’s diet (up to 70%; [1]). Predators in pond communities may exert unusually strong selection pressures on prey due to the limited possibilities for spatial refuge [2]. The freshwater isopod Asellus aquaticus has evolved cryptic pigmentation, presumably as an adaptation against visually oriented predators [1]. Additionally, isopods in proximity to a predator may halt activity to reduce predation risk, a behavioral response that is typically deployed only upon detection of either conspecific alarm cues or chemical cues from the predator itself (i.e., kairomones; [2,3,4]).
Crustaceans in general have well-developed chemosensory systems [5]. Freshwater amphipods decrease activity in response to a wide variety of fish, suggesting that the stimulatory chemical is inherent in most fish species [6,7]. However, our recent studies yielded the surprising result that pond-sourced Caecidotea communis (Say, 1818) isopods do not alter their rates of movements when exposed to chemical cues from three species of fish (golden shiners Notemigonus crysoleucas (Mitchill, 1814) and the sunfish Lepomis macrochirus Rafinesque, 1819 and Lepomis gibbosus (Linnaeus, 1758); [8]). However, these previous experiments [8] did not control for the specific time of day, which can affect a riparian species of isopod [9] and lacustrine individuals of our current study species [3]. Both A. aquaticus and C. communis isopods show negative phototaxis and photokinesis (as referenced in Table 7 of [10]). Our experimental isopods inhabit the littoral fringe of ponds that are typically well illuminated during the day, and the potential for vertical migration is limited. Because predatory fish can typically move much more rapidly than can isopods, even a distant fish might pose a dire threat to an isopod that has attracted notice. In these shallow aquatic systems, refuge from visually hunting predators in the daytime may require burrowing within the sediment or dense benthic layers of detrital leaves, with reduced or no movement to avoid attention from predators [11,12]. But visually oriented predatory fish often have reduced feeding rates in the dark [13,14,15], so night may allow isopods to forage with relative impunity.
Animal behavior has evolved in response to the combination and interaction of three aspects: direct responses to environmental stimuli (such as light, just discussed), endogenous circadian rhythms, and the influence of the environment on the circadian clock [12]. These aspects may favor similar behaviors and thus amplify the response. For instance, predictable daily fluctuations in predation risk due to light levels may drive adaptive predation-induced shifts in activity patterns, and eventually the evolution of endogenous circadian rhythms [12,16,17].
Numerous studies have noted that movements of aquatic invertebrates, and arthropods in particular, demonstrate circadian rhythms [18,19,20,21]. But the preponderance of studies of circadian rhythms in freshwater systems have focused on lake plankton, and their conclusions may have limited applicability to the benthic organisms of ponds that are restricted to the comparatively two-dimensional surface of the bottom. Even small isopods are typically much larger than zooplankton (such as Daphnia sp.), and so their movements in daylight might more easily catch the attention of visually oriented predators, suggesting that strong tendencies towards behavioral alterations in concert with the 24 h cycle would be adaptive. In freshwater lotic systems, nocturnally elevated rates of activity occur in crayfish [5,22], shrimp [23], mayflies, amphipods [6,24], and the river isopod Asellus sp. [25]. Relatively little is known about American species of isopods inhabiting ponds. These species admittedly represent a “decided minority” of the total known North American species (p. 439, [26]), and yet they can attain such dense populations as to be locally dominant taxa.
Isopods in general display a variety of circadian rhythms, with diurnal periodicity to metabolism [27], foraging behaviors [28], and locomotor activity [10,29,30,31,32,33,34,35]. Some intertidal isopods display circadian morphological alterations of pigment concentrations, perhaps to ensure correctly timed antipredator camouflage [36,37].
If either environmental light levels or endogenous circadian rhythms (or both) alter the response of C. communis isopods to fish kairomones, causing the cessation of isopod movement at some times of day, but elevated or unaltered rates at others, our previous surprising results of a lack of response by the isopods [8] may have been an artifact of data collection spanning multiple time periods. To examine this possibility, we conducted our current experiments at three discrete times within the 24 h daily cycle: early morning, mid-afternoon, and nighttime. We predicted that isopods exposed to kairomones from predatory fish in the light of midday would spend more time immobilized than those not exposed to kairomones so as to avoid detection. We predicted that early in the morning, when fish are likely most hungry from fasting overnight, the isopods exposed to kairomones would exhibit either extreme immobility (sustained stillness) or highly elevated rates of movement as they sought refuge. We were not sure which of these two responses would occur, as both may be adaptive, but either option would deviate greatly from background (non-kairomone) behaviors. However, we predicted that under the cover of nighttime darkness, isopods exposed to fish cues would not alter their movements. Finally, we also predicted that, in the absence of predatory cues, the isopods would move more rapidly in the daylight hours than under the cover of darkness at night, seeking proximity to shelters (which were not provided in the experimental arenas) as a safety precaution. Our experimental design combined the effects of light intensity and circadian rhythm (if they exist) in natural accordance (dark at night, natural light levels during the daytime). We reasoned that future experiments could distinguish between these two components (direct environmental stimuli and endogenous circadian rhythms).
2. Materials and Methods
2.1. Animal Collection and Cue Preparation
Isopods (Caecidotea communis (Say, 1818)) and two species of sunfish (bluegill and pumpkinseed, Lepomis macrochirus Rafinesque, 1819, and Lepomis gibbosus (Linnaeus, 1758), respectively) were collected in October and November 2020 from Meadow Pond in Graver Arboretum in Bath, PA (40°48′00.24″ N, 75°21′47.55″ W). This pond has contained healthy populations of both species of sunfish, as well as largemouth bass (Micropterus salmoides (Lacepède, 1802)) for at least a decade. Reducing bluegill populations in lakes can cause benthic macroinvertebrate density to increase [38], indicating that these fish feed voraciously on benthic invertebrates.
The fish were maintained in the laboratory in glass tanks. To ensure that the cue water contained digested prey metabolites from the fish (kairomones) and alarm cues from conspecifics, the sunfish were fed exclusively live isopods every other day during the experiments. Aquarium filters without inserts were used to ensure adequate turbulence for oxygenation without removing chemical cues.
Three community tanks, each filled with 37 L of aged tap water (to allow chlorine to off-gas for at least 48 h before use) and containing at least 15 fish, were maintained to reduce issues of pseudo-replication, but it was impossible to use different fish for each experimental replicate. Fish ranged in length from 2.7 cm to 10.6 cm (snout to tip of caudal fin), with most predators under 4 cm in length. Even the smallest fish consumed some of the isopods provided as prey, and multiple fish in each tank were capable of consuming even the largest isopods used in our experiment (Long, personal observations). Water removed from the tanks was used immediately in experiments. Our cue preparation likely produced a higher concentration of predator metabolites than is typically found in nature, although sunfish are plentiful in the pond. Because stronger concentrations of predator odors have elicited stronger antipredator responses in prey (e.g., [39,40]), we preferred to use hyper-concentrated cue preparations.
