COVID-19 Pandemic in Brazil: Clinical Manifestation and Effect of Comorbidities on Outcomes of Hospitalized SARI Cases †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source and Preparation
2.2. Methods
3. Results
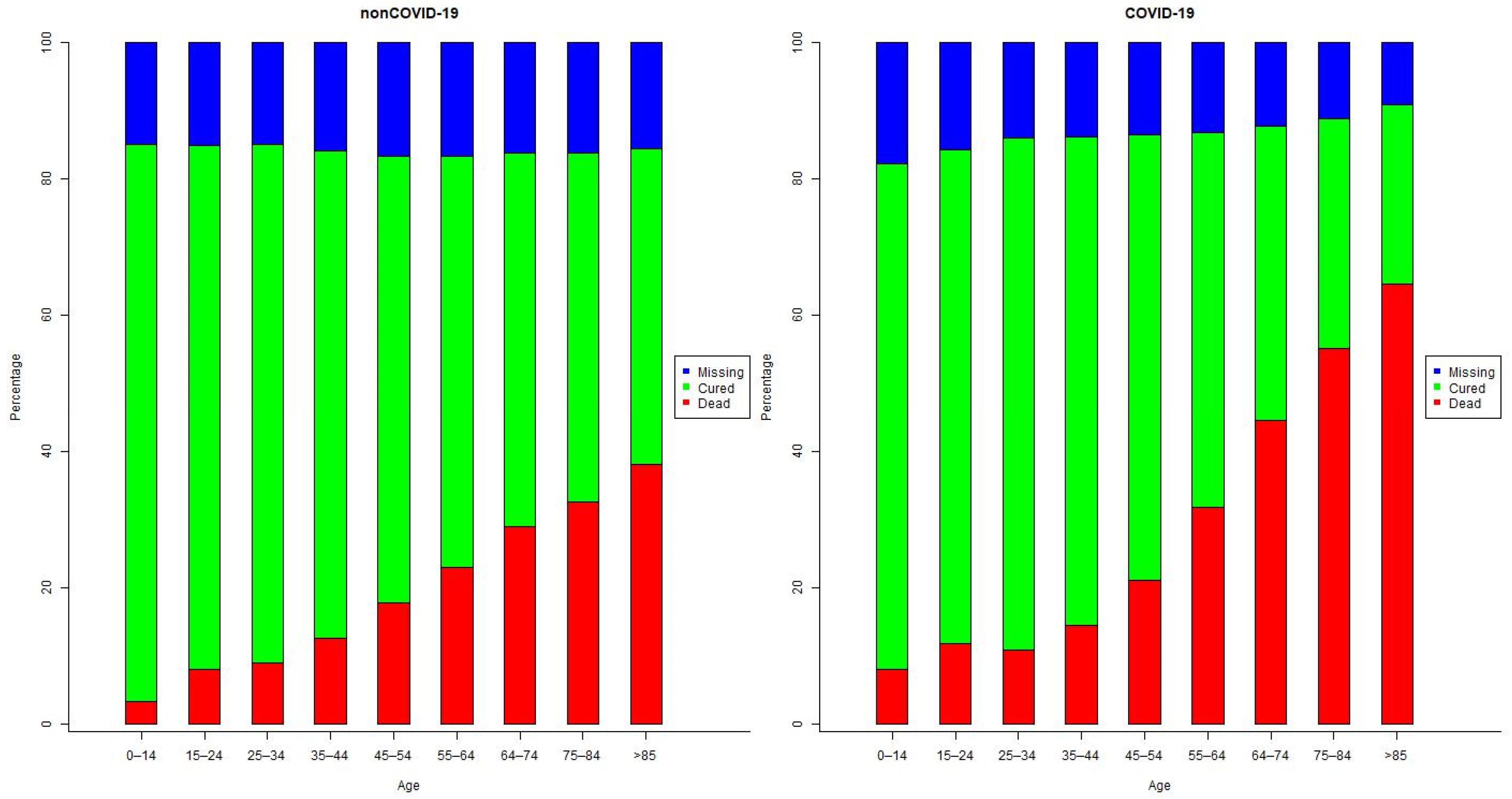
3.1. Demographic Characteristics of the Patients
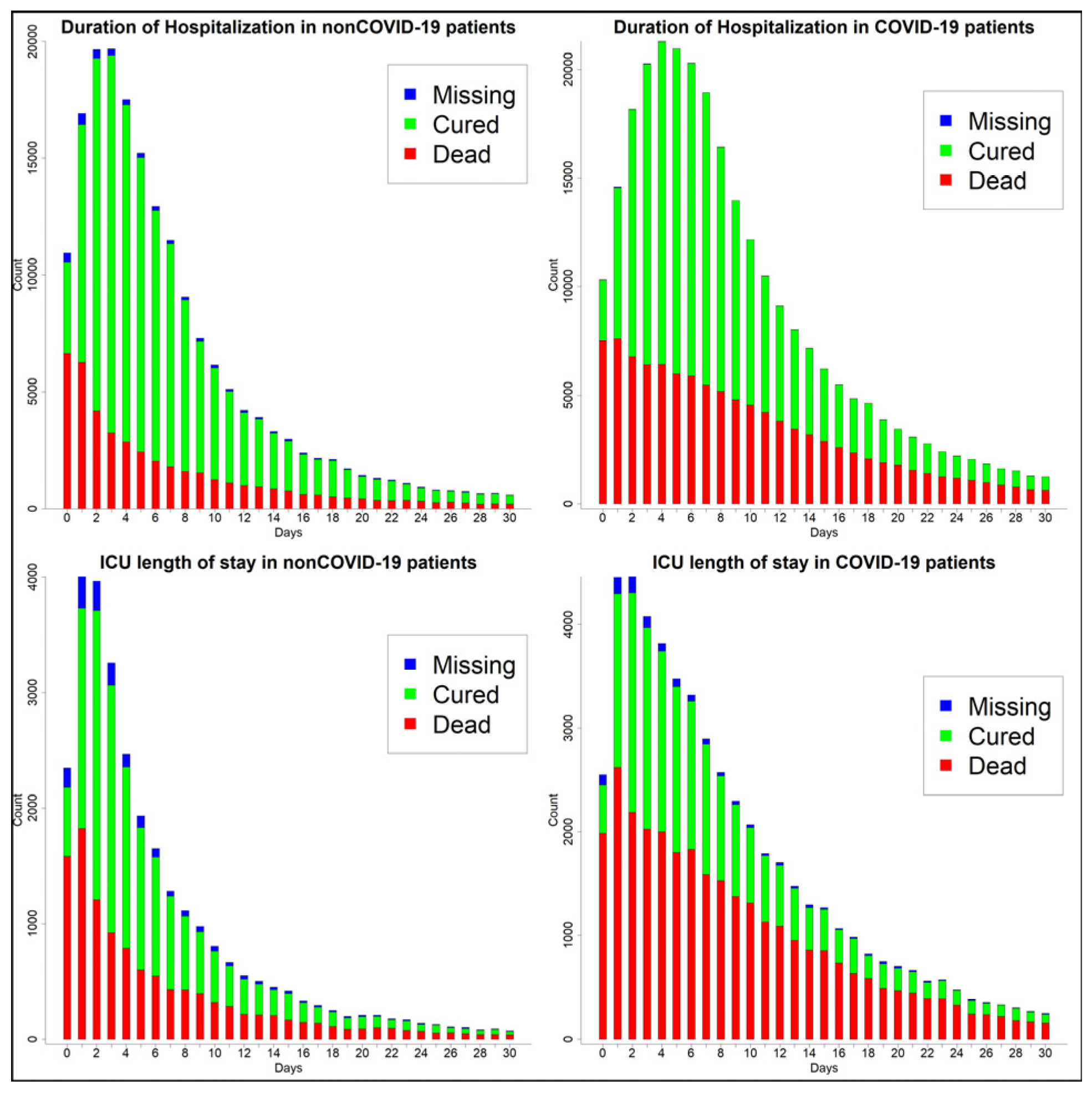
3.2. Clinical Manifestations and Outcomes by Group
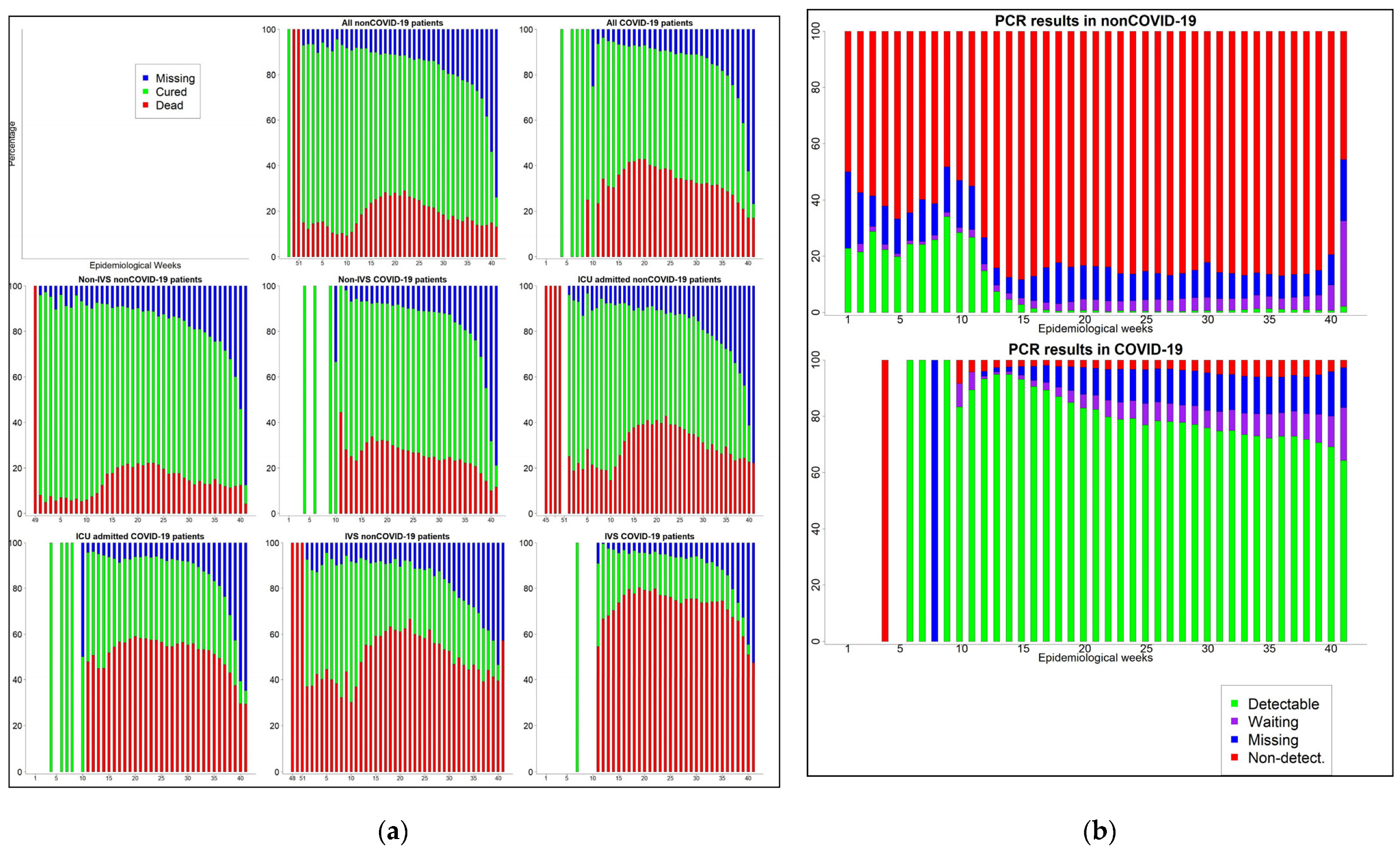
3.3. Time Trend of SARI Cases and the Performance of PCR Tests
3.4. Comorbidities and Their Effect on Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| None of Below | At Least One of Below | Severity |
|---|---|---|
| a, b, c, d, e, f, g, h, i | - | Asymptomatic |
| f, g, h, i | a, b, c, d, e | Mild |
| f, g, h, i | Severe |
| Non-COVID-19 | COVID-19 | |||||
|---|---|---|---|---|---|---|
| Dead (%) | Cured (%) | Missing (%) | Dead (%) | Cured (%) | Missing (%) | |
| Male | 29,102 (54.58) | 82,240 (51.85) | 21,441 (53.35) | 73,964 (58.04) | 106,905 (55.89) | 25,967 (56.21) |
| Female | 24,200 (45.39) | 76,290 (48.1) | 18,729 (46.6) | 53,440 (41.94) | 84,326 (44.09) | 20,218 (43.76) |
| Missing | 14 (0.03) | 80 (0.05) | 20 (0.05) | 26 (0.02) | 46 (0.02) | 12 (0.03) |
| Total | 53,316 | 158,610 | 40,190 | 127,430 | 191,277 | 46,197 |
References
- Gorbalenya, A.E.; Baker, S.C.; Baric, R.S.; de Groot, R.J.; Drosten, C.; Gulyaeva, A.A.; Haagmans, B.L.; Lauber, C.; Leontovich, A.M.; Neuman, B.W.; et al. The species Severe acute respiratory syndrome-related coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020, 5, 536–544. [Google Scholar] [CrossRef] [Green Version]
