Abstract
In this work, the possibility of cathodical electroanalytical determination of aesculetin and quercetin, the most representative polyphenolic coumarin and flavonoid, is theoretically described. The cathodic reaction is given by the electrochemical reduction of 2- and 4-pyrone rings simultaneously on a vanadium(III) oxyhydroxide-modified electrode at a pH correspondent to wine (3 < pH ≤ 7). Analysis of the mathematical model, corresponding to the reaction mechanism, lets us conclude that, although the oscillatory behavior remains highly probable, the cathodic electroanalytical process, based on VO(OH)-assisted reaction, may be even more efficient than anodic oxidation of the polyphenolic compounds in the same conditions.
1. Introduction
Douro wine is one of the symbols of Portugal. The Douro wine region was first demarked in the XVIII century. The wines produced in the area present their characteristic scent and flavor due to some aromatic lactones. However, the principal nutrient value of the vine is given by its polyphenolic composition [1].
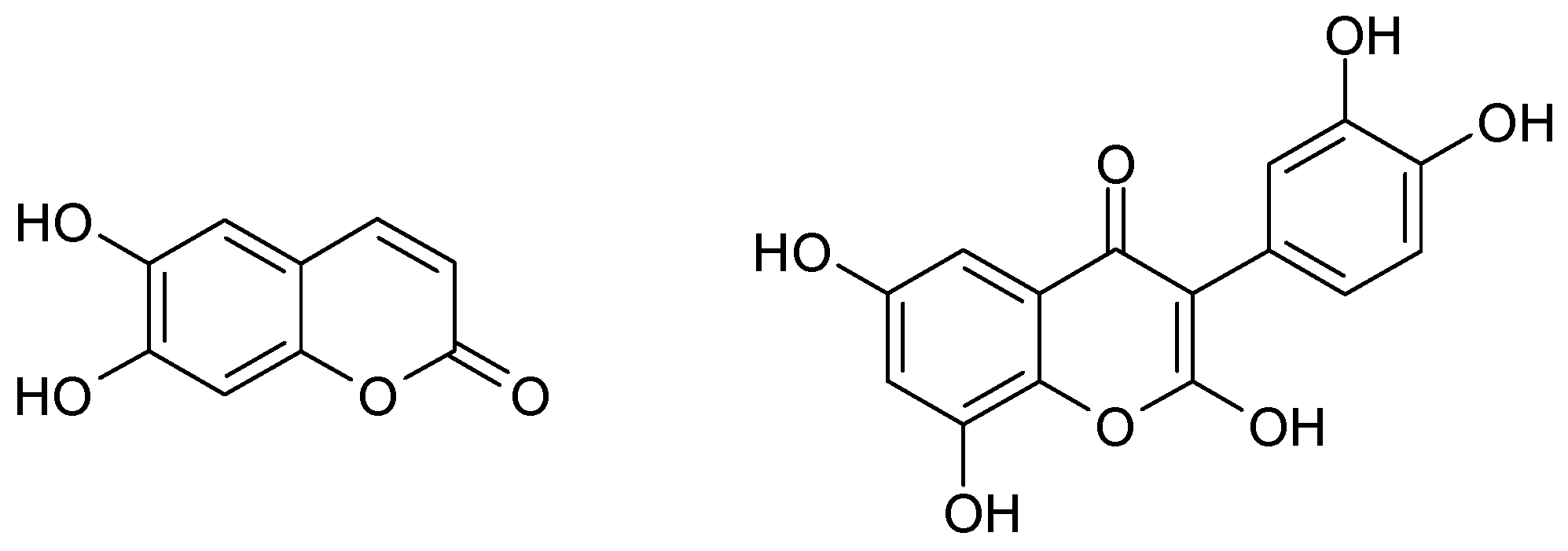
On the other hand, the chestnut C. Sativa is one of the most critical products and ingredients for the cuisine of Trás-os-Montes [2]. The districts of Vila Real and Bragança produce 25% of Portuguese chestnut. Its pulp and flowers also possess high concentrations of flavonoid (for example, quercetin) and coumarin (for instance, aesculetin) polyphenols, mainly those with hydroquinone moieties (Figure 1), which, in quinonic forms, act as antioxidants and preservatives, thereby being candidates for the substitution of sulfites in wine preparation.
