Insights from Density Functional Theory on the Feasibility of Modified Reactive Dyes as Dye Sensitizers in Dye-Sensitized Solar Cell Applications
Abstract
:1. Introduction
2. Computational Details
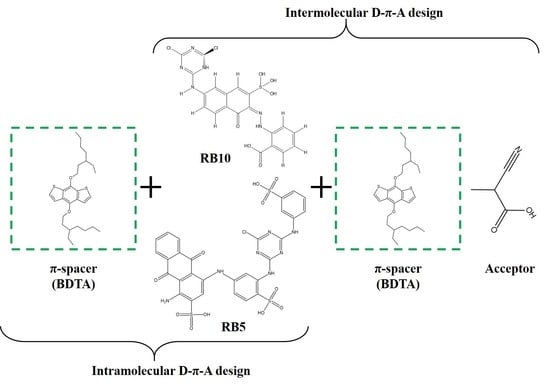
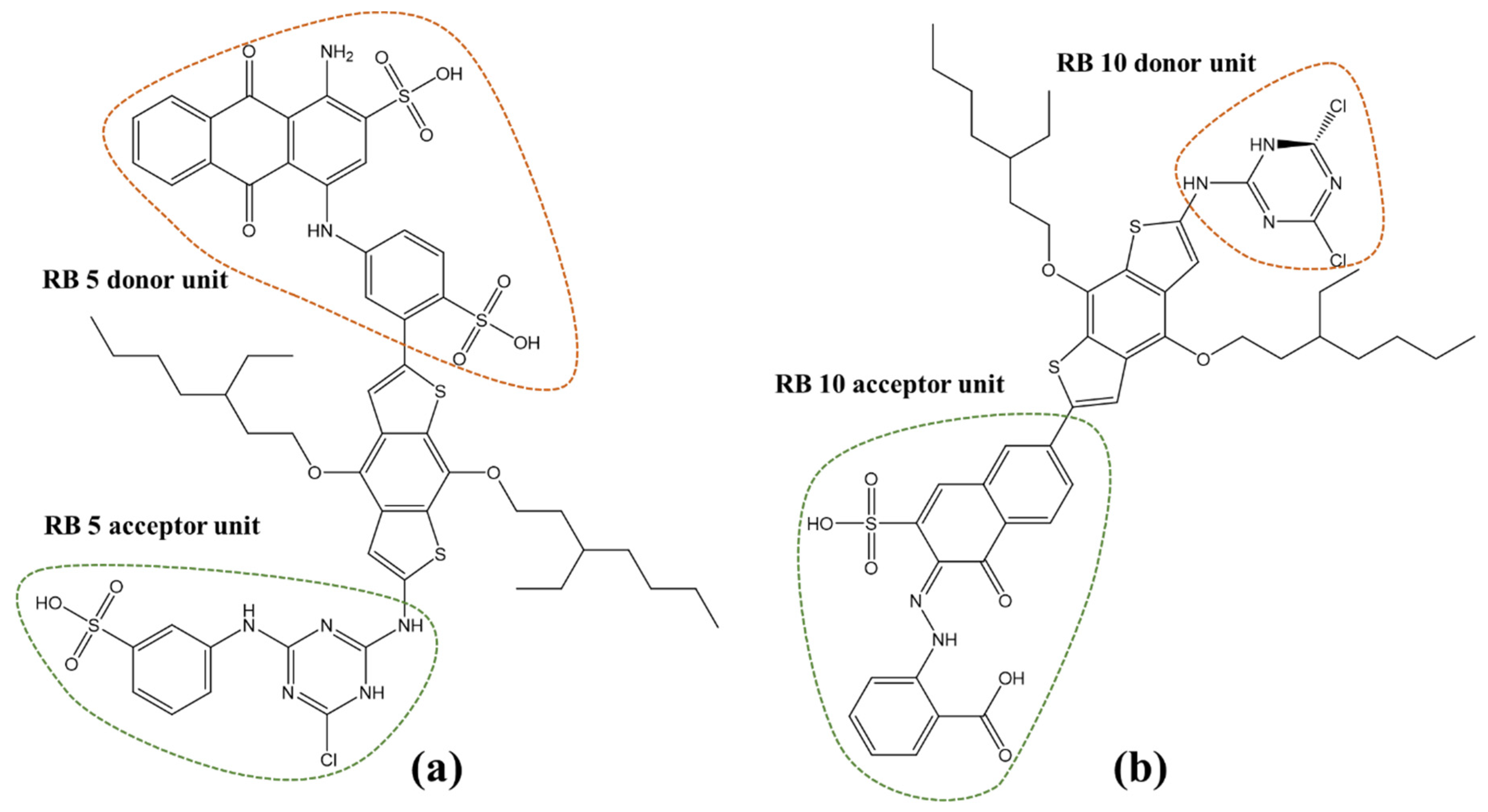
3. Design of RB 5 and RB 10-Based Dye Sensitizers
- (i)
- A suitable functional group (–Cl) was replaced in the dye molecules with an electron-donating –C2H5 (Et) group and an electron-withdrawing –NO2 group.
- (ii)
- (Intramolecular donor and acceptor units of the dyes were identified and connected with the BDTA spacer, as depicted in Figure 2.
- (iii)
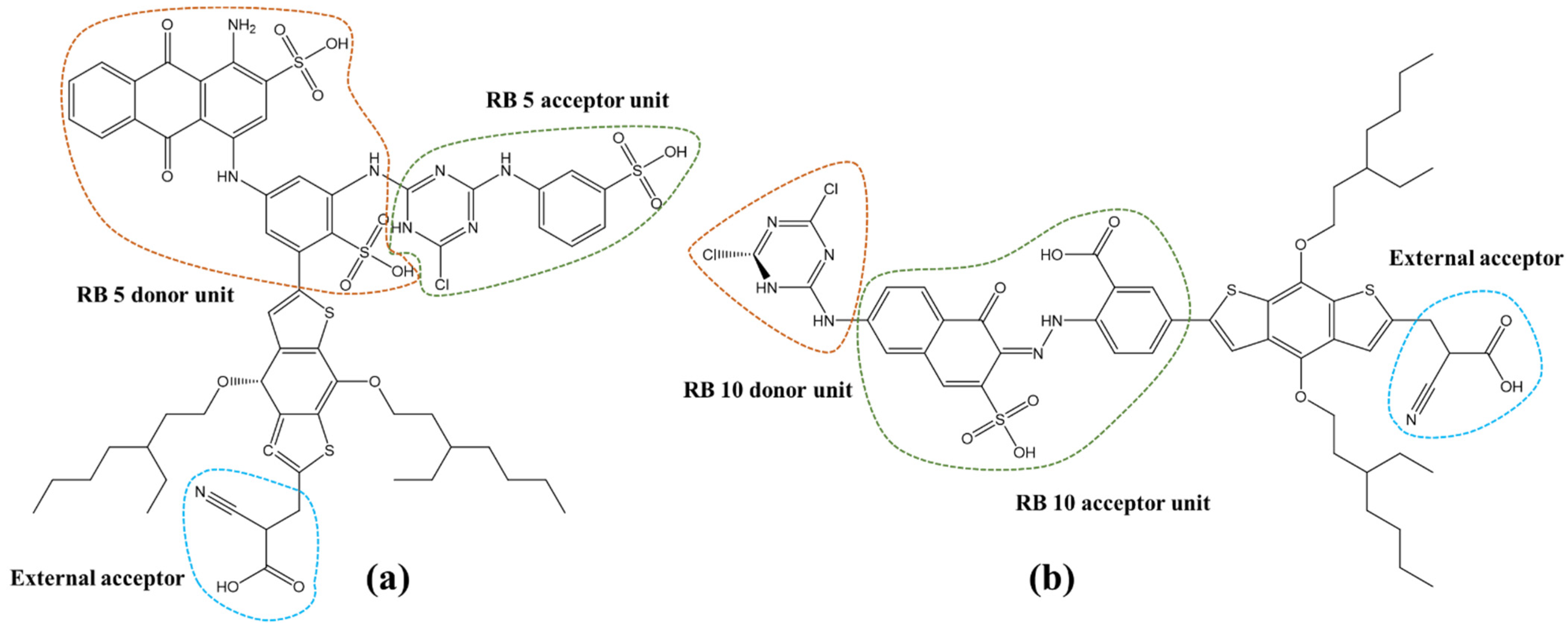
- The π-spacer was attached externally to the dye molecule at a suitable position, followed by the attachment of an acceptor group, cyanoacrylic acid (coded as A), as depicted in Figure 3.
