Nutritional Adequacy and Patient Perceptions of the Hospital Inpatient Haemodialysis Menu: A Mixed Methods Case Series
Abstract
:1. Introduction
2. Materials and Methods
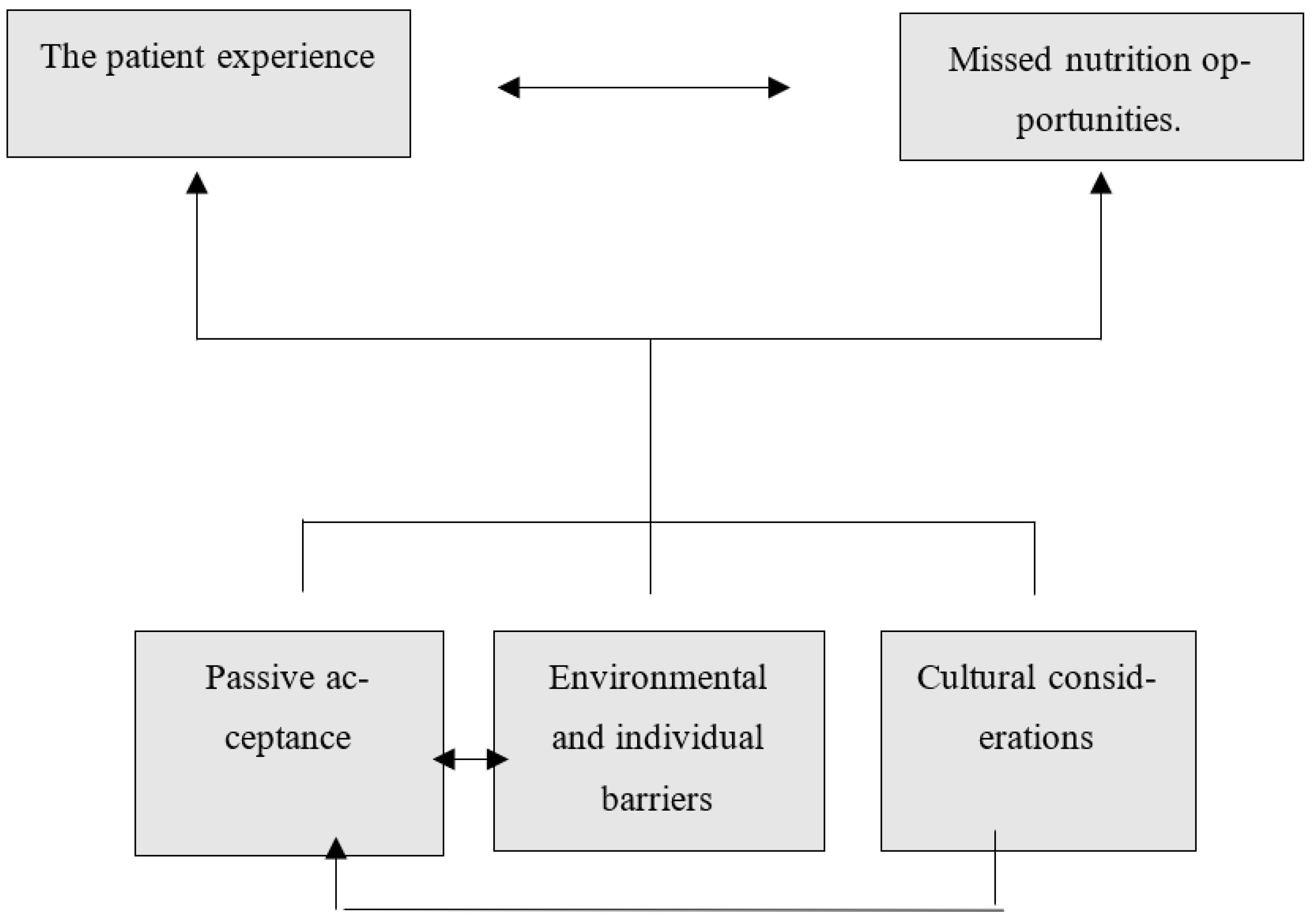
3. Results
“I’m in prison, cause I’m not very hungry” (Participant 5).