- Dhama, K.; Khan, S.; Tiwari, R.; Sircar, S.; Bhat, S.; Malik, Y.S.; Singh, K.P.; Chaicumpa, W.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J. Coronavirus Disease 2019-COVID-19. Clin. Microbiol. Rev. 2020, 33. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, P.; Wang, X.; Geng, C.; Chen, J.; Gong, Y. Molecular diagnosis of COVID-19: Current situation and trend in China (Review). Exp. Ther. Med. 2020, 20, 13. [Google Scholar] [CrossRef] [PubMed]
- Guglielmi, G. The explosion of new coronavirus tests that could help to end the pandemic. Nature 2020, 583, 506–509. [Google Scholar] [CrossRef] [PubMed]
- Hallal, P.C.; Hartwig, F.P.; Horta, B.L.; Silveira, M.F.; Struchiner, C.J.; Vidaletti, L.P.; Neumann, N.A.; Pellanda, L.C.; Dellagostin, O.A.; Burattini, M.N.; et al. SARS-CoV-2 antibody prevalence in Brazil: Results from two successive nationwide serological household surveys. Lancet Glob. Health 2020, 8, e1390–e1398. [Google Scholar] [CrossRef]
- Coronavirus. Available online: http://plataforma.saude.gov.br/coronavirus/dados-abertos/ (accessed on 12 November 2020).
- Ficha de Registro Individual-Casos de Síndrome Respiratória Aguda Grave Hospitalizado. Available online: http://plataforma.saude.gov.br/coronavirus/dados-abertos/sivep-gripe/ficha-SIVEP-GRIPE-SRAG-hospital-Sivepgripe.pdf (accessed on 12 November 2020).
- Clinical Spectrum of SARS-CoV-2 Infection. Available online: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/ (accessed on 12 November 2020).