The isopods were maintained in the laboratory in water and leaves from their natal pond at 4 °C (to sustain higher oxygen levels and comparable to contemporaneous natural temperatures) while exposed to a full-spectrum light on a 12 h light/dark cycle (turning on at 06:00 and off at 18:00) to parallel natural conditions. None of our experiments were conducted during the isopod breeding season. The distance to our field site precluded daily gathering of experimental animals, but all isopods were tested within ten days of field collection. The size ranges of the isopods used in experiments were as indicated in Table 1.

Table 1.
Specific aspects of experimental design. The “fish cue” treatment contained chemicals released into the water as sunfish fed on isopods. The “no fish cue” treatment was aged tap water. N is the number of replicates conducted. If an isopod never moved, that replicate was omitted from the analysis (reducing N).
Upon the conclusion of each series of experiments, animals were euthanized by freezing except for some fish maintained in a display tank and the isopods fed to the fish. Animals were not returned to their source ponds to avoid potentially introducing any non-native microbes from the laboratory, as we receive shipments of educational animals from distant locations. Permission for sample collections was granted by Graver Arboretum and Muhlenberg College; Iyengar and Long each possessed Pennsylvania state fishing licenses for animal collections, and our experimental design was approved by the IACUC committee of Muhlenberg College.
2.2. Study Design
The study design was nearly identical to Long and Iyengar [8], except that the current experiments were conducted at three different times of day: morning (between 08:00 and 10:00, so within three hours of sunrise), afternoon (between 13:00 and 15:00), and night (between 19:00 and 21:00, so at least one hour after sunset). During all trials, the overheard room lights were turned off. In the daytime trials, the incoming natural light from two windows provided enough illumination for photography. Natural variation in nocturnal light intensities, even moonlight, can impact the degree of circadian behaviors of marine isopods and other animals [17,35,41]. Therefore, we carefully avoided exposing the isopods to artificial white light during night trials. Instead, we illuminated the room and experimental arena with red light, as crustaceans typically cannot detect wavelengths close to red light [42,43]. Illumination was provided by a 50-watt infrared lamp positioned 0.91 m above each experimental arena. Preliminary tests indicated that the lamps did not change the temperature of the water over the course of the two-hour period of the experiment. The aged tap water for the control treatment and the fish tanks providing chemical cues were each 20 °C so that all experiments were conducted at the same temperature.
Isopods were placed individually in the center of a plastic arena (15 cm length, 11 cm width, 7 cm depth) containing a uniform sand benthos and water that either contained chemical cues (“cue” treatment; sourced directly from the fish tanks) or did not (“control” treatment; aged tap water). After a one-minute acclimation period, during which time the isopod was allowed to roam freely, an aerial photo of the container was taken every two seconds for a total of five minutes using an Olympus Touch TG-5 digital camera. For at least 30 min before use in experiments, isopods (still in 4 °C maintenance water) were placed in a 20 °C room to promote a gradual transition of the water to the higher temperature, avoiding temperature shock. Previous experiments demonstrated that the isopods moved at comparable rates whether after a one-minute or a 27 min acclimation period [8], and other aquatic arthropods (larval mayflies) significantly altered their behaviors within 5 min after the addition of fish chemicals [44]. Thus, our experimental time frame likely captured any relevant responses. Four cameras were used simultaneously so we could conduct two “cue” and two “control” replicates concurrently.
We calculated the rate of movement of each individual isopod over the five-minute series of experimental photographs using the manual tracking plug-in for the computer program ImageJ version IJ 1.46r [45,46,47]. We also calculated the proportion of time the isopod spent moving during trials by summing the number of photographs in which the animal moved at least a body’s length since the previous photograph (“moved”) and dividing that by the total number of photographs taken during the interval. Between each trial, we changed the water and sand substratum and rinsed the arena. To minimize the problem of pseudo-replication, we sequentially used chemical cues from each of the three source tanks containing fish.
No isopod was used in more than one trial. If an isopod never moved over the entire observation period, it was excluded from the analysis to avoid including data from injured or otherwise aberrant animals. This exclusion was rarely necessary (see Table 1 for exact numbers).
2.3. Evaluating the Efficacy of Our Experimental Design
To confirm that our experimental arena was accurately representing isopod behaviors rather than providing an uncomfortable environment that caused the animals to merely always move at top speed in an attempt to escape, we conducted a positive control experiment (in August, while the time-of-day studies were conducted in a previous autumn). We utilized the same experimental design described above, but instead of the cue of a predator, we used a food cue. This food cue was created by submerging naturally conditioned leaves and benthic detritus from the natal pond in a plastic tub containing 4 L of aged tap water for at least 24 h. The water was then decanted and poured through fine netting to capture particles before use in the experimental arena. Aged tap water (without the leaves and detritus) was used as the control treatment. We sequentially used water from at least two tubs of each type of treatment water to reduce issues of pseudo-replication.
Isopods were maintained for 48 h before experiments in pond water filtered through fine mesh to remove particulates. Our intent with the elapsed time was to allow the isopods to become hungry but not so food-deprived that they would reduce locomotor activities. Otherwise, the experiment proceeded during midday as described above.
2.4. Data Analysis
A two-way ANOVA was performed, with cue/control and the time of day (morning, afternoon, night) as the independent variables, and either rate of movement or proportion of time moved (in separate analyses) as the dependent variable. Utilizing only the subset of the data from the control treatments, a one-way ANOVA was calculated with the time of day as the independent variable, and either rate of movement or proportion of time moved (in separate analyses) as the dependent variable. Finally, our data from the experiment utilizing the positive control (food cue) was analyzed in a one-way ANOVA, with the rate of movement as the dependent variable and the cue/control treatment as the independent variable.
In all analyses, equal variance and normal distribution of residuals were assessed using, respectively, Levene’s test and the Kolmogorov test of the normality curve of residuals to confirm that the data met the assumptions inherent in parametric tests. All analyses, including those with proportion of time spent moving, met these assumptions without transformation. All statistical analyses were conducted using the computer program Data Desk (version 6.0; Data Description, Ithaca, NY, USA). We used p < 0.05 as the critical value in all statistical comparisons.