4. Results and Discussion
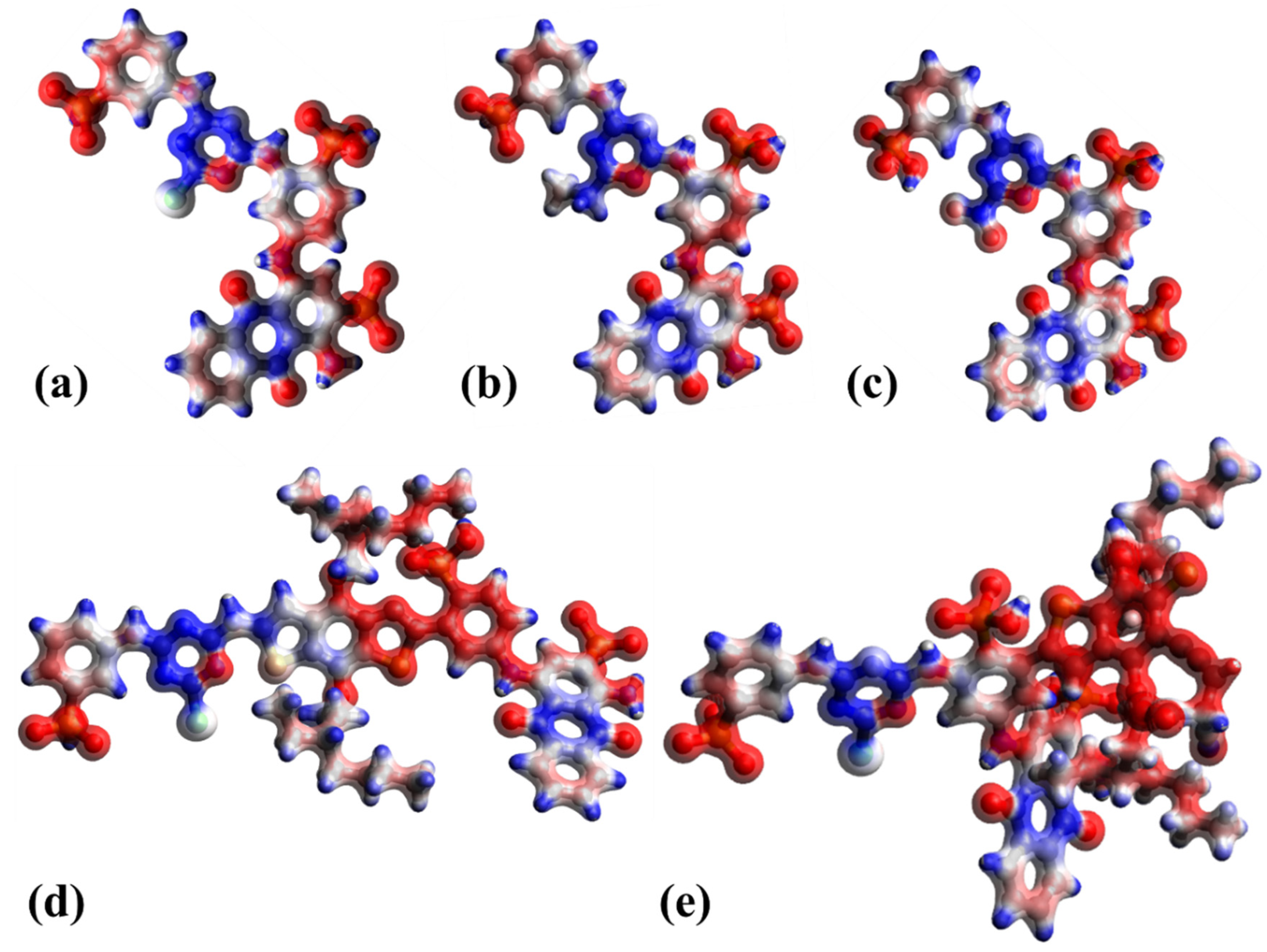
4.1. Modified Reactive Blue 5 as a Dye Sensitizer
4.2. Modified Reactive Brown 10 as Dye Sensitizer
4.3. Comparison of Modified Dyes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Satpute, S.D.; Jagtap, J.S.; Bhujbal, P.K.; Sonar, S.M.; Baviskar, P.K.; Jadker, S.R.; Pathan, H.M. Mercurochrome Sensitized ZnO/In2O3 Photoanode for Dye-Sensitized Solar Cell. ES Energy Environ. 2020, 9, 89–94. [Google Scholar] [CrossRef]
- Patel, R.V.; Yadav, A.; Winczek, J. Experimental Investigation and Mathematical Modelling of Heat Transfer Coefficient in Double Slope Solar Still. Strojniski Vestnik/J. Mech. Eng. 2021, 67, 369–379. [Google Scholar] [CrossRef]
- Ejaz, A.; Jamil, F.; Ali, H.M. A novel thermal regulation of photovoltaic panels through phase change materials with metallic foam-based system and a concise comparison: An experimental study. Sustain. Energy Technol. Assess. 2021, 49, 101726. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Ali, S.R.; Khan, S. Progress in dye sensitized solar cell by incorporating natural photosensitizers. Sol. Energy 2019, 181, 490–509. [Google Scholar] [CrossRef]
- Said, Z.; Ghodbane, M.; Tiwari, A.K.; Ali, H.M.; Boumeddane, B.; Ali, Z.M. 4E (Energy, Exergy, Economic, and Environment) examination of a small LFR solar water heater: An experimental and numerical study. Case Stud. Therm. Eng. 2021, 27, 101277. [Google Scholar] [CrossRef]
- Hassan, F.; Jamil, F.; Hussain, A.; Ali, H.M.; Janjua, M.M.; Khushnood, S.; Farhan, M.; Altaf, K.; Said, Z.; Li, C. Recent advancements in latent heat phase change materials and their applications for thermal energy storage and buildings: A state of the art review. Sustain. Energy Technol. Assess. 2021, 49, 101646. [Google Scholar] [CrossRef]
- Zhang, W.; Eperon, G.E.; Snaith, H.J. Metal halide perovskites for energy applications. Nat. Energy 2016, 1, 16048. [Google Scholar] [CrossRef]
- Liu, M.; Johnston, M.; Snaith, H. Efficient planar heterojunction perovskite solar cells by vapour deposition. Nature 2013, 501, 395–398. [Google Scholar] [CrossRef]
- Miles, R.W.; Zoppi, G.; Forbes, I. Inorganic photovoltaic cells. Mater. Today 2007, 10, 20–27. [Google Scholar] [CrossRef]
- McCandless, B.E.; Sites, J.R. Cadmium Telluride Solar Cells. In Handbook of Photovoltaic Science and Engineering; Wiley: Chichester, UK, 2010; pp. 600–641. [Google Scholar]
