Abstract
DNA double-strand breaks (DSBs) are known to be the most serious lesions in irradiated cells. Several protein pathways exist for repair. The mechanisms by which cells determine a specific pathway for repair remain poorly understood. DSB induction and repair can be spatiotemporally monitored by ionizing radiation-induced foci (IRIFs) and the formation of repair complexes. IRIF analyses revealed that DSB formation, repair and misrepair are strongly dependent on the radiation characteristics and the microarchitecture of the chromatin environment. However, the IRIF nano-architecture remains unknown, as does its impact on the decision-making process and follow-up protein recruitment. New insights into the relationship between the physical properties of radiation, environmental chromatin architecture, IRIF architecture and DSB repair mechanisms are presented using single-molecule localization microscopy. Ripley distance statistics and persistent homology calculations have shifted our ability to analyze chromatin and IRIF architectures from imaging to topology and structure calculations. We discuss these approaches for cancer treatment-relevant irradiation processes on selected cell systems and consider whether this “structuromics” can enhance our knowledge about the radiation response.
1. Introduction
The application of ionizing radiation has an increasing impact on bio-medical research, and cancer diagnosis and treatment. Nevertheless, there are a lot of open questions concerning the understanding of radiation DNA-damaging processes and repair mechanisms within the light of radio-sensitivity and, thus, individualized medical applications (for review, see [1]). The three-dimensional architecture of genomes on the micro-, meso- and nano-scale acts in combination with epigenetic modifications as an important player in gene regulation and, consequently, fundamental biological processes such as DNA damage response and repair (for review, see [2]). In this context, chromatin and protein nano-probing and super-resolution microscopy are powerful methods for topological analyses of genomic targets in native chromatin and repair protein complexes in single cells at resolutions of single antibodies, proteins, histones, short DNA segments, etc. [3].