3. Results
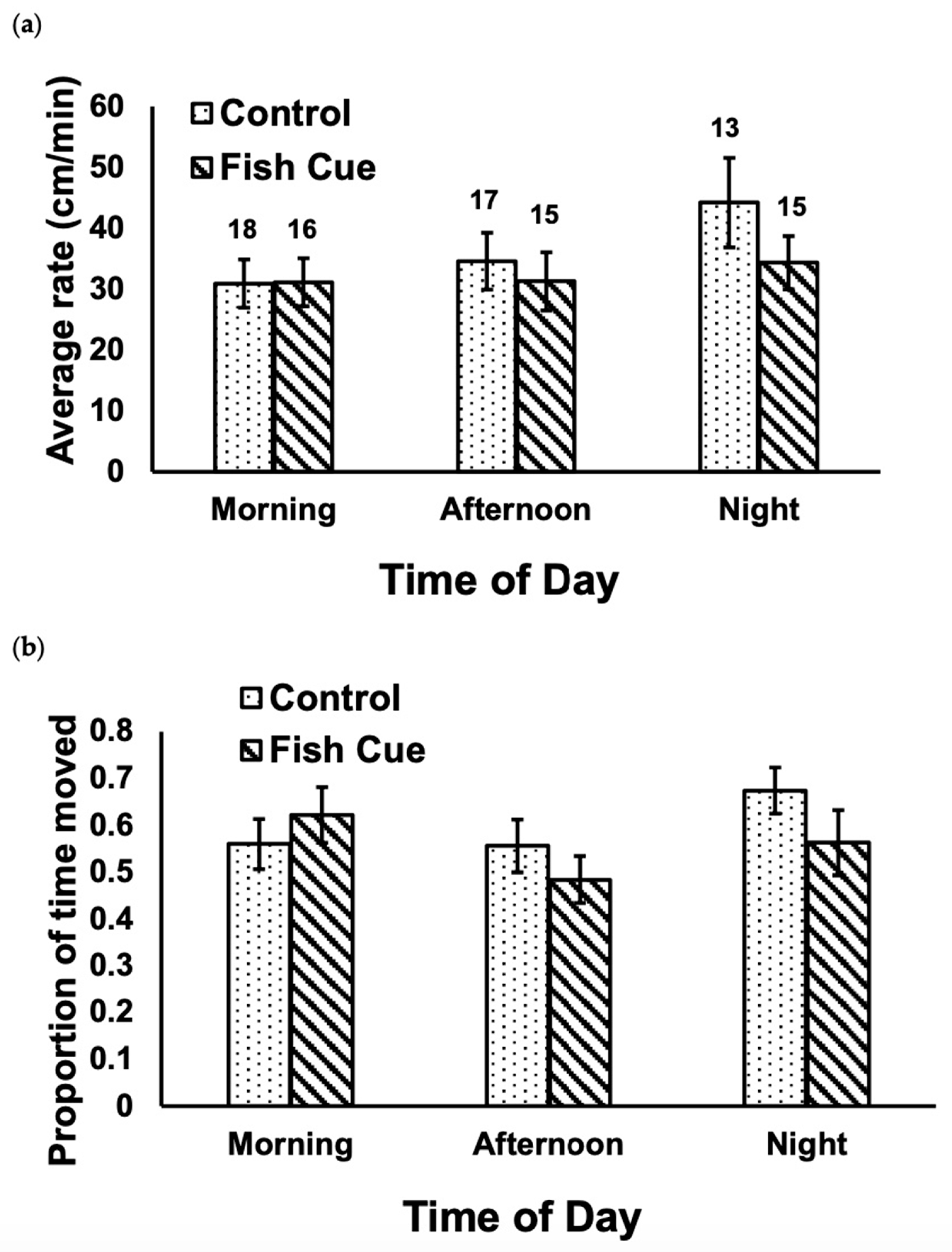
Cues from predatory fish did not significantly alter the rate of movement of isopods (Figure 1a; F1,88 = 1.21, p = 0.28), nor was there a significant effect of the time of day (F2,88 = 1.54, p = 0.22). The was no significant interaction between cue and time of day (Figure 1a; F2,88 = 0.55, p = 0.58). Similarly, for the proportion of time spent moving by isopods, there were no significant effects in terms of the chemical cues from fish (Figure 1b; F1,88 = 0.74, p = 0.39), the time of day (F2,88 = 1.54, p = 0.22), nor the interaction term (F2,88 = 1.28, p = 0.28). Within the control treatments, there was no significant effect of time of day on either the rate of movement (Figure 1a; F2,45 = 1.61, p = 0.21) or the proportion of time spent moving by the isopods (Figure 1b; F2,45 = 1.36, p = 0.27).
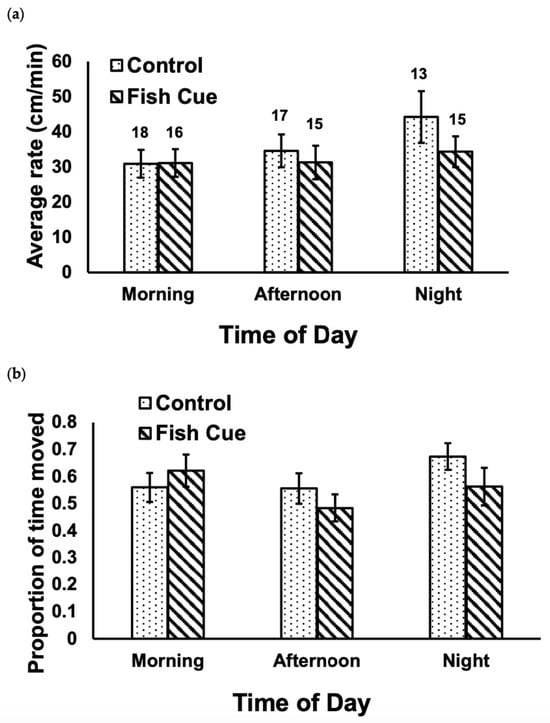
Figure 1.
There was no significant effect of fish cue (p = 0.28, p = 0.39), time of day (p = 0.22, p = 0.22), or the interaction term (p = 0.58, p = 0.28) on the average (a) rate of movement or (b) proportion of time spent moving by individual Caecidotea communis isopods (p-values reported in the respective order of (a,b)). The numbers above the bars in (a) indicate the number of replicates for each treatment; error bars are ±1SE. Examining only data from the control treatment, there was no significant difference among the three times of day in either (a) or (b): p = 0.21, p = 0.27, respectively.