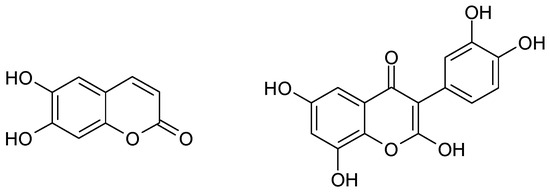
Figure 1.
Aesculetin and quercetin.
Another positive feature of those compounds is their contribution to polyphenolic wine profiles, which is why the development of an efficient method for their determination is actual, and the electroanalytical process applicable to polyphenolic compounds may be an exciting solution [3].
Both aesculetin and quercetin may be easily detected by an anodic process in which the hydroquinone moiety loses two protons, yielding the correspondent quinone. Nevertheless, the anodic process may lose selectivity, especially in the presence of carbocyclic polyphenols. Moreover, anodic oxidation is more favored in a neutral or neutralized medium, which is incompatible with wine pH (mildly acidic). Therefore, the cathodic process, in which the pyrogenic heterocyclic moiety is reduced in the presence of protons, may be helpful in the selective flavonoid and coumarin polyphenols’ determination in wine.
In this aspect, the use of chemically modified electrodes (CMEs), which constitute a flexible and modern tool for electroanalysis, becomes highly important, and vanadium (III) oxyhydroxide, a semiconducting material with highly expressed reducing properties, may serve as an exciting electrode modifier for this process. Nevertheless, the use of a novel modifier with new analytes requires behavior investigation to verify a priori the most probable mechanism of the reactions, leading to the appearance of the analytical signal, as well as demonstrating the possibility of electrochemical instabilities typical for similar systems [4] and the requirements of the most efficient analytical signal interpretation.
Therefore, this work aims to investigate the possibility of cathodical electroanalytical determination of aesculetin and quercetin over a VO(OH)-assisted modified electrode. The correspondent mathematical model will be thereby analyzed using linear stability theory and bifurcation analysis to investigate the requisites of the most efficient analytical signal interpretation, like the oscillatory and monotonic instability conditions. Additionally, the electroanalytical system’s behavior will be compared with that of similar ones [5].
2. System and Its Modeling
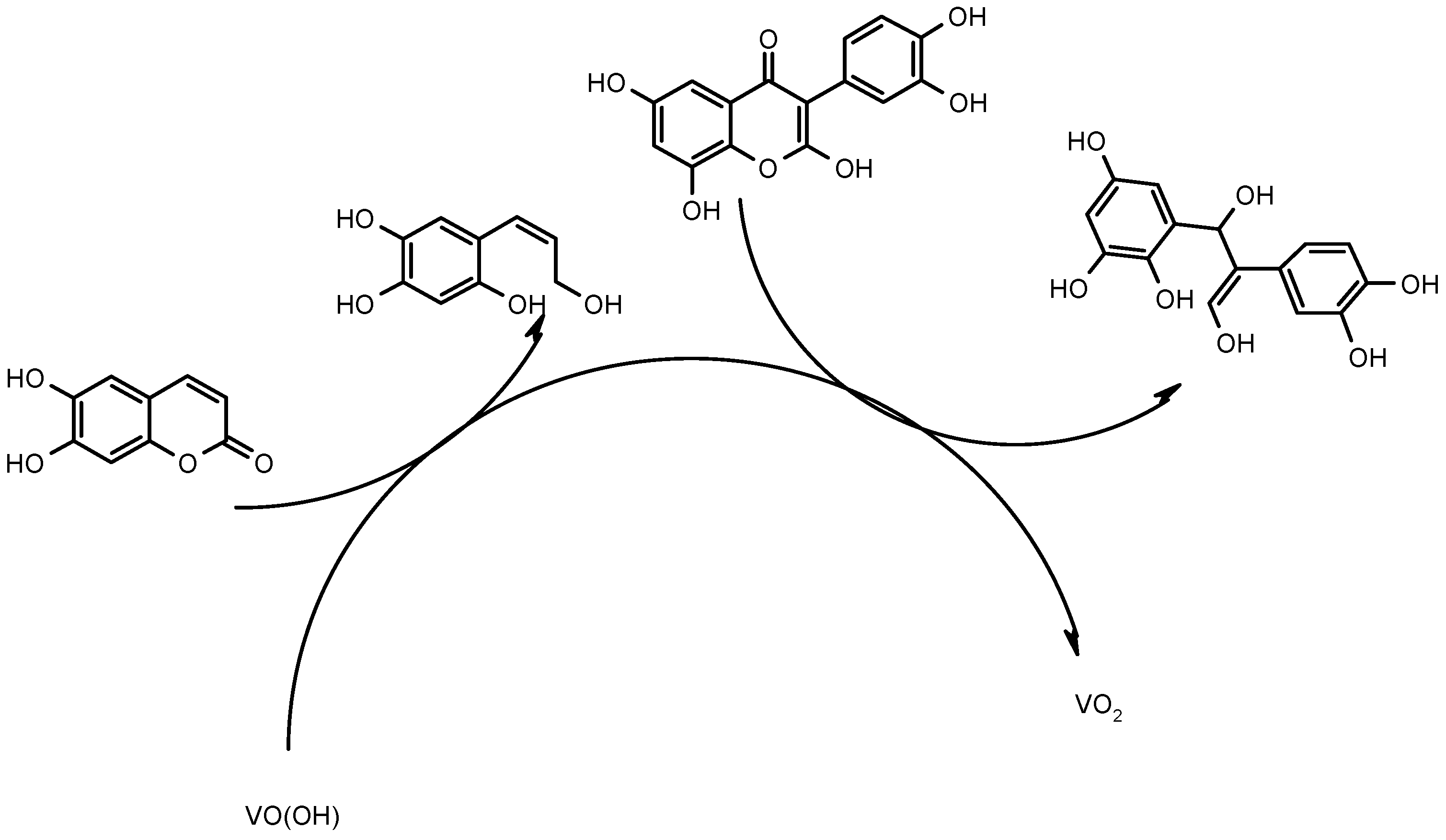