- Ameri, T.; Li, N.; Brabec, C.J. Highly efficient organic tandem solar cells: A follow up review. Energy Environ. Sci. 2013, 6, 2390–2413. [Google Scholar] [CrossRef]
- Riede, M.; Uhrich, C.; Widmer, J.; Timmreck, R.; Wynands, D.; Schwartz, G.; Gnehr, W.-M.; Hildebrandt, D.; Weiss, A.; Hwang, J.; et al. Efficient Organic Tandem Solar Cells based on Small Molecules. Adv. Funct. Mater. 2011, 21, 3019–3028. [Google Scholar] [CrossRef]
- Meng, L.; Zhang, Y.; Wan, X.; Li, C.; Zhang, X.; Wang, Y.; Ke, X.; Xiao, Z.; Ding, L.; Xia, R.; et al. Organic and solution-processed tandem solar cells with 17.3% efficiency. Science 2018, 361, 1094–1098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tvrdy, K.; Kamat, P. Quantum Dot Solar Cells. In Comprehensive Nanoscience and Technology; Elsevier BV: Amsterdam, The Netherlands, 2011; pp. 257–275. [Google Scholar]
- Ning, Z.; Gong, X.; Comin, R.; Walters, G.; Fan, F.; Voznyy, O.; Yassitepe, E.; Buin, A.K.; Hoogland, S.; Sargent, E.H. Quantum-dot-in-perovskite solids. Nature 2015, 523, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Rao, H.; Mora-Seró, I.; Bisquert, J.; Zhong, X. Quantum dot-sensitized solar cells. Chem. Soc. Rev. 2018, 47, 7659–7702. [Google Scholar] [CrossRef]
- Dette, C.; Osorio, M.A.P.; Kley, C.S.; Punke, P.; Patrick, C.; Jacobson, P.; Giustino, F.; Jung, S.J.; Kern, K. TiO2 Anatase with a Bandgap in the Visible Region. Nano Lett. 2014, 14, 6533–6538. [Google Scholar] [CrossRef]
- Feldt, S.M.; Gibson, E.A.; Gabrielsson, E.; Sun, L.; Boschloo, G.; Hagfeldt, A. Design of Organic Dyes and Cobalt Polypyridine Redox Mediators for High-Efficiency Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2010, 132, 16714–16724. [Google Scholar] [CrossRef]
- Kavan, L. Electrochemistry and dye-sensitized solar cells. Curr. Opin. Electrochem. 2017, 2, 88–96. [Google Scholar] [CrossRef]
- Sugathan, V.; John, E.; Sudhakar, K. Recent improvements in dye sensitized solar cells: A review. Renew. Sustain. Energy Rev. 2015, 52, 54–64. [Google Scholar] [CrossRef]
- Ruhane, T.; Islam, M.T.; Rahaman, S.; Bhuiyan, M.; Islam, J.M.; Newaz, M.; Khan, K.; Khan, M.A. Photo current enhancement of natural dye sensitized solar cell by optimizing dye extraction and its loading period. Optik 2017, 149, 174–183. [Google Scholar] [CrossRef]
- Mao, X.; Zhou, R.; Zhang, S.; Ding, L.; Wan, L.; Qin, S.; Chen, Z.; Xu, J.; Miao, S. High Efficiency Dye-sensitized Solar Cells Constructed with Composites of TiO2 and the Hot-bubbling Synthesized Ultra-Small SnO2 Nanocrystals. Sci. Rep. 2016, 6, srep19390. [Google Scholar] [CrossRef] [Green Version]