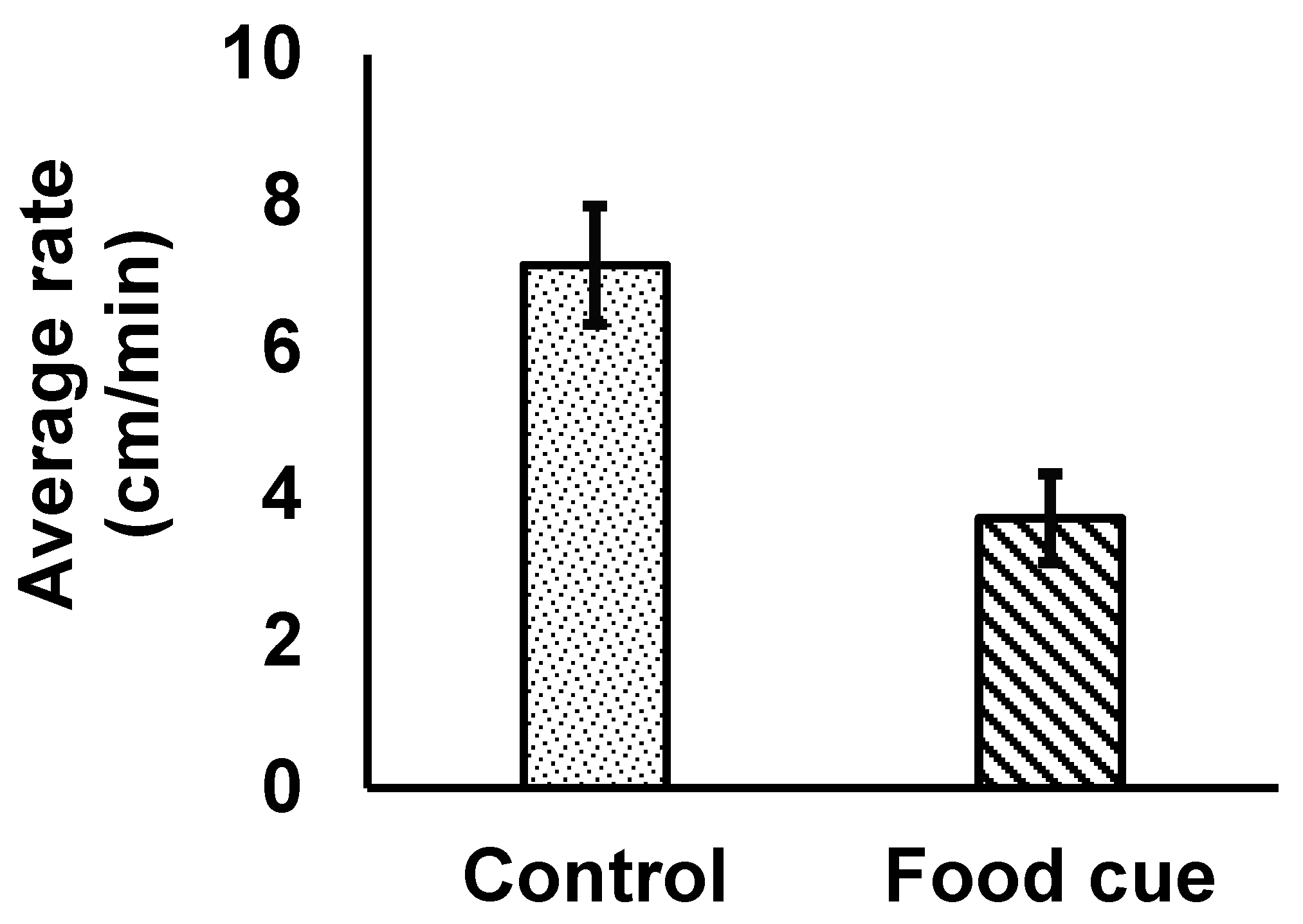
In contrast, isopods moved statistically significantly more quickly in the control water than in the positive control (water containing food cues; Figure 2; F1,14 = 9.04, p = 0.0094), demonstrating that our experimental design was effective.
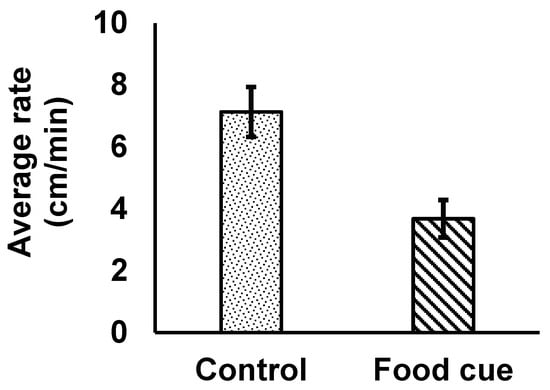
Figure 2.
The average (±1SE) rate of movement by individual Caecidotea communis isopods was significantly slower when animals were immersed in aged tap water containing food chemical cues (“food cue” with diagonal patterning in the figure) than in aged tap water without such cues (“control” with speckled patterning in the figure). N = 8 for both treatments.
4. Discussion
Contrary to our original predictions but congruent with our previous findings [8], we found no evidence at any time of day that the isopods changed their movement rates or proportion of time spent moving in response to chemical cues from sympatric predators. These results may indicate that C. communis isopods move at similar rates because they are unresponsive to both predator cues and increased visibility. Alternatively, the isopods may move at a maximum rate during both the daytime and nighttime even in the absence of predator cues, ostensibly to find shelters (not provided in the test arenas). However, the former appears more likely, given that some C. communis will climb into cages containing sunfish predators in fishless ponds at Graver Arboretum [48].
Our current result is even more surprising given that lacustrine C. communis increased sheltering when exposed to bluegill fish cues in the laboratory (ref. [3]; a non-peer-reviewed publication). Because captive sunfish consumed isopods similar in size to those in our experiment, the difference in results was not because the isopods in our experiments had attained a size-refuge from predators. Whether the difference between our consistent results and those of Saunders [3] is due to source habitat (pond versus lake), response examined (speed/proportion of time spent moving versus sheltering), or group size (solitary versus 10 isopods) is unknown but warrants further investigation. Because others (e.g., [6,49]) have proposed that the fish kairomones inducing defensive behaviors in freshwater amphipods and isopods are basic secretions inherent across most piscine taxa, variability among isopod populations from different ecosystems appears more likely than variability among the cues from the fish predators. However, due to the lack of peer review and the much smaller number of replicates in Saunders (ref. [3]: only three trials versus at least 13 trials herein), we hesitate to conclude that lacustrine populations of isopods react differently than pond populations to fish chemical cues without additional confirmatory experimentation.