Thus far, little is known [4,5] about the impact of the chromatin architecture on DNA double-strand break (DSB) induction and repair pathway selection at individual damage sites. How does a cell nucleus manage DSBs and re-organize the chromatin to generate functionally intact repair units? Is there a radio-sensitivity-related difference in this reaction? In order to answer these questions, we investigated spatial and topological parameters of chromatin and repair foci during a time period of repair to highlight key aspects related to these questions. Nano-probing of radiation-induced chromatin damage sites and the recruited DNA repair proteins in combination with super-resolution single-molecule localization microscopy (SMLM) [6,7,8] is a powerful method for geometric and topological analyses of these structures in single cells and single DSB sites and, thus, to study mechanisms of their formation and repair pathway regulation (Figure 1). In the following study, we analyzed the nano-architecture and -dynamics of DNA damage markers, chromatin markers and DNA repair protein complexes in 3D-conserved nuclei of different cell types after exposure to various types (low-LET, high-LET; α-, β-particles) and doses of ionizing radiation.
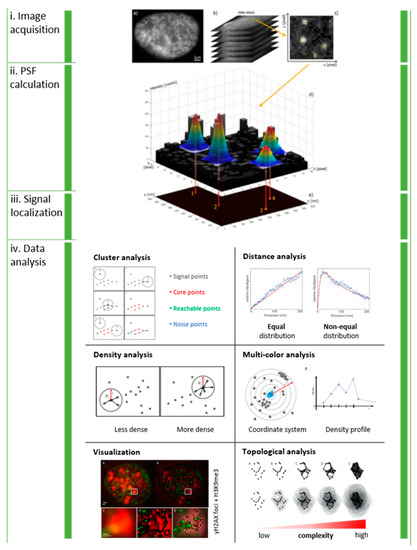
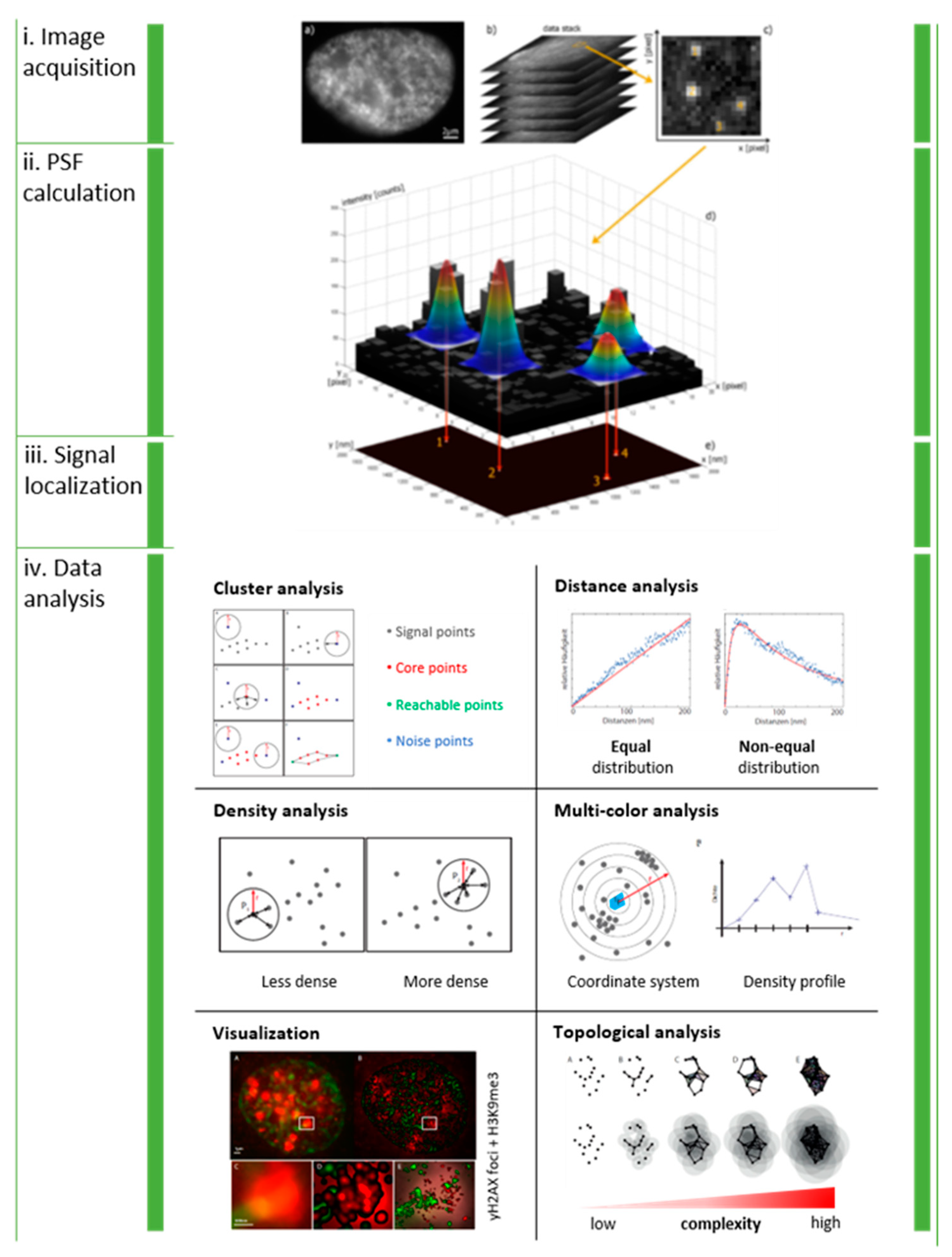
Figure 1.
General workflow of SMLM and data analysis: Serial images are acquired from the same region of interest (i). The point-spread function of each blinking molecule in each single image frame is Gaussian fitted to estimate the intensity maximum (ii), which represents the idealized lateral coordinates of the signal (iii). The result is a data table containing the coordinates of all detected signal points. The matrix representation of data allows mathematical and statistical analysis of clustering, distance distributions, signal densities, multi-color signal distributions, enhanced visualization and topology (iv). Note: This figure and the figure legend were originally published under a CC BY license in [1].