The electrochemical determination of aesculetin and quercetin by VO(OH)-assisted cathodic resolution is given by the electrochemical reduction process, leading to the rupture of both rings and yielding the appearance of two clear analytical signals. As for trivalent vanadium, it is oxidized to the tetravalent form, thereby being regenerated in the electrochemical stage, according to the reaction (1):
VO2 + H+ + e− → VO(OH)
Schematically, the electroanalytical process is depicted in Figure 2.
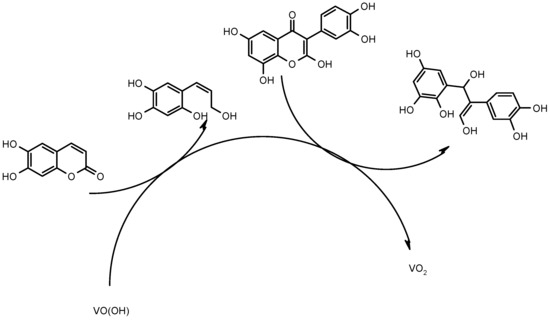
Figure 2.
The schematic representation of aesculetin and quercetin electrochemical determination on VO(OH).
Therefore, taking into account the above-cited statements and some assumptions [5], we describe the behavior of this system using the trivariate equation set (2):
in which a and q are aesculetin and quercetin concentrations in the pre-surface layer; and q0 are their bulk concentrations, and D are diffusion coefficients, is the pre-surface layer thickness, v is the vanadium (IV) oxide surface coverage degree, V is its maximal surface concentration, and the parameters r are the correspondent reaction rates, calculated as follows (3–5):
Herein, the parameters k stand for the correspondent reaction rate constants, F is the Faraday number, is the potential slope, related to the zero-charge potential, R is the universal gas constant, and T is the absolute temperature.
Considering that the phenolic compounds are less ionized in the mildly acidic medium than in neutral or alkaline, which is characteristic for anodic oxidation [5], the cathodic process in mildly acidic media, corresponding to wine pH, tends to be more stable. Therefore, as shown below, the cathodic determination for aesculetin and quercetin in wine is preferable.
3. Results and Discussion
We investigate the system with aesculetin and quercetin electrochemical determination in wine, analyzing the equation set (2) and considering the algebraic relations (3–5) using the linear stability theory. The steady-state Jacobian matrix members for this system may be described as follows:
In which
As mentioned above, the phenolic compounds are less ionized in mildly acidic media, corresponding to wine. Therefore, the ionization multipliers previously mentioned do not constitute the reaction rate expressions, and the ionization factor, responsible for the enhanced probability of the oscillatory behavior in neutral and basic media, will be absent in this case. This makes the system more stable and the analytical signal easier to interpret, as shown below.