- Yi, Z.; Zeng, Y.; Wu, H.; Chen, X.; Fan, Y.; Yang, H.; Tang, Y.; Yi, Y.; Wang, J.; Wu, P. Synthesis, surface properties, crystal structure and dye-sensitized solar cell performance of TiO2 nanotube arrays anodized under different parameters. Results Phys. 2019, 15, 102609. [Google Scholar] [CrossRef]
- Anouar, H.; Elhassan, A.; Hourch AEl Kacemi, K.E. Electronic and Optical Properties of Reactive Orange 16 azo dye. Int. J. Innov. Appl. Stud. 2014, 8, 1447–1454. [Google Scholar]
- Yadav, V.; Chaudhary, S.; Negi, C.M.S.; Gupta, S.K. Textile dyes as photo-sensitizer in the dye sensitized solar cells. Opt. Mater. 2020, 109, 110306. [Google Scholar] [CrossRef]
- Louis, H.; Onyebuenyi, I.B.; Odey, J.O.; Igbalagh, A.T.; Mbonu, M.T.; Eno, E.A.; Pembere, A.M.S.; Offiong, O.E. Synthesis, characterization, and theoretical studies of the photovoltaic properties of novel reactive azonitrobenzaldehyde derivatives. RSC Adv. 2021, 11, 28433–28446. [Google Scholar] [CrossRef]
- Inamdar, Y.; Beedri, N.; Kodam, K.; Shaikh, A.; Pathan, H. Aggregation of ZnO Nanocrystallites Using Polyol Process for Dye (Reactive Red) Sensitized Solar Cell. Macromol. Symp. 2015, 347, 52–57. [Google Scholar] [CrossRef]
- Sharma, K.; Sharma, V.; Sharma, S.S. Dye-Sensitized Solar Cells: Fundamentals and Current Status. Nanoscale Res. Lett. 2018, 13, 381. [Google Scholar] [CrossRef]
- Janjua, M.R.S.A.; Khan, M.U.; Khalid, M.; Ullah, N.; Kalgaonkar, R.; Alnoaimi, K.; Baqader, N.; Jamil, S. Theoretical and Conceptual Framework to Design Efficient Dye-Sensitized Solar Cells (DSSCs): Molecular Engineering by DFT Method. J. Clust. Sci. 2021, 32, 243–253. [Google Scholar] [CrossRef]
- Tian, H.; Yang, X.; Chen, R.; Zhang, R.; Hagfeldt, A.; Sun, L. Effect of Different Dye Baths and Dye-Structures on the Performance of Dye-Sensitized Solar Cells Based on Triphenylamine Dyes. J. Phys. Chem. C 2008, 112, 11023–11033. [Google Scholar] [CrossRef]
- Xie, X.; Liu, Z.; Li, W.; Bai, F.-Q.; Lee, E.-C.; Zhang, H.-X. Theoretical study on organic dyes with tunable π-spacers for dye-sensitized solar cells: Inspired by the organic polymer photovoltaics. Chem. Phys. Lett. 2019, 719, 39–44. [Google Scholar] [CrossRef]
- Xie, M.; Hao, L.; Jia, R.; Wang, J.; Bai, F.-Q. Theoretical study on the influence of electric field direction on the photovoltaic performance of aryl amine organic dyes for dye-sensitized solar cells. New J. Chem. 2019, 43, 651–661. [Google Scholar] [CrossRef]