2. Spatial Analysis of Repair Cluster Formation after Low-LET Photon Irradiation
We used various tools for such investigations based on image-free high-precision SMLM, nano-scaled molecule distribution analyses, appropriate metrics following Ripley’s distance frequencies and cluster formation analyses [9] and topological quantifications employing persistence homology calculations [10]. In low-linear energy transfer (LET) X-irradiated HeLa cell nuclei, DSB-induced γH2AX foci being visible by standard fluorescence microscopy were subdivided into three–four equally sized (diameter of about 400 nm), functionally relevant fluorescence tag-containing clusters. The number of clusters increased with the radiation dose (0–2 Gy) and decreased with the repair time (0.5–8 h). This behavior corresponded to the detected number of γH2AX antibody labeling tags, as seen by SMLM nanoscopy [9].
Persistence homologies of components and holes can be calculated as a measure of the topology of repair clusters and be compared for their similarities [10]. The data obtained for HeLa cells and SkBr3 breast cancer cells suggested topological similarities in repair cluster formation associated with heterochromatin, indicating a well-defined, non-random molecule topology at given time points during repair. As to the size of the γH2AX clusters, there was a persistent similarity independent of the repair time. As long as detectable clusters were found, size and topology seem to be maintained.
MRE11 clusters analyzed in fibroblasts and MCF-7 breast cancer cells were embedded into the corresponding γH2AX clusters. With a diameter of about 200 nm, the MRE11 clusters were considerably smaller than the γH2AX clusters (diameter of about 400 nm). Again, the cluster sizes of the repair proteins were independent of the repair time after radiation exposure and seem to also be independent of the cell type [11]. In fibroblasts irradiated with a dose of 2 Gy, γH2AX clusters showed a high averaged topological similarity for persistent homologies within the first 3 h after irradiation. After 2 h, the averaged similarity of MRE11 clusters considerably increased and was maintained until 48 h post-irradiation (Hahn et al., manuscript in preparation). In contrast, randomly occurring γH2AX and MRE11 clusters in the non-irradiated control cells did not show any significant similarity.
3. Spatial Analysis of Repair Cluster Formation within α-Particle Tracks and β-Particle Damage Sites
SMLM in combination with Ripley cluster analysis of single-molecule signal-point density regions of DSB repair markers was applied to investigate the nano-structure of DNA damage foci tracks in leukocytes exposed, in solution, to Ra-223 decay products [12]. Alpha-particle-induced single DNA damage tracks in chromatin were efficiently outlined by γH2AX that formed large foci composed of numerous 60–80 nm-sized tag clusters. The tracks contained 60–70% of all γH2AX point signals in a nucleus, while less than 30% of 53BP1, MRE11 or p-ATM signals were located inside γH2AX damage tracks. 53BP1 formed clusters whose tags were interspersed with the γH2AX cluster tags, indicating that both proteins are part of the larger chromatin domain around DSB DNA damage. MRE11 and p-ATM tags, on the other hand, formed focal nanoclusters of about 20 nm peak size embedded into the γH2AX clusters. On average, about 12 (± 9) MRE11 nanoclusters were found within the γH2AX-marked alpha-particle track. In cell nuclei where the alpha-particle track did not obviously completely cross the nucleus, the amount of 53BP1 and MRE11 clusters increased at the end of the track; thereby, the signal ratio of 53BP1/γH2AX and MRE11/γH2AX remained constant.
The nano-structure of DNA damage foci of Lu-177 in-solution exposed leukocytes was also analyzed (Scherthan and Hausmann, unpublished). Preliminary results indicated an average cluster radius of about 300 nm for all types of repair-associated protein clusters analyzed (γH2AX, MRE11, 53BP1). However, the signal density in the clusters was highly variable; an issue that is under further investigation.
4. Spatial Analysis of Repair Cluster Formation within Damage Tracks of High-LET 15N Ions
SMLM has been successfully applied to study the DNA repair and cluster formation of γH2AX and 53BP1 molecules under high-LET radiation conditions [13]. 53BP1 foci were investigated in differently radio-resistant cell types, the moderately radio-resistant neonatal human dermal fibroblast cell line (NHDF) and the highly radio-resistant U87 glioblastoma cell line [14]. Specimens of both cell types were exposed to high-LET 15N ion radiation at doses of 1.3 Gy (10° irradiation scheme) and 4.0 Gy (90° irradiation scheme) at the particle irradiation facility of the Joint Institute for Nuclear Research, Dubna, Russia.