As known, the primary condition for Hopf bifurcation, which corresponds to the oscillatory behavior, is the presence of the positive elements in the Jacobian main diagonals (7), (11), and (15). In the absence of the polyphenol ionization and its impact on the DEL ionic force during the chemical stages, the unique positive element in this system is , if j > 0, thereby being correspondent to the DEL influences of the electrochemical stage, which is common for all the similar systems [5]. As observed experimentally, the oscillation frequency and amplitude will depend on the background electrolyte composition [4].
As for steady-state stability, the electroanalytical system for cathodic reduction tends to be more stable than for anodic oxidation in a neutral or slightly alkaline medium. Applying the Routh–Hurwitz criterion and avoiding cumbersome expressions, we rewrite the Jacobian determinant as follows (16):
Applying the Det J < 0 requisite, salient from the criterion, and changing the signs to the opposite, we obtain the stability requisite, expressed as follows (17):
Defining an efficient electroanalytical system controlled by diffusional and kinetical factors, but primarily by diffusional factors. The topological region, correspondent to steady-state stability, which, in electroanalytical terms, corresponds to the linear dependence between the electrochemical parameter and concentration, is more comprehensive than for the anodic oxidation in neutral or alkaline medium. On the other hand, in acidic media, a proton-leaving reaction of hydroquinone electrooxidation becomes slow and ineffective, which makes the cathodic reduction more efficient for aesculetin and quercetin electroanalytical determination in wine.
As for the detection limit, defined by the monotonic instability correspondent to the saddle-node bifurcation, it occurs at the condition of Det J = 0, or the following (18):
At this point, the stable, steady states are divided from the unstable states. The system chooses one of the multiple unstable steady-states, destroyed when the conditions are changed and not necessarily restored in the return to the bifurcation point.
The same procedure may be efficiently used to determine quercetin and aesculetin in vines and vine-based drinks. In certain conditions, an additional analytical signal for flavoring substances like vitispiran and sotolone may be provided.
4. Conclusions
From the theoretical investigation for quercetin and aesculetin VO(OH)-assisted electrochemical determination in wine, it was possible to conclude that the cathodic decision in wine is more stable, efficient, and selective than the anodic oxidation in the same conditions or in neutral or neutralized media. The electroanalytical process is diffusion-controlled chiefly, and the topological region of the linear dependence between the electrochemical parameter and concentration becomes vaster than for the anodic electrooxidation.
Author Contributions
All authors have contributed equally to the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analysed in this study.
Acknowledgments
Volodymyr V. Tkach acknowledges the Engineering Faculty of the University of Porto and the University of Trás-os-Montes and Alto Douro for their support during these difficult times for Ukraine and its research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Azevedo, J.; Pinto, J.; Teixeira, N.; Oliveira, J.; Cabral, M.; Guedes de Pinho, P.; Lopes, P.; Mateus, N.; de Freitas, V. The Impact of the Storage Conditions and Bottle Orientation on the Evolution of Phenolic and Volatile Compounds of Vintage Port Wine. Foods 2022, 11, 2770. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.A.; Pedrosa, M.C.; Ueda, J.M.; Ferreira, E.; Rodrigues, P.; Heleno, S.A.; Carocho, M.; Prieto, M.A.; Ferreira, I.C.; Barros, L. Improving the Physicochemical Properties of a Traditional Portuguese Cake—“Económicos” with Chestnut Flour. Food Funct. 2022, 15, 8243–8253. [Google Scholar] [CrossRef] [PubMed]
- Martínez, S.; Fuentes, C.; Carballo, J. Antioxidant Activity, Totel Phenolic Content and Total Flavonoid Content in Sweet Chestnut (C. sativa) Cultivars Grown in Northwest Spain under Different Environmental Conditions. Foods 2022, 11, 3519. [Google Scholar] [CrossRef] [PubMed]
- Bazzaoui, M.; Bazzaoui, E.A.; Martins, L.; Martins, J.I. Electropolymerization of pyrrole on zinc–lead–silver alloys electrodes in acidic and neutral organic media. Synth. Met. 2002, 130, 73–83. [Google Scholar] [CrossRef]
- Tkach, V.; Kushnir, M.; Kopiika, V.; Luganska, O.V.; Omelyanchik, L.O.; Kormosh, Z.O.; Kucher, M.M.; Garcia, J.R.; Palamarek, K.V.; Bagrii, K.L.; et al. Theoretical Description of Sotolone Electrochemical Determination in Wine in Basic Media over an Undoped Conducting Polymer. Biointerface Res. Appl. Chem. 2023, 13, 470. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).