- Delgado-Montiel, T.; Soto-Rojo, R.; Baldenebro-López, J.; Glossman-Mitnik, D. Theoretical Study of the Effect of Different π Bridges Including an Azomethine Group in Triphenylamine-Based Dye for Dye-Sensitized Solar Cells. Molecules 2019, 24, 3897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maurya, I.C.; Singh, S.; Srivastava, P.; Maiti, B.; Bahadur, L. Natural dye extract from Cassia fistula and its application in dye-sensitized solar cell: Experimental and density functional theory studies. Opt. Mater. 2019, 90, 273–280. [Google Scholar] [CrossRef]
- Gao, F.; Yang, C.-L.; Jiang, G. Effects of the coupling between electrode and GQD-anthoxanthin nanocomposites for dye-sensitized solar cell: DFT and TD-DFT investigations. J. Photochem. Photobiol. A Chem. 2021, 407, 113080. [Google Scholar] [CrossRef]
- Al-Temimei, F.A.; Alkhayatt, A.H.O. A DFT/TD-DFT investigation on the efficiency of new dyes based on ethyl red dye as a dye-sensitized solar cell light-absorbing material. Optik 2020, 208, 163920. [Google Scholar] [CrossRef]
- Prajongtat, P.; Suramitr, S.; Nokbin, S.; Nakajima, K.; Mitsuke, K.; Hannongbua, S. Density functional theory study of adsorption geometries and electronic structures of azo-dye-based molecules on anatase TiO2 surface for dye-sensitized solar cell applications. J. Mol. Graph. Model. 2017, 76, 551–561. [Google Scholar] [CrossRef]
- Pounraj, P.; Mohankumar, V.; Pandian, M.S.; Ramasamy, P. The effect of different π-bridge configuration on bi-anchored triphenylamine and phenyl modified triphenylamine based dyes for dye sensitized solar cell (DSSC) application: A theoretical approach. J. Mol. Graph. Model. 2018, 79, 235–253. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Shao, L.; Li, H.; Yan, R.; Wang, X.; Hou, L. Indolo[3,2-b]carbazole-based multi-donor–π–acceptor type organic dyes for highly efficient dye-sensitized solar cells. J. Power Source 2016, 319, 39–47. [Google Scholar] [CrossRef]
- Qian, X.; Yan, R.; Xu, C.; Shao, L.; Li, H.; Hou, L. New efficient organic dyes employing indeno[1,2-b]indole as the donor moiety for dye-sensitized solar cells. J. Power Source 2016, 332, 103–110. [Google Scholar] [CrossRef]
- Guo, K.; Yan, K.; Lu, X.; Qiu, Y.; Liu, Z.; Sun, J.; Yan, F.; Guo, W.; Yang, S. Dithiafulvenyl Unit as a New Donor for High-Efficiency Dye-Sensitized Solar Cells: Synthesis and Demonstration of a Family of Metal-Free Organic Sensitizers. Org. Lett. 2012, 14, 2214–2217. [Google Scholar] [CrossRef]