At given time points up to 24 h post-irradiation, SMLM of fluorescently tagged 53BP1 molecules was performed, and clusters were determined as sub-units of repair foci. The formation and relaxation of these clusters was studied during the repair period. The results revealed a higher ratio of 53BP1 proteins being recruited into clusters in NHDF cells (less radio-resistant) as compared to U87 cells (more radio-resistant) with a different starting situation and different levels of distribution of repair proteins prior to DNA damage induction. This relation of 53BP1 inside and outside the track clusters remained different for both cell types during the whole repair time observed. Hence, the 53BP1 ratio within and outside repair foci could be seen as a measure of the “just-in-time” availability of 53BP1 proteins but did not reflect the absolute number of 53BP1 proteins available. The speed of cluster formation and relaxation differed for the two cell types, indicating the recruitment of the existing proteins in the cell nucleus (higher in U87 cells) rather than a de novo production. A certain number of the clusters remained persistent for even longer than 24 h post-irradiation, and the number of these remaining clusters varied for the cell lines; persistent foci/clusters could thus causatively be linked to the cell type-specific radioresistance [13,14].
Appropriate metrics following Ripley’s distance frequencies and cluster formation analyses revealed equally sized clusters in NHDF cells during the repair period analyzed. For U87 cells, the cluster formation resulted in more variable clusters. Comparing the topology of repair clusters by persistence homology calculations suggested similarities of repair cluster formation along given particle tracks [15]. The resulting components and holes were compared, and their similarity was calculated. For both cell types, the similarity measure, as determined by the Jaccard index [10,15], was very high for the components (>0.9 means high similarity), independent of the irradiation scheme (10°, 90°). This indicates that the complex, ion-induced damages were marked with specific repair cluster setups. On the other hand, the similarity measure for the holes was below 0.5 for both cell types and irradiation schemes; thereby, the similarity of holes was, on average, higher for NHDF than for U87 cells. In general, it can be concluded that the normal (non-transformed), more radio-sensitive NHDF cells revealed higher topological similarity in 53BP1 clustering than the more radio-resistant U87 cancer cells [15].
5. Dose-Dependent ALU Signaling
Multi-color nano-probing using specific antibodies for heterochromatin (H3K9me3) and specific oligonucleotides uniquely binding to the ALU consensus sequences (17-mer oligonucleotide) [7] and SMLM were applied to study pan-nuclear chromatin rearrangements in the cell nuclei of SkBr3 breast cancer cells [6,16]. Counting of signals of dispersed genomic Alu sequences resulted in decreasing linear quadratic dose–effect curves for low- to higher dose ranges, which may be related to DNA breakage events preferentially in these regions. In the low-dose range (< 500 mGy), a linear approximation was possible [6]; thereby, the fit values of the curves were the same within the error ranges. In addition, the spatial distribution changes in anti-H3K9me3-labeled heterochromatin clusters indicated a dose-independent chromatin relaxation upon radiation exposure.
6. Conclusions
We demonstrated efficient applicability of SMLM to study radiation-induced damage and the associated repair protein recruitment to damage sites in space and time. The results revealed a cell type- and radiation type-specific nano-architecture of DNA damage foci with respect to γH2AX, Mre11 or 53BP1 and their dynamic molecular rearrangements during repair processes. Correlations with radio-sensitivity were revealed after 15N ion irradiation. SMLM studies thus contributed and will further contribute to the molecular understanding of cellular radiation responses at the sub-light microscopic chromatin levels, indicating that chromatin and the repair-focused nano-architecture might contribute to the repair pathway decision. Showing how the chromatin architecture around complex damage sites and the repair-focused nano-architecture may impact ongoing repair processes, our studies pave the way to the molecular understanding of the cellular radiation response and its regulation in cancer and non-cancer cells.