4.1. Individuals of Caecidotea Communis Display Cathemerality
Contrary to our predictions, locomotory behaviors were extremely consistent at all three time periods of the day examined (morning, midday, and night). This pattern fits the definition of cathemerality: animals active throughout both light and dark phases of the 24 h cycle (sometimes called metaturnality; [50]) rather than adhering to diurnal, nocturnal, or crepuscular patterns of activity–rest patterns. While originally noted as an unusual behavior for mammals, reports of cathemerality are increasing, suggesting that it may be more prevalent in vertebrates than originally considered [50]. This behavior has been reported for some fish [51,52,53] and in at least 34 species of arthropods (ants, moths, and spiders; [54]), but we were unable to find any published literature applying this term to crustaceans. Thus, our study may be the first to document cathemerality in an aquatic isopod.
The temperature in these laboratory studies was the same in the day and night. However, water’s high specific heat dampens daily temperature fluctuations in ponds within temperate deciduous forests, and the multiple layers of autumnal leaves covering the bottom may prevent incoming light rays from warming the benthos. Further research investigating whether this pattern of consistent activity occurs in nature for C. communis and other detritivores within ponds is of interest.
Cathemerality may be driven by the combination of selection pressures from both predators and energy needs. While sunfish typically do not feed at night due to reduced visual acuity [55], fishermen note that bluegills will bite at lures at any time of the day year round [56]. Bluegills often move to warmer water in winter, such as shallow waters receiving direct sunlight [56], which coincides with where we collected the isopods. Therefore, the risk of predation by bluegill for the isopods may be more constant across the 24 h cycle than assumed in our original hypothesis. A lack of diel cycles may render individual isopods “temporally cryptic”, as irregular, flexible activity patterns are less predictable for predators [57]. Furthermore, a shift to diurnal foraging despite higher predation threats has been reported in some fish because feeding only at night could not meet energetic requirements [52]. Cathemerality may allow animals longer foraging times than provided by either diurnal or nocturnal activity patterns. While this hypothesis has been proposed to explain the increased tendency to cathemerality of larger-bodied mammals [58], it may also apply to larger-bodied detritivores. Due to the lower assimilation efficiency typical of aquatic detritivores compared with carnivores [59], supporting the appreciable body mass of isopods may require more hours feeding than available during the night. Thus, cathemerality in C. communis isopods may function both as an anti-predator adaptation and a foraging benefit.
4.2. Circadian Rhythms and Inducible Alterations in Movement Rate Might Not Be Adaptive
While circadian rhythms have been demonstrated in shore crabs [60], terrestrial isopods [10,29,32], and isopods of sandy beaches [31,34,35], they appear to be lacking in our pond isopods. Perhaps in lentic systems circadian rhythms are less important, as the extreme temperature fluctuations present in the terrestrial systems just referenced will be dampened or non-existent, and rapid light attenuation in murky conditions might render external light cycles irrelevant. While many fish are visually oriented, they also often use other sensory modalities in predation to detect concealed prey or find prey in the dark [61]. Indeed, isopods and amphipods in streams reduced movements in response to sunfish but only at night, not in the daytime [9,62]). Brochu and Aubin-Horth [63] were surprised to discover that wild-caught threespine sticklebacks were mostly nocturnal and that this species, like numerous other fish species, showed intraspecific differences in the strength of daily activity rhythms. Some individuals were nocturnal, others diurnal, and others were active around the clock [63]. If many fish species, especially sunfish and largemouth bass, have extreme amounts of intraspecific variability in activity timing, circadian rhythms and negative photoactivity may fail to protect isopods from predation.
The diurnal patterns of the entire suite of potential predators, not just the fish, drive adaptations in isopod prey. Many insects are active in very dim light, with eyes adapted to promote success in nocturnal environments [64]. Belostomatids, which occur in Meadow Pond, can capture equal numbers of amphipods in light and dark treatments [65], while dragonflies capture more isopods at night [3]. Backswimmers and the larvae of some diving beetles use mechanoreception to effectively find prey in the dark [14,66,67], and some water scorpions shift to active nocturnal hunting from daytime ambush strategies [68,69]. At night these predatory insects may more easily detect the pressure waves from lumbering larger-bodied isopods than smaller prey, reducing the difference in predation risk between daytime and nighttime for isopods in ponds with many invertebrate predators. If adaptations against one predatory strategy (e.g., of visually oriented fish) cause increased susceptibility to other predator strategies (e.g., mechanoreception of benthic macroinvertebrates), effective responses to fish predators may be precluded. Therefore, the lack of response by the isopods in our experiments may have been because the isopods chose a middle-of-the-road defensive strategy or perhaps one that is optimized for metabolic expenditures, rather than focusing on reacting to fish.
As a final note on this subject, Saunders [3] concluded there was evidence of circadian differences in the behavior of lacustrine C. communis because cues from dragonfly larvae caused a reduction in the number of unsheltered isopods in the day, but this response to cues was not seen at night. However, the daytime difference reported by Saunders (ref. [3]; Figure 12 therein) appears largely to be the result of the erratic behavior of animals in the control treatment in the first few days, so neither a response to dragonflies nor a circadian difference appears likely in natural situations.
4.3. Our Experimental Design Can Induce Behavioral Responses
To eliminate the possibility that we did not detect alterations in isopod movements in the presence of fish cues only because our experimental arena provided a stressful alien environment that precluded natural responses, we conducted a positive control experiment: exposing hungry isopods to the scents of food. It was impossible to utilize a negative stimulus to confirm the efficacy of our experimental design because we have not found such a stimulus—we had predicted fish kairomones would be one of the strongest of such stimuli. Therefore, we focused on presenting a positive cue instead. We predicted that the isopods would demonstrate kinesis; thus, animals in the food cue, a favorable environment, would slow their movements and perhaps turn more often (as terrestrial isopods do in humid, rather than dry, environments; [70]). Animals using the strategy of kinesis stay longer in good conditions and more quickly leave poor conditions, which is especially useful for exploring areas with patches and fluctuations in food distribution [71]. This slower-movement response when detecting food cues should allow the isopods to be more likely to remain in the favorable area until they encounter the source from which the cue emanated.
Indeed, the isopods immersed in aged tap water that had recently contained leaves and detritus significantly reduced their rates of movement compared to isopods in aged tap water without food cues. This behavioral alteration suggests that the isopods were activating kinetic responses. Thus, with our experiments using fish cues in aged tap water, the arena did not present such adverse conditions that the isopods did not show a response due to arena-related stress.