- Agresti, A. Logistic Regression. In An Introduction to Categorical Data Analysis; Wiley: Hoboken, NJ, USA, 2007; pp. 106–109. [Google Scholar]
- Agresti, A. Generalized Linear Models for Count Data. In An Introduction to Categorical data Analysis, 2nd ed.; Wiley: Hoboken, NJ, USA, 2007; pp. 74–83. [Google Scholar]
- de Souza, W.M.; Buss, L.F.; Candido, D.d.S.; Carrera, J.-P.; Li, S.; Zarebski, A.E.; Pereira, R.H.M.; Prete, C.A.; de Souza-Santos, A.A.; Parag, K.V.; et al. Epidemiological and clinical characteristics of the COVID-19 epidemic in Brazil. Nat. Hum. Behav. 2020, 4, 856–865. [Google Scholar] [CrossRef] [PubMed]
- Carli, G.; Cecchi, L.; Stebbing, J.; Parronchi, P.; Farsi, A. Is asthma protective against COVID-19? Allergy 2021, 76, 866–868. [Google Scholar] [CrossRef] [PubMed]

| Variable | Categories | COVID-19 (%) | Non-COVID-19 (%) |
|---|---|---|---|
| Sex | Male | 206,836 (56.7) | 132,783 (52.7) |
| Female | 157,984 (43.3) | 119,219 (47.3) | |
| Missing | 84 | 114 | |
| Race | White | 17,771 (6.5) | 13,024 (6.6) |
| Black | 124,863 (45.8) | 98,909 (50.3) | |
| Other | 129,858 (47.7) | 84,559 (43.0) | |
| Missing | 92,412 | 55,624 | |
| Education | No education | 10,194 (8.1) | 10,203 (12.1) |
| 1st cycle | 35,244 (28.0) | 28,773 (34.0) | |
| 2nd cycle | 23,557 (18.7) | 15,649 (18.5) | |
| High school | 39,030 (31.0) | 21,615 (25.6) | |
| University | 18,111 (14.36) | 8335 (9.86) | |
| Missing | 238,768 | 167,541 |
| Criteria | Detectable | Not Detect/Inconcl. | Waiting | Missing | Total | |