“They look appetising, they look good and you put it in your mouth and it’s like ehhh” (Participant 5).
“I don’t mind fish, but not poached in water…It comes out of water and goes back in water” (Participant 7).
“Sometimes my carer brings the food for me, because I don’t like what’s on the menu” (Participant 2).
“…And because he can speak to my mum about what he wants … if there’s anything wrong with it then he can say this and that. But he doesn’t feel like he has that option sometimes with … the hospital food” (Carer of Participant 3).
“Because I always ask for something and I don’t always get it” (Participant 2).
“It’s probably good for me you know” (Participant 5).
“Everything’s like healthy you know, what can you do” (Participant 8).
“I like juice but they don’t like me to have that. They say I’m not supposed to have juice” (Participant 2).
“You can’t ask for what I want” (Participant 8).
“Yeah so that’s the thing, when he comes back after an hour the food might go cold” (Carer of Participant 1).
“They’re busy okay so no one can really help you to get you up” (Participant 8).
“It’s not human to be laying in bed and trying to eat” (Participant 7).
“He’s just not very accepting. He doesn’t enjoy the Western sort of cuisine, he just prefers the oriental sort of cuisine that my mum makes” (Carer of Participant 3).
“They do different way for our benefit you know” (Participant 8).
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ash, S.; Campbell, K.L.; Bogard, J.; Millichamp, A. Nutrition prescription to achieve positive outcomes in chronic kidney disease: A systematic review. Nutrients 2014, 6, 416–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bossola, M.; Di Stasio, E.; Viola, A.; Cenerelli, S.; Leo, A.; Santarelli, S.; Monteburini, T. Dietary Daily Sodium Intake Lower than 1500 mg Is Associated with Inadequately Low Intake of Calorie, Protein, Iron, Zinc and Vitamin B1 in Patients on Chronic Hemodialysis. Nutrients 2020, 12, 260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogacka, A.; Sobczak-Czynsz, A.; Kucharska, E.; Madaj, M.; Stucka, K. Analysis of nutrition and nutritional status of haemodialysis patients. Rocz. Panstw. Zakl. Hig. 2018, 69, 165–174. [Google Scholar] [PubMed]
- Neven, E.; De Schutter, T.M.; Behets, G.J.; Gupta, A.; D’Haese, P.C. Iron and vascular calcification. Is there a link? Nephrol. Dial. Transplant. 2011, 26, 1137–1145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jankowska, M.; Rutkowski, B.; Dębska-Ślizień, A. Vitamins and microelement bioavailability in different stages of chronic kidney disease. Nutrients 2017, 9, 282. [Google Scholar] [CrossRef] [Green Version]
- Hendriks, F.K.; Smeets, J.S.; Broers, N.J.; van Kranenburg, J.M.; van der Sande, F.M.; Kooman, J.P.; van Loon, L.J. End-Stage Renal Disease Patients Lose a Substantial Amount of Amino Acids during Hemodialysis. J. Nutr. 2020, 150, 1160–1166. [Google Scholar] [CrossRef] [Green Version]