4.4. Other Responses May Be More Adaptive
Because freshwater isopods have responded to chemical cues from predators in other studies (e.g., [4,49]), we hesitate to conclude that our animals could not detect the chemical cues. Rather than altering rates of movement, our pond isopods may respond to predatory fish through other responses, such as increasing sheltering, grouping behaviors, or altering their location within the pond.
The slow-moving nature of our isopods may have caused the animals to disregard the potential cover of nightfall and lack of predatory cues and incessantly search for possible shelter to find a refuge option should one be needed (as shelters provided effective protection against fish for A. aquaticus; [61]). Additionally, prey in groups may benefit from the dilution effect and the confusion effect groups [17], so the constant movements of our solitary pond isopods may have reflected group-seeking behaviors. It would be of interest in future studies to examine whether animals that had encountered a shelter would be (a) more likely to move more slowly outside of the shelter when in proximity to the safe haven, and (b) if predator cue was introduced to the arena, whether the isopods would then head directly for the shelter, indicating that they spatially remembered where it existed. Lastly, our experimental design did not provide a depth gradient to the benthos. Thus, we could not observe any diel vertical migration, as performed by numerous zooplankton (e.g., [72,73]) but rarely examined in benthic species. Because descent to depth via travel over short horizontal benthic distances is possible in many ponds, future experiments examining this aspect would be intriguing.
5. Conclusions
Our results demonstrate that C. communis collected from a pond environment display insensitivity to predator chemical cues and cathemerality, challenging traditional assumptions about isopod behavior in freshwater ecosystems. Cathemerality may provide both anti-predator and foraging adaptations for these relatively large-bodied detritivores. The possibility that isopods living within ponds react differently to chemicals from fish predators than do lacustrine and stream isopods, perhaps due to the elevated diversity and density of macroinvertebrate predators in ponds, is intriguing and warrants further study.
Author Contributions
Both authors participated in all stages of this research and manuscript writing, with initial draft writing and substantially more investigation by E.C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received funding only from Muhlenberg College, including a John E. Trainer grant to E.C. Long.
Institutional Review Board Statement
All applicable institutional and/or national guidelines for the care and use of animals were followed. The use of fish as reported in these experiments was approved by the IACUC committee of Muhlenberg College.
Data Availability Statement
Data will be provided by the corresponding author upon receipt of a reasonable request.
Acknowledgments
We thank the John E. Trainer grant of Muhlenberg College for funding to E.C. Long, the Graver Arboretum (T. Shotzbarger in particular) for permission to collect animals, T. Iyengar and B. Cardonick for collection assistance, and K. Conn and T. Sime-Ngando for comments on the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lafuente, E.; Lürig, M.D.; Rövekamp, M.; Matthews, B.; Buser, C.; Vorburger, C.; Räsänen, K. Building on 150 years of knowledge: The freshwater isopod Asellus aquaticus as an integrative eco-evolutionary model system. Front. Ecol. Evol. 2021, 9, 748212. [Google Scholar] [CrossRef]
- Vandekerkhove, J.; Namiotko, T.; Hallmann, E.; Martens, K. Predation by macroinvertebrates on Heterocypris incongruens (Ostracoda) in temporary ponds: Impacts and responses. Fundam. Appl. Limnol. 2012, 181, 39–47. [Google Scholar] [CrossRef]
- Saunders, B.D. Behavioral Response of the Amphipod Gammarus fasciatus and the Isopod Asellus communis to Fish (Lepomis macrochirus) and Dragonfly (Gomphidae) Predators. Master’s Thesis, The College of William and Mary, Williamsburg, VA, USA, 1981. [Google Scholar] [CrossRef]
- Bengtsson, G. Energetic costs of amino acids exudation in the interactions between the predator Gammarus pulex L. and the prey Asellus aquaticus L. J. Chem. Ecol. 1982, 8, 1271–1281. [Google Scholar] [CrossRef]
- Cushing, C.E.; Allan, J.D. Streams: Their Ecology and Life; Academic Press: San Diego, CA, USA, 2001. [Google Scholar]
- Williams, D.D.; Moore, K.A. The role of semiochemicals in benthic community relationships of the lotic amphipod Gammarus pseudolimnaeus: A laboratory analysis. Oikos 1985, 44, 280–286. [Google Scholar] [CrossRef]
- Wudkevich, K.; Wisenden, B.D.; Chivers, D.P.; Smith, R.J.F. Reactions of Gammarus lacustris to chemical stimuli from natural predators and injured conspecifics. J. Chem. Ecol. 1997, 23, 1163–1173. [Google Scholar] [CrossRef]
- Long, E.C.; Iyengar, E.V. Effects of chemical cues from two piscine predators, natal predator regime, and time since cue introduction, on the movements of aquatic isopods (Caecidotea communis). Hydrobiologia 2022, 849, 1–12. [Google Scholar] [CrossRef]
- Holomuzki, J.R.; Short, T.M. Ontogenetic shifts in habitat use and activity in a stream-dwelling isopod. Ecography 1990, 13, 300–307. [Google Scholar] [CrossRef]
- Warburg, M.R. Behavioral adaptations of terrestrial isopods. Am. Zool. 1968, 8, 545–559. [Google Scholar] [CrossRef]
- Hansson, L.-A.; Becares, E.; Fernández-Aláez, M.; Fernández-Aláez, C.; Kairesalo, T.; Miracle, M.R.; Romo, S.; Stephen, D.; Vakkilainen, K.; van de Bund, W.; et al. Relaxed circadian rhythm in zooplankton along a latitudinal gradient. Oikos 2007, 116, 585–591. [Google Scholar] [CrossRef]
- Kronfeld-Schor, N.; Dayan, T. Partitioning of time as an ecological resource. Ann. Rev. Ecol. Evol. Syst. 2003, 34, 153–181. [Google Scholar] [CrossRef]
- Zaret, T.M.; Suffern, J.S. Vertical migration in zooplankton as a predator avoidance mechanism. Limnol. Oceanogr. 1976, 21, 804–813. [Google Scholar] [CrossRef]