|---|---|---|---|---|---|---|
| COVID-19 | Lab. | 282,206 (83.1) | 8731 (2.6) | 17,593 (5.2) | 31,040 (9.1) | 339,570 (93.1) |
| E. Linking | 178 (7.8) | 272 (11.8) | 651 (28.3) | 1196 (52.1) | 2297 (0.6) | |
| Clinical | 407 (5.5) | 1004 (13.6) | 1596 (21.6) | 4398 (59.4) | 7405 (2.0) | |
| Other | 162 (2.3) | 3576 (51.6) | 1004 (14.5) | 2185 (31.5) | 6927 (1.9) | |
| Missing | 4474 (51.4) | 432 (5.0) | 1868 (21.5) | 1931 (22.2) | 8705 (2.4) | |
| Non-COVID-19 | Lab | 4771 (2.2) | 203,640 (91.9) | 5721 (2.6) | 7404 (3.3) | 221,536 (87.9) |
| E. Linking | 0 (0.0) | 498 (20.9) | 332 (13.9) | 1553 (65.2) | 2383 (0.9) | |
| Clinical | 13 (0.1) | 2432 (13.6) | 2025 (11.3) | 13,452 (75.1) | 17,922 (7.1) | |
| Other | 0 (0.0) | 354 (50.4) | 77 (11.0) | 271 (38.6) | 702 (0.3) | |
| Missing | 105 (1.1) | 6082 (63.5) | 1424 (14.9) | 1962 (20.5) | 9573 (3.8) |
| Severity | ||||
|---|---|---|---|---|
| Asymptomatic (%) | Mild (%) | Severe (%) | Missing | |
| Chronic Heart diseases | ||||
| Yes | 177 (32.9) | 10,756 (21.19) | 180,798 (33.31) | 4145 (18.13) |
| No | 361 (67.1) | 40,008 (78.81) | 362,054 (66.69) | 18,721 (81.87) |
| Chronic Lung diseases | ||||
| Yes | 7 (1.3) | 894 (1.76) | 30,530 (5.62) | 401 (1.75) |
| No | 531 (98.7) | 49,870 (98.24) | 512,322 (94.38) | 22,465 (98.25) |
| Diabetes | ||||
| Yes | 93 (17.29) | 8311 (16.37) | 128,058 (23.59) | 3056 (13.36) |
| No | 445 (82.71) | 42,453 (83.63) | 414,794 (76.41) | 19,810 (86.64) |
| Asthma | ||||
| Yes | 8 (1.49) | 913 (1.8) | 22,619 (4.17) | 222 (0.97) |