- Piccini, S.; Fairburn, A.; Gill, E.; Budgeon, C.A.; O’Sullivan, T. Predictors of malnutrition in Australian haemodialysis patients and comparison of dietary protein intakes to national guidelines. Ren. Soc. Australas. J. 2014, 10, 133–140. [Google Scholar]
- Akhlaghi, Z.; Sharifipour, F.; Nematy, M.; Safarian, M.; Malekahmadi, M.; Barkhidarian, B.; Norouzy, A. Assessment of nutritional status in maintenance hemodialysis patients: A multicenter cross-sectional study in Iran. Semin. Dial. 2021, 34, 77–82. [Google Scholar] [CrossRef]
- Lee, J.E.; Kim, H.-J.; Lee, M.J.; Kwon, Y.E.; Kyung, M.-S.; Park, J.-T.; Lee, J.P.; Kim, S.-H.; Kim, J.-H.; Oh, H.J.; et al. Comparison of dietary intake patterns in hemodialysis patients by nutritional status: A cross-sectional analysis. Kidney Res. Clin. Pract. 2020, 39, 202–212. [Google Scholar] [CrossRef]
- Bataille, S.; Landrier, J.-F.; Astier, J.; Cado, S.; Sallette, J.; Giaime, P.; Sampol, J.; Sichez, H.; Ollier, J.; Gugliotta, J.; et al. Haemodialysis patients with diabetes eat less than those without: A plea for a permissive diet. Nephrology 2017, 22, 712–719. [Google Scholar] [CrossRef] [Green Version]
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.-J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J.; et al. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. Am. J. Kidney Dis. 2020, 76, S1–S107. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.I.; Wang, W.; Chan, E.Y.; Mohamed, F.; Chen, H.C. Dietary and fluid restriction perceptions of patients undergoing haemodialysis: An exploratory study. J. Clin. Nurs. 2017, 26, 3664–3676. [Google Scholar] [CrossRef]
- Opiyo, R.O.; Nyawade, S.A.; McCaul, M.; Nyasulu, P.S.; Lango, D.B.; Were, A.J.O.; Nabakwe, E.C.; Bukania, Z.N.; Olenja, J.M. Perceptions on Adherence to Dietary Prescriptions for Adults with Chronic Kidney Disease on Hemodialysis: A Qualitative Study. Diseases 2020, 8, 29. [Google Scholar] [CrossRef] [PubMed]
- Marshall, A.P.; Takefala, T.; Williams, L.T.; Spencer, A.; Grealish, L.; Roberts, S. Health practitioner practices and their influence on nutritional intake of hospitalised patients. Int. J. Nurs. Sci. 2019, 6, 162–168. [Google Scholar] [CrossRef]
- Larby, A.; Roberts, S.; Desbrow, B. Accuracy and adequacy of food supplied in therapeutic diets to hospitalised patients: An observational study: Accuracy and adequacy of hospital therapeutic diets. Nutr. Diet. 2016, 73, 342–347. [Google Scholar] [CrossRef] [Green Version]
- Keller, H.; Allard, J.; Vesnaver, E.; Laporte, M.; Gramlich, L.; Bernier, P.; Davidson, B.; Duerksen, D.; Jeejeebhoy, K.; Payette, H. Barriers to food intake in acute care hospitals: A report of the Canadian Malnutrition Task Force. J. Hum. Nutr. Diet. 2015, 28, 546–557. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.; Brady, A.-M.; Byrne, G. An overview of mixed methods research—Revisited. J. Res. Nurs. 2016, 21, 623–635. [Google Scholar] [CrossRef]
- Agency for Clinical Innovation (Ed.) Nutrition Standards for Adult Inpatients in NSW Hospitals; Agency for Clinical Innovation: St Leonards, Australia, 2011.