- Xia, H.-Q.; Kong, C.-P.; Wang, J.; Bai, F.-Q.; Zhang, H.-X. Design of D–A–π–A organic dyes with different acceptor and auxiliary acceptor for highly efficient dye-sensitized solar cells: A computational study. RSC Adv. 2014, 4, 50338–50350. [Google Scholar] [CrossRef]
- Ni, J.-S.; Yen, Y.-C.; Lin, J.T. Organic sensitizers with a rigid dithienobenzotriazole-based spacer for high-performance dye-sensitized solar cells. J. Mater. Chem. A 2016, 4, 6553–6560. [Google Scholar] [CrossRef]
- Irfan, A.; Aftab, H.; Al-Sehemi, A.G. Push–pull effect on the geometries, electronic and optical properties of thiophene based dye-sensitized solar cell materials. J. Saudi Chem. Soc. 2014, 18, 914–919. [Google Scholar] [CrossRef] [Green Version]
- Kakiage, K.; Aoyama, Y.; Yano, T.; Oya, K.; Kyomen, T.; Hanaya, M. Fabrication of a high-performance dye-sensitized solar cell with 12.8% conversion efficiency using organic silyl-anchor dyes. Chem. Commun. 2015, 51, 6315–6317. [Google Scholar] [CrossRef]
- Ganesan, P.; Yella, A.; Holcombe, T.W.; Gao, P.; Rajalingam, R.; Al-Muhtaseb, S.A.; Graetzel, M.; Nazeeruddin, M.K. Unravel the Impact of Anchoring Groups on the Photovoltaic Performances of Diketopyrrolopyrrole Sensitizers for Dye-Sensitized Solar Cells. ACS Sustain. Chem. Eng. 2015, 3, 2389–2396. [Google Scholar] [CrossRef]
- Matta, S.K.; Kakiage, K.; Makuta, S.; Veamatahau, A.; Aoyama, Y.; Yano, T.; Hanaya, M.; Tachibana, Y. Dye-Anchoring Functional Groups on the Performance of Dye-Sensitized Solar Cells: Comparison between Alkoxysilyl and Carboxyl Groups. J. Phys. Chem. C 2014, 118, 28425–28434. [Google Scholar] [CrossRef]
- Victor, A.; Pulidindi, I.N.; Kim, T.H.; Gedanken, A. Design of a selective solid acid catalyst for the optimization of glucose production from Oryza sativa straw. RSC Adv. 2016, 6, 31–38. [Google Scholar] [CrossRef]
- Ikpesu, J.E.; Iyuke, S.E.; Daramola, M.; Okewale, A.O. Synthesis of improved dye-sensitized solar cell for renewable energy power generation. Sol. Energy 2020, 206, 918–934. [Google Scholar] [CrossRef]
- Linares-Flores, C.; Schott, E.; Claveria-Cadiz, F.; Zarate, X. Energy conversion process of substituted phthalocyanines with potential application to DSSC: A theoretical study. Theor. Chim. Acta 2018, 137, 52. [Google Scholar] [CrossRef]