| No | 530 (98.51) | 49,851 (98.2) | 520,233 (95.83) | 22,644 (99.03) |
| Immunodeficiency | ||||
| Yes | 29 (5.39) | 1905 (3.75) | 18,308 (3.37) | 534 (2.34) |
| No | 509 (94.61) | 48,859 (96.25) | 524,544 (96.63) | 22,332 (97.66) |
| Chronic Renal diseases | ||||
| Yes | 22 (4.09) | 1828 (3.6) | 24,458 (4.51) | 714 (3.12) |
| No | 516 (95.91) | 48,936 (96.4) | 518,394 (95.49) | 22,152 (96.88) |
| Chronic Liver diseases | ||||
| Yes | 8 (1.49) | 474 (0.93) | 5,714 (1.05) | 176 (0.77) |
| No | 530 (98.51) | 50,290 (99.07) | 53,7138 (98.95) | 22690 (99.23) |
| Obesity | ||||
| Yes | 11 (2.04) | 976 (1.92) | 22,390 (4.12) | 359 (1.57) |
| No | 527 (97.96) | 49,788 (98.08) | 520,462 (95.88) | 22,507 (98.43) |
| ICU Admission | Non-IVS vs. No VS | IVS vs. No VS | Dead vs. Cured | |||||
|---|---|---|---|---|---|---|---|---|
| β | OR | β | OR | β | OR | β | OR | |
| COVID-19 * | 0.27c | 1.31 | 0.27 c | 1.31 | 0.39 c | 1.47 | 0.62 b | 1.85 |
| VS * -Non-IVS | - | - | - | - | - | - | 0.37 b | 1.45 |
| -IVS | - | - | - | - | - | - | 2.70 c | 14.9 |
| Severity § | ||||||||
| Asymp. | 1.06 c | 2.9 | −0.06 | 0.94 | 1.01 | 2.76 | −0.32 c | 0.74 |
| Severe | 0.65 c | 1.91 | 1.58 | 4.84 | 1.84 | 6.34 | 0.45 c | 1.56 |
| Heart dise.* | 0.23 c | 1.26 | 0.26 c | 1.3 | 0.3 c | 1.35 | −0.03 b | 0.97 |
| Lung dise. * | 0.24 c | 1.27 | 0.28 c | 1.32 | 0.47 c | 1.61 | 0.08 c | 1.08 |
| Diabetes * | 0.18 | 1.19 | 0.14 c | 1.15 | 0.3 c | 1.36 | 0.15 c | 1.16 |
| Asthma * | −0.16 c | 0.85 | 0.26 c | 1.29 | −0.04 | 0.96 | −0.33 c | 0.72 |