- Ash, S.; Campbell, K.; MacLaughlin, H.; McCoy, E.; Chan, M.; Anderson, K.; Corke, K.; Dumont, R.; Lloyd, L.; Meade, A.; et al. Evidence based practice guidelines for the nutritional management of chronic kidney disease. Nutr. Diet. 2006, 63, S33–S45. [Google Scholar] [CrossRef] [Green Version]
- Desbrow, B.; Bauer, J.; Blum, C.; Kandasamy, A.; McDonald, A.; Montgomery, K. Assessment of nutrition status in haemodialysis patients using the patient-generated subjective global assessment. J. Ren. Nutr. 2005, 15, 211–216. [Google Scholar] [CrossRef]
- National Health and Medical Research Council (Ed.) Nutrient Reference Values; National Health and Medical Research Council: Canberra, Australia, 2017. [Google Scholar]
- Green, J.; Willis, K.; Hughes, E.; Small, R.; Welch, N.; Gibbs, L.; Daly, J. Generating best evidence from qualitative research: The role of data analysis. Aust. N. Z. J. Public Health 2007, 31, 545–550. [Google Scholar] [CrossRef]
- World Health Organization. Vienna Declaration on Nutrition and Noncommunicable Diseases in the Context of Health 2020; World Health Organization: Copenhagen, Denmark, 2020. [Google Scholar]
- Trang, S.; Fraser, J.; Wilkinson, L.; Steckham, K.; Oliphant, H.; Fletcher, H.; Tzianetas, R.; Arcand, J. A multi-center assessment of nutrient levels and foods provided by hospital patient menus. Nutrients 2015, 7, 9256–9264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rattray, M.; Desbrow, B.; Roberts, S. Comparing nutritional requirements, provision and intakes among patients prescribed therapeutic diets in hospital: An observational study. Nutrition 2017, 39–40, 50–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Australian Commission on Safety and Quality in Health Care (Ed.) Hospital-Acquired Complication Malnutrition; Australian Commission on Safety and Quality in Health Care: Sydney, Australia, 2018. [Google Scholar]
- Mudge, A.; Ross, L.; Young, A.; Isenring, E.; Banks, M. Helping understand nutritional gaps in the elderly (HUNGER): A prospective study of patient factors associated with inadequate nutritional intake in older medical inpatients. Clin. Nutr. 2011, 30, 320–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- dos Santos, R.G.; Scatone, N.K.; Malinovski, J.; Sczip, A.C.; de Oliveira, J.C.; Morais, J.G.; Ramos, C.I.; Nerbass, F.B. Higher Frequency of Fruit Intake Is Associated With a Lower Risk of Constipation in Hemodialysis Patients: A Multicenter Study. J. Ren. Nutr. 2021, 31, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-M.; Kang, B.C.; Kim, H.-J.; Kyung, M.-S.; Oh, H.J.; Kim, J.-H.; Kwon, O.; Ryu, D.-R. Comparison of hemodialysis and peritoneal dialysis patients’ dietary behaviors. BMC Nephrol. 2020, 21, 91. [Google Scholar] [CrossRef] [Green Version]
- Raimann, J.; Levin, N.; Craig, R.; Sirover, W.; Kotanko, P.; Handelman, G. Is vitamin C intake too low in dialysis patients? Semin. Dial. 2013, 26, 1–5. [Google Scholar] [CrossRef]
- Handelman, G. Vitamin C deficiency in dialysis patients—Are we perceiving the tip of an iceburg. Nephrol. Dial. Transplant. 2006, 22, 328–331. [Google Scholar] [CrossRef] [Green Version]