- Zarate, X.; Schott, E.; Gomez, T.; Arratia-Pérez, R. Theoretical Study of Sensitizer Candidates for Dye-Sensitized Solar Cells: Peripheral Substituted Dizinc Pyrazinoporphyrazine–Phthalocyanine Complexes. J. Phys. Chem. A 2013, 117, 430–438. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Unny, D.; Sivanadanam, J.; Mandal, S.; Aidhen, I.S.; Ramanujam, K. Effect of Flexible, Rigid Planar and Non-Planar Donors on the Performance of Dye-Sensitized Solar Cells. J. Electrochem. Soc. 2018, 165, H845–H860. [Google Scholar] [CrossRef]
- Becke, A.D. A new mixing of Hartree–Fock and local density-functional theories. J. Chem. Phys. 1993, 98, 1372–1377. [Google Scholar] [CrossRef]
- Chen, X.-K.; Coropceanu, V.; Brédas, J.-L. Assessing the nature of the charge-transfer electronic states in organic solar cells. Nat. Commun. 2018, 9, 5295. [Google Scholar] [CrossRef] [Green Version]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Divya, V.V.; Suresh, C.H. Density functional theory study on the donating strength of donor systems in dye-sensitized solar cells. New J. Chem. 2020, 44, 7200–7209. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Tenderholt, A.L.; Langner, K.M. cclib: A library for package-independent computational chemistry algorithms. J. Comput. Chem. 2008, 29, 839–845. [Google Scholar] [CrossRef]
- Sobhy, M.; Sofo, J.; Castleman, A. A density-functional study of the structural, electronic, and vibrational properties of Ti8C12 metallocarbohedrynes with relevance to ultrafast time-resolved spectroscopy. Femtochem. VII 2006, VII, 80–84. [Google Scholar] [CrossRef]
- Fu, G.C. Transition-Metal Catalysis of Nucleophilic Substitution Reactions: A Radical Alternative to SN1 and SN2 Processes. ACS Central Sci. 2017, 3, 692–700. [Google Scholar] [CrossRef] [Green Version]
- Balis, N.; Verykios, A.; Soultati, A.; Constantoudis, V.; Papadakis, M.; Kournoutas, F.; Drivas, C.; Skoulikidou, M.-C.; Gardelis, S.; Fakis, M.; et al. Triazine-Substituted Zinc Porphyrin as an Electron Transport Interfacial Material for Efficiency Enhancement and Degradation Retardation in Planar Perovskite Solar Cells. ACS Appl. Energy Mater. 2018, 1, 3216–3229. [Google Scholar] [CrossRef]
- Bisht, R.; Fairoos, M.K.M.; Singh, A.K.; Nithyanandhan, J. Panchromatic Sensitizer for Dye-Sensitized Solar Cells: Unsymmetrical Squaraine Dyes Incorporating Benzodithiophene π-Spacer with Alkyl Chains to Extend Conjugation, Control the Dye Assembly on TiO2, and Retard Charge Recombination. J. Org. Chem. 2017, 82, 1920–1930. [Google Scholar] [CrossRef]