Author Contributions
Conceptualization, M.H. and M.F.; methodology, M.H. and H.S.; software, M.H. and D.W.H.; investigation, C.N., H.H., R.W., H.S. and G.P.; resources, M.H., H.S. and M.F.; data curation, M.H., C.N. and G.P.; writing—original draft preparation, M.H.; writing—review and editing, M.F., H.S. and D.W.H.; visualization, C.N. and R.W.; supervision, M.H., H.S., G.P., G.H. and M.F.; project administration, I.F. and M.H.; funding acquisition, M.H. and M.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the projects GACR 20-04109J (M.F. and M.H.) and GACR 19-09212S (M.F.), and projects of the Czech Government Plenipotentiary and Project 3 + 3 for cooperation with JINR Dubna to M.F. (MEYS CR, 2020). The Czech–German collaboration was supported by the Heidelberg University Mobility Grant for International Research Cooperation within excellence initiative II of the Deutsche Forschungsgemeinschaft (DFG), project DAAD-19-03 (M.F. and M.H.), and DFG grant H1601/16-1 to M.H.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The article reviews methods and data of several projects. For data availability, see the statements of the original articles (see list of references).
Acknowledgments
The authors greatly acknowledge the successful collaboration with Felix Bestvater, German Cancer Research Center (DKFZ), Heidelberg, and Christoph Cremer, Institute for Pharmacy and Molecular Biotechnology, Heidelberg University. In addition, the authors express their deep thanks to all members of the Laboratory of Radiation Biology and other colleagues from the Joint Institute for Nuclear Research (JINR), Dubna, Russia, involved in high-LET cell irradiation and sample preparation. Last but not least, the members of the Department of Cell Biology and Radiobiology, IBP, Brno, CR, and the members and students of the Experimental Biophysics Group, KIP, Heidelberg University, who contributed to the acquisition of a large part of the scientific data discussed in the present article are greatly appreciated.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lee, J.-H.; Hausmann, M. Super-Resolution Radiation Biology: From Bio-Dosimetry towards Nano-Studies of DNA Repair Mechanisms; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Falk, M.; Hausmann, M. A Paradigm Revolution or Just Better Resolution—Will Newly Emerging Superresolution Techniques Identify Chromatin Architecture as a Key Factor in Radiation-Induced DNA Damage and Repair Regulation? Cancers 2020, 13, 18. [Google Scholar] [CrossRef] [PubMed]
- Eberle, J.P.; Rapp, A.; Krufczik, M.; Eryilmaz, M.; Gunkel, M.; Erfle, H.; Hausmann, M. Super-resolution microscopy tech-niques and their potential for applications in radiation biophysics. In Super-resolution Microscopy—Methods and Proto-cols. Meth. Molec. Biol. 2017, 1663, 1–13. [Google Scholar]
- Falk, M.; Hausmann, M.; Lukášová, E.; Biswas, A.; Hildenbrand, G.; Davídková, M.; Krasavin, E.; Kleibl, Z.; Falková, I.; Ježková, L.; et al. Determining OMICS spatiotemporal dimensions using exciting new nanoscopy techniques to asses complex cell responses to DNA damage—PART A (Radiomics). Crit. Rev. Eukaryot. Gene Express. 2014, 24, 205–223. [Google Scholar] [CrossRef] [PubMed]
- Falk, M.; Hausmann, M.; Lukášová, E.; Biswas, A.; Hildenbrand, G.; Davídková, M.; Krasavin, E.; Kleibl, Z.; Falková, I.; Ježková, L.; et al. Determining OMICS spatiotemporal dimensions using exciting new nanoscopy techniques to asses complex cell responses to DNA damage—PART B (Structuromics). Crit. Rev. Eukaryot. Gene Express. 2014, 24, 225–247. [Google Scholar] [CrossRef] [PubMed]