- Naithani, S.; Whelan, K.; Thomas, J.; Gulliford, M.C.; Morgan, M. Hospital inpatients’ experiences of access to food: A qualitative interview and observational study. Health Expect. 2008, 11, 294–303. [Google Scholar] [CrossRef] [Green Version]
- Nkwonta, C.A.; Dawson, R.M.; Adegboyega, A. “I don’t think I have a chance to get it”: International university student HPV knowledge and preventive behaviors. J. Am. Coll. Health 2020, 70, 240–247. [Google Scholar] [CrossRef]
- Aasen, E.M.; Kvangarsnes, M.; Heggen, K. Perceptions of patient participation amongst elderly patients with end-stage renal disease in a dialysis unit. Scand. J. Caring Sci. 2012, 26, 61–69. [Google Scholar] [CrossRef] [Green Version]
- McLean, R.M.; Xie, Z.; Nelson, V.; Nosa, V.; Thein, H.; Po’e-Tofaeono, A.; Walker, R.; Wyeth, E.H. Experiences of New Zealand Haemodialysis Patients in Relation to Food and Nutrition Management: A Qualitative Study. Nutrients 2021, 13, 2299. [Google Scholar] [CrossRef] [PubMed]
- Snyder, H.J.; Fletcher, K.E. The Hospital Experience Through the Patients’ Eyes. J. Patient Exp. 2020, 7, 408–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, A.; Saraiva, C.; Esteves, A.; Gonçalves, C. Evaluation of Hospital Food Waste—A Case Study in Portugal. Sustainability 2020, 12, 6157. [Google Scholar] [CrossRef]
- Barker, L.A.; Gout, B.S.; Crowe, T.C. Hospital malnutrition: Prevalence, identification and impact on patients and the healthcare system. Int. J. Environ. Res. Public Health 2011, 8, 514–527. [Google Scholar] [CrossRef] [Green Version]
- Saglimbene, V.M.; Su, G.; Wong, G.; Natale, P.; Ruospo, M.; Palmer, S.C.; Craig, J.C.; Carrero, J.J.; Strippoli, G.F. Dietary intake in adults on hemodialysis compared with guideline recommendations. J. Nephrol. 2021, 34, 1999–2007. [Google Scholar] [CrossRef] [PubMed]
- Arenas Moya, D.; Plascencia Gaitán, A.; Ornelas Camacho, D.; Arenas Márquez, H. Hospital Malnutrition Related to Fasting and Underfeeding: Is It an Ethical Issue? Nutr. Clin. Pract. 2016, 31, 316–324. [Google Scholar] [CrossRef]
| Nutrient | Reference Value | Requirement for 76 kg Reference Individual | Average Haemodialysis Diet Menu Provision | p-Value |
|---|---|---|---|---|
| Energy | 126 kJ/kg | 9576 kJ/day | 8767 kJ ± 362 | <0.001 |
| Protein | 1.2 g/kg | 91 g/day | 90 g ± 6 | 0.40 |
| Sodium | <100 mmol/day | <100 mmol/day | 72 mmol/day ± 9 | <0.001 |
| Potassium | 1 mmol/kg | 76 mmol/day | 64 mmol/day ± 4 | <0.001 |
| Phosphate | 32 mmol/day | 32 mmol/day | 46 mmol/day ± 2 | <0.001 |
| Iron | 8 mg/day | 8 mg/day | 14 mg/day ± 1 | <0.001 |
| Zinc | 14 mg/day | 14 mg/day | 14 mg/day ± 2 | 0.46 |
| Vitamin C | 45 mg/day | 45 mg/day | 33 mg/day ± 10 | <0.001 |
| Folate | 400 ug/day | 400 ug/day | 439 mg/day ± 27 | <0.001 |
| Fibre | 30 g/day | 30 g/day | 26 g/day ± 3 | <0.001 |
| Study ID 1 | Study ID 2 | Study ID 3 | Study ID 4 | Study ID 5 | Study ID 6 | Study ID 7 | Study ID 8 | |
|---|---|---|---|---|---|---|---|---|
| Gender | M | F | M | M | M | M | M | M |
| Age | 78 | 66 | 76 | 64 | 71 | 60 | 76 | 78 |
| Weight (kg) | 65 | 39.4 | 58 | 66 | 96 | 65 | 100 | 71 |
| BMI (kg/m2) | 22.8 | 17.5 | 24.1 | 27.5 | 33.2 | 25.4 | 33.3 | 24.6 |