- Pophristic, V.; Goodman, L. Hyperconjugation not steric repulsion leads to the staggered structure of ethane. Nature 2001, 411, 565–568. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Mushahary, B.C.; Kalita, D.J. Rational Design of Bay-Annulated Indigo (BAI)-Based Oligomers for Bulk Heterojunction Organic Solar Cells: A Density Functional Theory (DFT) Study. ACS Omega 2020, 5, 8321–8333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, K.D.; Song, H.M.; Lee, M.J.; Pastore, M.; Anselmi, C.; De Angelis, F.; Nazeeruddin, M.K.; Gräetzel, M.; Kim, H.K. Coumarin dyes containing low-band-gap chromophores for Dye-sensitised solar cells. Dye. Pigment. 2011, 90, 304–310. [Google Scholar] [CrossRef]
- Chitumalla, R.K.; Jang, J. Density functional theory study on ruthenium dyes and dye@TiO2 assemblies for dye sensitized solar cell applications. Sol. Energy 2018, 159, 283–290. [Google Scholar] [CrossRef]


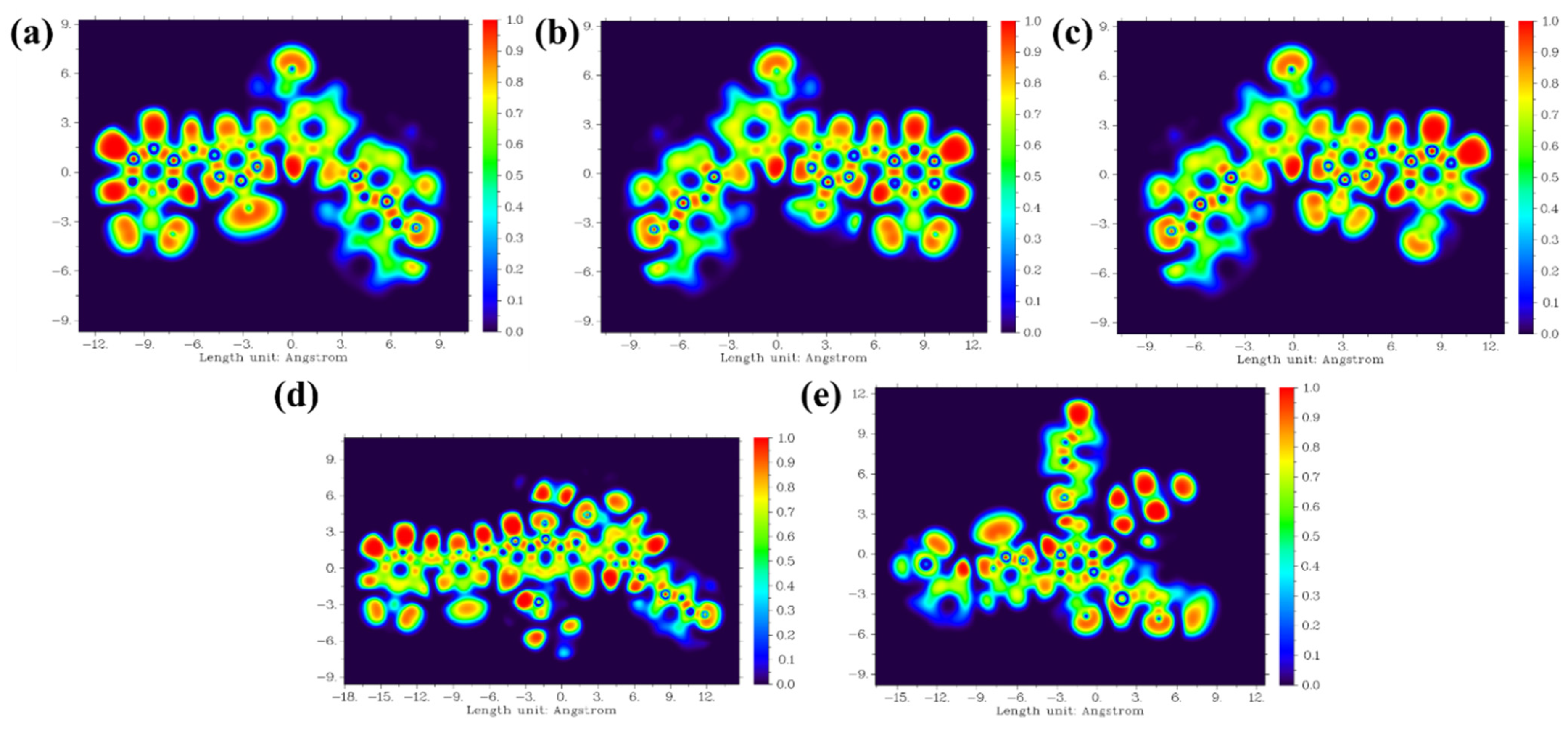

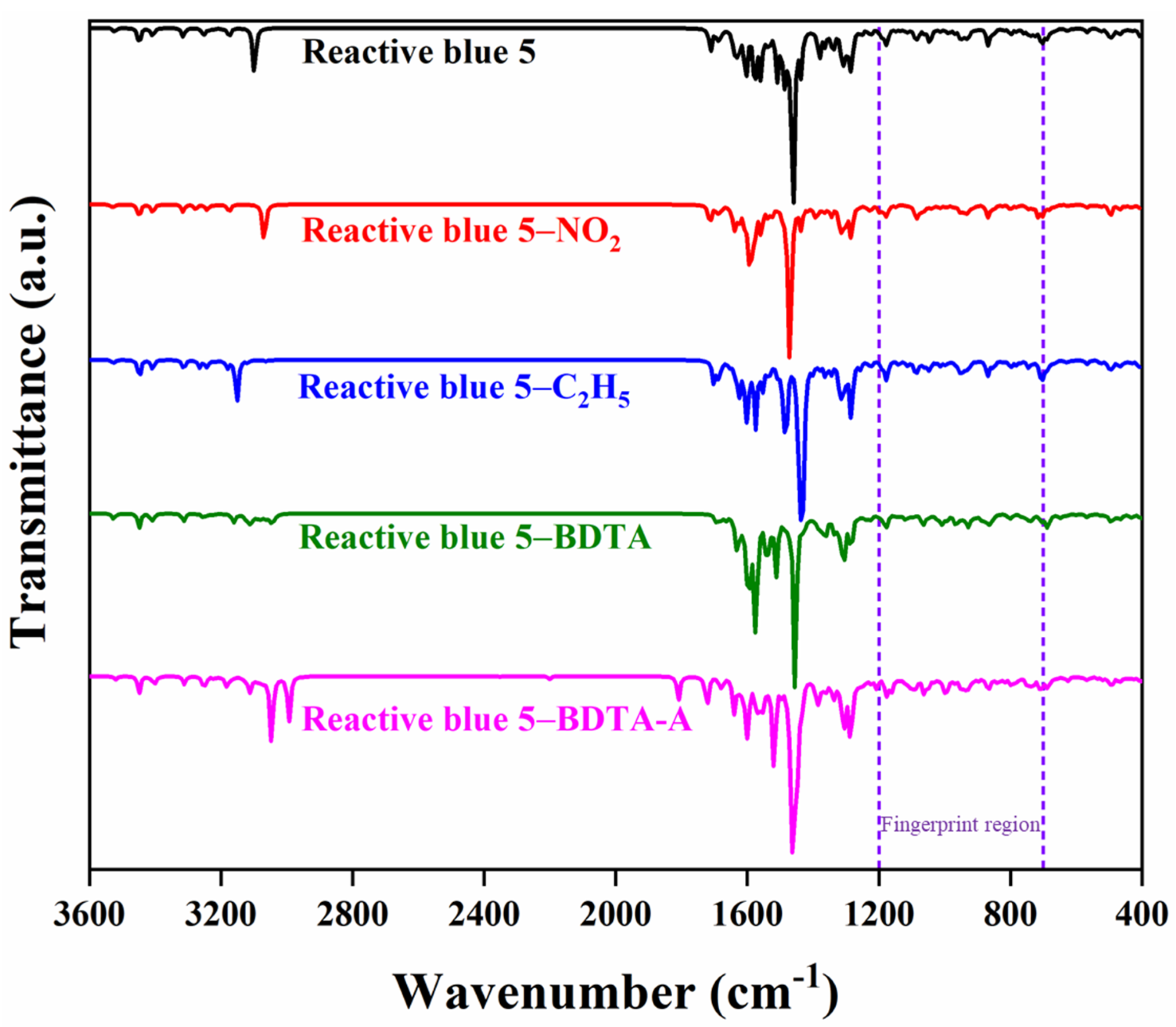
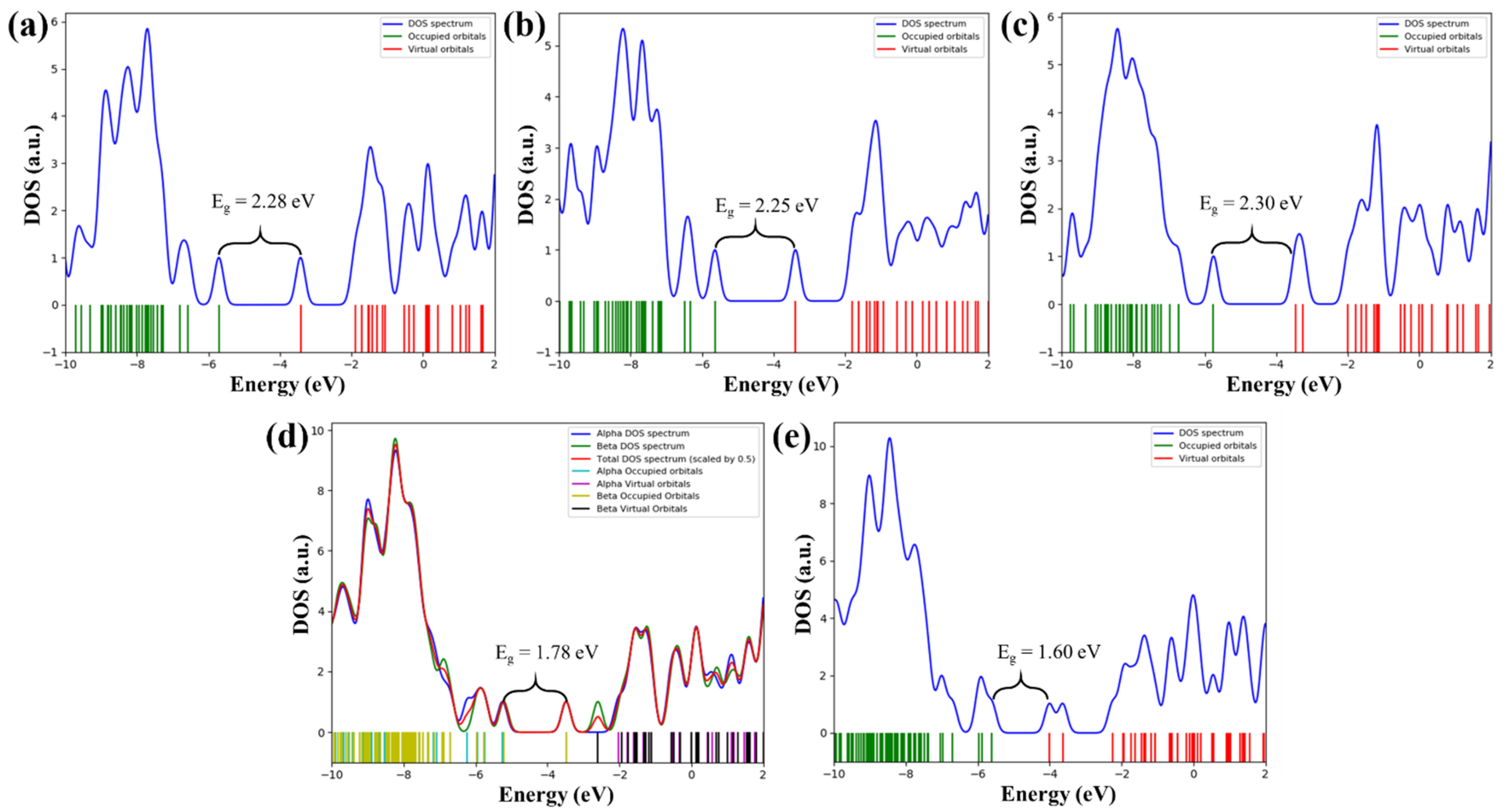

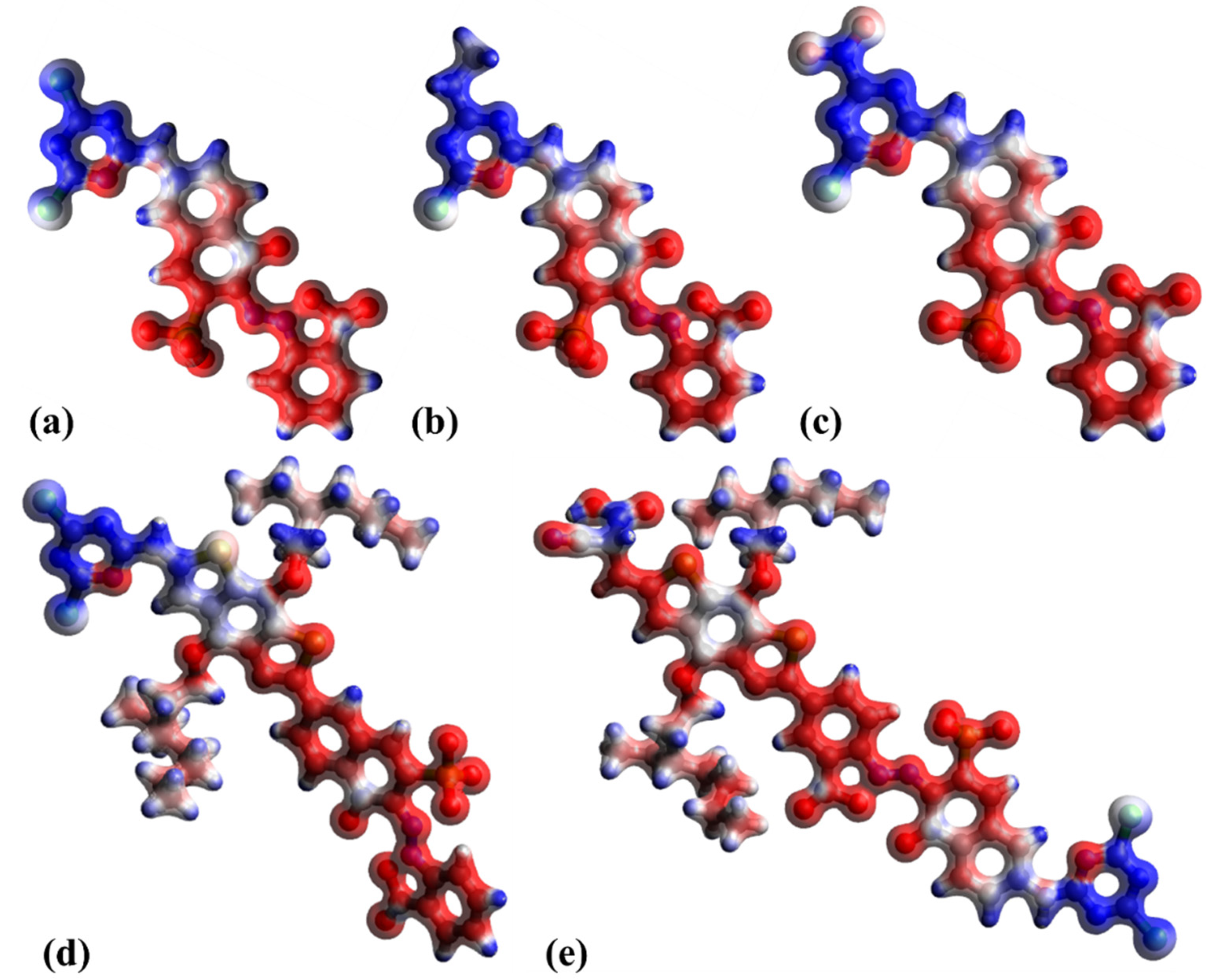
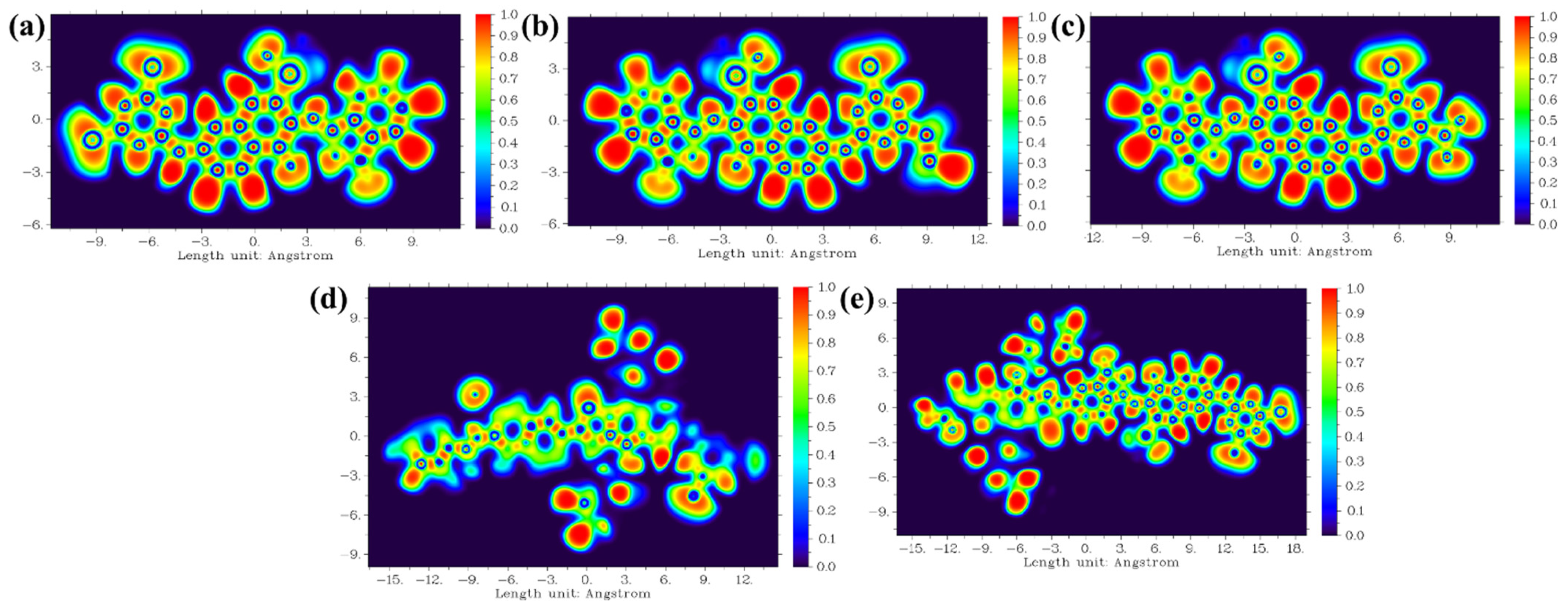
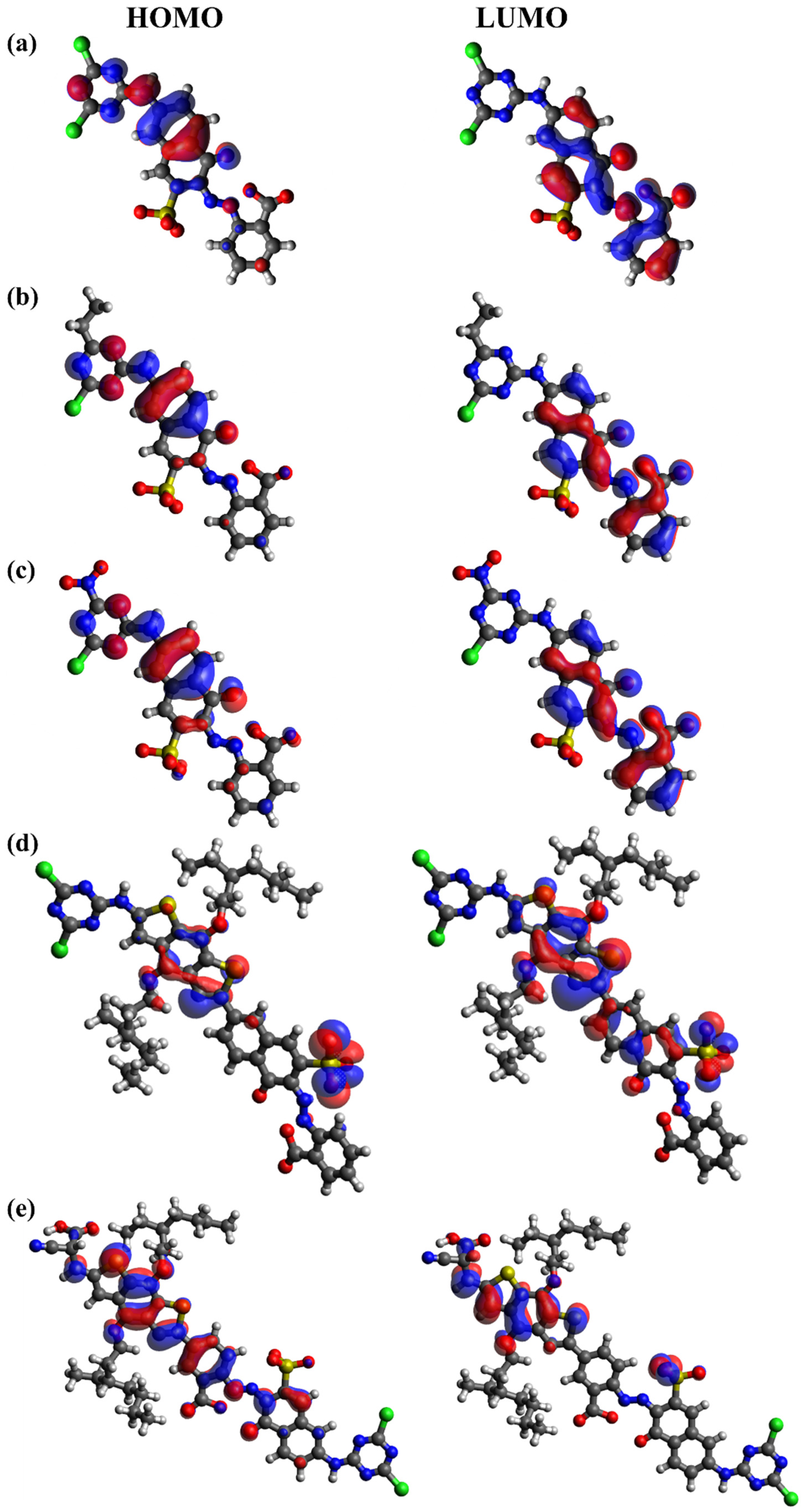

| RB 5 | RB 5–Et | RB 5–NO2 | RB 5–BDTA | RB 5–BDTA–A | |
|---|---|---|---|---|---|
| HOMO (eV) | −5.71 | −5.64 | −5.76 | −5.26 | −5.61 |
| LUMO (eV) | −3.43 | −3.39 | −3.46 | −3.48 | −4.01 |
| HLG (eV) | 2.28 | 2.25 | 2.30 | 1.78 | 1.60 |
| RB 10 | RB 10–Et | RB 10–NO2 | RB 10–BDTA | RB 10–BDTA–A | |
|---|---|---|---|---|---|
| HOMO (eV) | −6.50 | −6.36 | −6.58 | −5.57 | −5.29 |
| LUMO (eV) | −3.49 | −3.33 | −3.66 | −5.22 | −5.04 |
| HLG (eV) | 3.01 | 3.03 | 2.92 | 0.35 | 0.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dindorkar, S.S.; Yadav, A. Insights from Density Functional Theory on the Feasibility of Modified Reactive Dyes as Dye Sensitizers in Dye-Sensitized Solar Cell Applications. Solar 2022, 2, 12-31. https://doi.org/10.3390/solar2010002
Dindorkar SS, Yadav A. Insights from Density Functional Theory on the Feasibility of Modified Reactive Dyes as Dye Sensitizers in Dye-Sensitized Solar Cell Applications. Solar. 2022; 2(1):12-31. https://doi.org/10.3390/solar2010002
Chicago/Turabian StyleDindorkar, Shreyas S., and Anshul Yadav. 2022. "Insights from Density Functional Theory on the Feasibility of Modified Reactive Dyes as Dye Sensitizers in Dye-Sensitized Solar Cell Applications" Solar 2, no. 1: 12-31. https://doi.org/10.3390/solar2010002
APA StyleDindorkar, S. S., & Yadav, A. (2022). Insights from Density Functional Theory on the Feasibility of Modified Reactive Dyes as Dye Sensitizers in Dye-Sensitized Solar Cell Applications. Solar, 2(1), 12-31. https://doi.org/10.3390/solar2010002