- Gergs, A.; Hoeltzenbein, N.I.; Ratte, H.T. Diurnal and nocturnal functional response of juvenile Notonecta maculata considered as a consequence of shifting predation behaviour. Behav. Process. 2010, 85, 151–156. [Google Scholar] [CrossRef]
- Bolton, D.; Mayer-Pinto, M.; Clark, G.F.; Dafforn, K.A.; Brassil, W.A.; Becker, A.; Johnston, E.L. Coastal urban lighting has ecological consequences for multiple trophic levels under the sea. Sci. Total Environ. 2017, 576, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yerushalmi, S.; Green, R.M. Evidence for the adaptive significance of circadian rhythms. Ecol. Lett. 2009, 12, 970–981. [Google Scholar] [CrossRef]
- Rubenstein, D.R.; Alcock, J. Animal Behavior, 11th ed.; Sinauer Associates: New York, NY, USA, 2019. [Google Scholar]
- Aréchiga, H.; Fernández-Quiróz, F.; Fernández de Miguel, F.; Rodríguez-Sosa, L. The circadian system of crustaceans. Chronobiol. Int. 1993, 10, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Buskey, E.J. Factors affecting feeding selectivity of visual predators on the copepod Acartia tonsa: Locomotion, visibility and escape responses. Hydrobiologia 1994, 292/293, 447–453. [Google Scholar] [CrossRef]
- Ringelberg, J. Diel Vertical Migration of Zooplankton in Lakes and Oceans: Causal Explanations and Adaptive Significances; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Yokomizo, T.; Takahashi, Y. Plasticity of circadian and circatidal rhythms in activity and transcriptomic dynamics in a freshwater snail. Heredity 2024, 132, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, J.M.; Genco, M.C.; Marlow, E.D.; Benton, J.L.; Beltz, B.S.; Sandeman, D.C. Brain photoreceptor pathways contributing to circadian rhythmicity in crayfish. Chronobiol. Int. 2009, 26, 1136–1168. [Google Scholar] [CrossRef]
- Johnson, S.L.; Covich, A.P. The importance of night-time observations for determining habitat preferences of stream biota. Regul. River 2000, 16, 91–99. [Google Scholar] [CrossRef]
- Holt, C.S.; Waters, T.F. Effect of light intensity on the drift of stream invertebrates. Ecology 1967, 48, 225–234. [Google Scholar] [CrossRef]
- Moon, H.P. An investigation of the movements of fresh-water invertebrate faunas. J. Anim. Ecol. 1940, 9, 76–83. [Google Scholar] [CrossRef]
- Pennak, R.W. Fresh-Water Invertebrates of the United States, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1978. [Google Scholar]
- Wright, J.C.; Peña-Peralta, M. Diel variation in ammonia excretion, glutamine levels, and hydration status in two species of terrestrial isopods. J. Comp. Physiol. B 2005, 175, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Broly, P.; Deneubourg, J.-L. Behavioural contagion explains group cohesion in a social crustacean. PLoS Comput. Biol. 2015, 11, e1004290. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.T.F.; Larimer, J.L. Circadian wheel-running behavior in the isopod, Armadillidium vulgare. J. Exp. Zool. 1979, 209, 73–80. [Google Scholar] [CrossRef]
- Williams, J. The endogenous locomotor activity rhythm of four supralittoral peracarid crustaceans. J. Mar. Biolog. Assoc. UK 1983, 63, 481–492. [Google Scholar] [CrossRef]
- Quilter, C.G.; Lewis, R.D. Clock control of foraging in the isopod Scyphax ornatus Dana. N. Z. J. Zool. 1989, 16, 373–382. [Google Scholar] [CrossRef]
- Refinetti, R. Circadian rhythm of locomotor activity in the pill bug, Armadillidium vulgare (Isopoda). Crustaceana 2000, 73, 575–583. [Google Scholar] [CrossRef]
- Ammar, K.N.; Morgan, E. Preliminary observations on the natural variation in the endogenous rhythm of the desert isopod Hemilepistus reaumurii. Eur. J. Soil Biol. 2005, 41, 63–68. [Google Scholar] [CrossRef]
- Cheeseman, J.F.; Fewster, R.M.; Walker, M.M. Circadian and circatidal clocks control the mechanism of semilunar foraging behaviour. Sci. Rep. 2017, 7, 3780. [Google Scholar] [CrossRef]
- Duarte, C.; Quintanilla-Ahumada, D.; Anguita, C.; Manriquez, P.H.; Widdicombe, S.; Pulgar, J.; Silva-Rodriquez, E.A.; Miranda, C.; Manriquez, K.; Quijon, P.A. Artificial light pollution at night (ALAN) disrupts the distribution and circadian rhythm of a sandy beach isopod. Environ. Pollut. 2019, 248, 565–573. [Google Scholar] [CrossRef]
- Armitage, K.B. Chromatophore behavior in the isopod Ligia occidentalis Dana, 1853. Crustaceana 1960, 1, 193–207. [Google Scholar] [CrossRef]
- Willmer, P.G.; Baylis, M.; Simpson, C.L. The roles of colour change and behaviour in the hygrothermal balance of a littoral isopod, Ligia oceanica. Oecologia 1989, 78, 349–356. [Google Scholar] [CrossRef]
- DeBoom, C.S.; Wahl, D.H. Piscivore enhancement effects on food webs depend on planktivore body size and species composition in replicated whole lake experiments. Hydrobiologia 2014, 736, 31–49. [Google Scholar] [CrossRef]
- Ferrari, M.C.O.; Messier, F.; Chivers, D.P. Can prey exhibit threat-sensitive generalization of predator recognition? Extending the predator recognition continuum hypothesis. Proc. Biol. Sci. 2008, 275, 1811–1816. [Google Scholar] [CrossRef]
- Brown, G.E.; Macnaughton, C.J.; Elvidge, C.K.; Ramnarine, I.; Godin, J.J. Provenance and threat-sensitive predator avoidance patterns in wild-caught Trinidadian guppies. Behav. Ecol. Sociobiol. 2009, 63, 699–706. [Google Scholar] [CrossRef]
- Anderson, N. Depressant effect of moonlight on activity of aquatic insects. Nature 1966, 209, 319–320. [Google Scholar] [CrossRef]
- Marshall, N.J.; Cronin, T.W.; Frank, T.M. Visual adaptations in crustaceans: Chromatic, developmental, and temporal aspects. In Sensory Processing in Aquatic Environments; Collin, S.P., Marshall, N.J., Eds.; Springer: New York, NY, USA, 2003. [Google Scholar] [CrossRef]
- Frank, T.M.; Johnsen, S.; Cronin, T.W. Light and vision in the deep-sea benthos: II. Vision in deep-sea crustaceans. J. Exp. Biol. 2012, 215, 3344–3353. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, A.R.; Peckarsky, B.L.; Taylor, B.W. Rapid size-specific changes in the drift of Baetis bicaudatus (Ephemeroptera) caused by alterations in fish odour concentration. Oecologia 1999, 118, 256–264. [Google Scholar] [CrossRef]
- Cordelières, F.P. Manual Tracking. 2005. Available online: https://imagej.nih.gov/ij/plugins/track/ManualTrackingplugin.pdf (accessed on 29 April 2020).