- Hausmann, M.; Ilić, N.; Pilarczyk, G.; Lee, J.-H.; Logeswaran, A.; Borroni, A.P.; Krufczik, M.; Theda, F.; Waltrich, N.; Bestvater, F.; et al. Challenges for Super-Resolution Localization Microscopy and Biomolecular Fluorescent Nano-Probing in Cancer Research. Int. J. Mol. Sci. 2017, 18, 2066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.-H.; Tchetgna, F.L.D.; Krufczik, M.; Schmitt, E.; Cremer, C.; Estvater, F.B.; Hausmann, M. COMBO-FISH: A Versatile Tool Beyond Standard FISH to Study Chromatin Organization by Fluorescence Light Microscopy. OBM Genet. 2018, 3, 1. [Google Scholar] [CrossRef]
- Zhang, Y.; Máté, G.; Müller, P.; Hillebrandt, S.; Krufczik, M.; Bach, M.; Kaufmann, R.; Hausmann, M.; Heermann, D.W. Radiation Induced Chromatin Conformation Changes Analysed by Fluorescent Localization Microscopy, Statistical Physics, and Graph Theory. PLoS ONE 2015, 10, e0128555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hausmann, M.; Wagner, E.; Lee, J.-H.; Schrock, G.; Schaufler, W.; Krufczik, M.; Papenfuss, F.; Port, M.; Bestvater, F.; Scherthan, H. Super-resolution localization microscopy of radiation-induced histone H2AX-phosphorylation in relation to H3K9-trimethylation in HeLa cells. Nanoscale 2018, 10, 4320–4331. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, A.; Krufczik, M.; Heermann, D.W.; Hausmann, M. Using Persistent Homology as a New Approach for Super-Resolution Localization Microscopy Data Analysis and Classification of γH2AX Foci/Clusters. Int. J. Mol. Sci. 2018, 19, 2263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eryilmaz, M.; Schmitt, E.; Krufczik, M.; Theda, F.; Lee, J.-H.; Cremer, C.; Bestvater, F.; Schaufler, W.; Hausmann, M.; Hildenbrand, G. Localization Microscopy Analyses of MRE11 Clusters in 3D-Conserved Cell Nuclei of Different Cell Lines. Cancers 2018, 10, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scherthan, H.; Lee, J.-H.; Maus, E.; Schumann, S.; Muhtadi, R.; Chojowski, R.; Port, M.; Lassmann, M.; Bestvater, F.; Hausmann, M. Nanostructure of Clustered DNA Damage in Leukocytes after In-Solution Irradiation with the Alpha Emitter Ra. Cancers 2019, 11, 1877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Depes, D.; Lee, J.-H.; Bobkova, E.; Jezkova, L.; Falkova, I.; Bestvater, F.; Pagacova, E.; Kopecna, O.; Zadneprianetc, M.; Bacikova, A.; et al. Single-molecule localization microscopy as a promising tool for γH2AX/53BP1 foci exploration. Eur. Phys. J. 2018, 72, 158. [Google Scholar] [CrossRef]
- Bobkova, E.; Depes, D.; Lee, J.-H.; Jezkova, L.; Falkova, I.; Pagacova, E.; Kopecna, O.; Zadneprianetc, M.; Bacikova, A.; Kulikova, E.; et al. Recruitment of 53BP1 Proteins for DNA Repair and Persistence of Repair Clusters Differ for Cell Types as Detected by Single Molecule Localization Microscopy. Int. J. Mol. Sci. 2018, 19, 3713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hausmann, M.; Neitzel, C.; Bobkova, E.; Nagel, D.; Hofmann, A.; Chramko, T.; Smirnova, E.; Kopečná, O.; Pagáčová, E.; Boreyko, A.; et al. Single Molecule Localization Microscopy Analyses of DNA-Repair Foci and Clusters Detected Along Particle Damage Tracks. Front. Phys. 2020, 8, 473. [Google Scholar] [CrossRef]
- Krufczik, M.; Sievers, A.; Hausmann, A.; Lee, J.-H.; Hildenbrand, G.; Schaufler, W.; Hausmann, M. Combining Low Temperature Fluorescence DNA-Hybridization, Immunostaining, and Super-Resolution Localization Microscopy for Nano-Structure Analysis of ALU Elements and Their Influence on Chromatin Structure. Int. J. Mol. Sci. 2017, 18, 1005. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).