| Immunosu. * | 0.27 c | 1.31 | −0.02 | 0.98 | 0.28 c | 1.32 | 0.77 c | 2.17 |
| Renal dise. * | 0.48 c | 1.62 | −0.05 a | 0.95 | 0.32 c | 1.38 | 0.47 c | 1.6 |
| Liver dise. * | 0.33 c | 1.4 | −0.06 | 0.94 | 0.38 | 1.46 | 0.68 c | 1.97 |
| Obesity * | 0.48 c | 1.62 | 0.33 c | 1.38 | 0.68 c | 1.98 | 0.13 c | 1.14 |
| Age | 0.01 | 1.01 | 0.01 | 1.01 | 0.02 c | 1.02 | 0.04 | 1.05 |
| Female † | −0.11 | 0.89 | −0.06 c | 0.94 | −0.18 c | 0.83 | −0.16 | 0.85 |
| Race ¶ -White | 0.08 c | 1.09 | 0.01 | 1 | −0.11 a | 0.9 | −0.36 | 0.7 |
| -Other | 0.001 | 1 | 0.001 | 1 | 0.05 | 1.06 | 0.06 | 1.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keko, M.; Peace, K.E. COVID-19 Pandemic in Brazil: Clinical Manifestation and Effect of Comorbidities on Outcomes of Hospitalized SARI Cases. Med. Sci. Forum 2021, 4, 37. https://doi.org/10.3390/ECERPH-3-09070
Keko M, Peace KE. COVID-19 Pandemic in Brazil: Clinical Manifestation and Effect of Comorbidities on Outcomes of Hospitalized SARI Cases. Medical Sciences Forum. 2021; 4(1):37. https://doi.org/10.3390/ECERPH-3-09070
Chicago/Turabian StyleKeko, Mario, and Karl E. Peace. 2021. "COVID-19 Pandemic in Brazil: Clinical Manifestation and Effect of Comorbidities on Outcomes of Hospitalized SARI Cases" Medical Sciences Forum 4, no. 1: 37. https://doi.org/10.3390/ECERPH-3-09070
APA StyleKeko, M., & Peace, K. E. (2021). COVID-19 Pandemic in Brazil: Clinical Manifestation and Effect of Comorbidities on Outcomes of Hospitalized SARI Cases. Medical Sciences Forum, 4(1), 37. https://doi.org/10.3390/ECERPH-3-09070