- Ferreira, T.; Rasband, W.S. ImageJ User Guide—IJ 1.46. 2010–2012. Available online: https://imagej.nih.gov// (accessed on 29 April 2020).
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Iyengar, E.V.; Hoffman, A.R.; Russell, J.C. Benthic pond macroinvertebrates coexist with nearby potentially predatory fish. Biol. Bull. 2024, 246, 11–21. [Google Scholar] [CrossRef]
- Short, T.M.; Holomuzki, J.R. Indirect effects of fish on foraging behaviour and leaf processing by the isopod Lirceus fontinalis. Freshw. Biol. 1992, 27, 91–97. [Google Scholar] [CrossRef]
- Tattersall, I. The concept of cathemerality: History and definition. Folia Primatol. 2006, 77, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Alanärä, A.; Brännäs, E. Diurnal and nocturnal feeding activity in Arctic char (Salvelinus alpinus) and rainbow trout (Oncorhynchus mykiss). Can. J. Fish. Aquat. Sci. 1997, 54, 2894–2900. [Google Scholar] [CrossRef]
- Metcalfe, N.B.; Steele, G.I. Changing nutritional status causes a shift in the balance of nocturnal to diurnal activity in European minnows. Funct. Ecol. 2001, 15, 304–309. [Google Scholar] [CrossRef]
- Fox, R.J.; Bellwood, D.R. Unconstrained by the clock? Plasticity of diel activity rhythm in a tropical reef fish, Siganus lineatus. Funct. Ecol. 2011, 25, 1096–1105. [Google Scholar] [CrossRef]
- Cox, D.T.C.; Gaston, K.J. Cathemerality: A key temporal niche. Biol. Rev. Camb. Philos. Soc. 2024, 99, 329–347. [Google Scholar] [CrossRef]
- MNDNR: Minnesota Department of Natural Resources. How to Catch a Sunfish. 2024. Available online: https://www.dnr.state.mn.us/gofishing/how-catch-sunfish.html (accessed on 23 December 2024).
- Courtillet, A. Tailored Tackle: The Best Time to Fish for Bluegill and Sunfish. 2021. Available online: https://tailoredtackle.com/the-best-time-to-fish-for-bluegill/ (accessed on 23 December 2024).
- Colquhoun, I.C. Anti-predator strategies of cathemeral primates: Dealing with predators of the day and the night. In Primate Anti-Predator Strategies. Developments in Primatology: Progress and Prospects; Gursky, S.L., Nekaris, K.A.I., Eds.; Springer: Boston, MA, USA, 2007. [Google Scholar]
- van Schaik, C.P.; Griffiths, M. Activity periods of Indonesian rain forest mammals. Biotropica 1996, 28, 105–112. [Google Scholar] [CrossRef]
- Welch, H.E. Relationships between assimiliation efficiencies and growth efficiencies for aquatic consumers. Ecology 1968, 49, 755–759. [Google Scholar] [CrossRef]
- Reid, D.G.; Naylor, E. Are there separate circatidal and circadian clocks in the shore crab Carcinus maenas? Mar. Ecol. Prog. Ser. 1989, 52, 1–6. [Google Scholar] [CrossRef]
- Hay, A.M. Foraging Behaviour of the Ruffe (Gymnocephalus cernuus) and Predator Avoidance by the Freshwater Isopod Asellus aquaticus: Implications for Predator-Prey Interactions. Ph.D. Thesis, Division of Environmental and Evolutionary Biology, University of Glasgow, Glasgow, Scotland, 1999. ProQuest Number: 13818650. [Google Scholar]
- Holomuzki, J.R.; Hoyle, J.D. Effect of predatory fish presence on habitat use and diel movement of the stream amphipod, Gammarus minus. Freshw. Biol. 1990, 24, 509–517. [Google Scholar] [CrossRef]
- Brochu, M.-P.; Aubin-Horth, N. Shedding light on the circadian clock of the threespine stickleback. J. Exp. Biol. 2021, 224, jeb242970. [Google Scholar] [CrossRef] [PubMed]
- Greiner, B. Adaptations for nocturnal vision in insect apposition eyes. Int. Rev. Cytol. 2006, 250, 1–46. [Google Scholar]
- Runck, C.; Blinn, D.W. Role of Belostoma bakeri (Heteroptera) in the trophic ecology of a fishless desert spring. Limnol. Oceanogr. 1994, 39, 1800–1812. [Google Scholar] [CrossRef]
- Gilbert, J.J.; Hampton, S.E. Diel vertical migrations of zooplankton in a shallow, fishless pond: A possible avoidance-response cascade induced by notonectids. Freshw. Biol. 2001, 46, 611–621. [Google Scholar] [CrossRef]
- Inoda, T.; Inoda, Y.; Rullan, J.K. Larvae of the water scavenger beetle, Hydrophilus acuminatus (Coleoptera: Hydrophilidae) are specialist predators of snails. Eur. J. Entomol. 2015, 112, 145–150. [Google Scholar] [CrossRef]
- Blinn, D.W.; Pinney, C.; Sanderson, M.W. Nocturnal planktonic behavior of Ranatra montezuma Polhemus (Nepidae: Hemiptera) in Montezuma Well, Arizona. J. Kans. Entomol. 1982, 55, 481–484. [Google Scholar]
- Blinn, D.W.; Runck, C.; Davies, R.W. The impact of prey behaviour and prey density on the foraging ecology of Ranatra montezuma (Heteroptera): A serological examination. Can. J. Zool. 1993, 71, 387–391. [Google Scholar] [CrossRef]
- Gunn, D.L. The humidity reactions of the wood-louse, Porcellio scaber (Latreille). J. Exp. Biol. 1937, 14, 178–186. [Google Scholar] [CrossRef]
- Gorban, A.N.; Çabukoǧlu, N. Basic model of purposeful kinesis. Ecol. Complex. 2018, 33, 75–83. [Google Scholar] [CrossRef]
- Loose, C.J.; Dawidowicz, P. Trade-offs in diel vertical migration by zooplankton: The costs of predator avoidance. Ecology 1994, 75, 2255–2263. [Google Scholar] [CrossRef]
- De Meester, L.; Dawidowicz, P.; Van Gool, E.; Loose, C.J. Ecology and evolution of predator-induced behavior of zooplankton: Depth selection behavior and diel vertical migration. In The Ecology and Evolution of Inducible Defenses; Tollrian, R., Harvell, C.D., Eds.; Princeton University Press: Princeton, NJ, USA, 1999; pp. 160–176. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).