| PG-SGA score | A | B | B | C | B | C | B | C |
| Food provided from Home | Yes | Ad hoc | Yes | Nil | Nil | Nil | Nil | Nil |
| Study ID 1 | Study ID 2 | Study ID 3 | Study ID 4 | Study ID 5 | Study ID 6 | Study ID 7 | Study ID 8 | Mean | SD | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Energy (MJ) | Energy: 105–146 kJ/kg [11] | ||||||||||
| EER | 6.9–9.7 | 4.1–5.8 | 6.1–8.5 | 7.2–10 | 10–14.1 | 6.8–9.5 | 10–13.9 | 6.8–9.5 | |||
| Actual | 5.5 | 4.5 | 2.6 | 5.1 | 3.9 | 1.1 | 3.8 | 4.2 | 3.8 | 1.4 | |
| % | 80 | 100 | 43 | 71 | 39 | 16 | 38 | 62 | |||
| Protein (g) [11] | Protein: 1.0–1.2 g/kg [11] | ||||||||||
| ER | 66–79 | 39–47 | 58–70 | 69–82 | 96–115 | 65–78 | 95–114 | 65–78 | |||
| Actual | 62 | 54 | 22 | 75 | 39 | 11 | 42 | 45 | 44 | 21 | |
| % | 93 | 115 | 37 | 100 | 41 | 17 | 45 | 69 | |||
| Saturated fat (% energy) | Saturated fat recommended intake: <7% [11] | ||||||||||
| ER | 13 | 8 | 11 | 13 | 19 | 13 | 18 | 15 | |||
| EI | 15 | 13 | 13 | 19 | 8 | 4 | 11 | 16 | 12 | 5 | |
| % | 115 | 160 | 118 | 150 | 42 | 31 | 61 | 106 | |||
| Carbohydrate (g) | Carbohydrates recommended: 50–60% of energy intake [11] | ||||||||||
| ER | 206–248 | 123–148 | 181–218 | 215–258 | 300–361 | 204–244 | 298–357 | 204–244 | |||
| Actual | 168 | 116 | 77 | 12 | 136 | 36 | 104 | 97 | 44 | 18 | |
| % | 81 | 94 | 42 | 6 | 45 | 17 | 35 | 48 | |||
| Fibre (g/day) | Fibre recommended: Men: 30 g/day, Women: 25 g/day [21] | ||||||||||
| ER | 30 | 25 | 30 | 30 | 30 | 30 | 30 | 30 | |||
| Actual | 13 | 15 | 6 | 14 | 17 | 6 | 10 | 7 | 11 | 4 | |
| % | 45 | 60 | 20 | 46 | 55 | 20 | 34 | 22 | |||
| Potassium (mmol) a | Potassium recommended: Limit to 1 mmol/kg/Ideal body weight/day if hyperkalemic [11] | ||||||||||
| ER | 66 | 35 | 58 | 69 | 96 | 65 | 95 | 65 | |||
| Actual | 43 | 35 | 13 | 41 | 32 | 17 | 30 | 23 | 29 | 11 | |
| % | 66 | 100 | 23 | 60 | 33 | 26 | 32 | 36 | |||
| Phosphate (mmol) | ER | Phosphorus recommended: 32 mmol/day [19] if hyperphosphatemia [11] | |||||||||
| Actual | 29 | 29 | 15 | 33 | 15 | 7 | 18 | 17 | 20 | 9 | |
| % | 91 | 91 | 47 | 103 | 47 | 22 | 56 | 53 | |||
| Sodium (mmol) b | ER | Sodium recommended: <100 mmol day [11] | |||||||||
| Actual | 50 | 39 | 18 | 58 | 33 | 16 | 45 | 48 | 38 | 15 | |
| % | 50 | 39 | 18 | 58 | 33 | 16 | 45 | 48 | |||
| Calcium (mg) | ER | Calcium recommended: <1000 mg/day [21] | |||||||||
| Actual | 566 | 414 | 309 | 577 | 206 | 194 | 217 | 270 | 344 | 157 | |
| % | 57 | 41 | 31 | 58 | 21 | 19 | 22 | 27 | |||
| Iron (mg) | ER | 8 mg/day [21] | |||||||||
| Actual | 9 | 10 | 3 | 12 | 7 | 3 | 9 | 5 | 7 | 3 | |
| % | 113 | 126 | 32 | 145 | 92 | 41 | 116 | 59 | |||
| Zinc (mg) | Zinc recommended: Men: 14 mg/day, Women: 8 mg/day [21] | ||||||||||
| ER | 14 | 8 | 14 | 14 | 14 | 14 | 14 | 14 | |||
| Actual | 6 | 6 | 2 | 12 | 7 | 2 | 8 | 3 | 6 | 3 | |
| % | 46 | 76 | 16 | 89 | 50 | 17 | 55 | 21 | |||
| Folate (μg) | ER | Folate recommended: 400 μg/day [21] | |||||||||
| Actual | 295 | 220 | 86 | 313 | 245 | 130 | 310 | 223 | 228 | 83 | |
| % | 74 | 55 | 21 | 78 | 61 | 33 | 77 | 56 | |||
| Vitamin C (mg) | ER | Vitamin C recommended: 45 mg/day [21] | |||||||||
| Actual | 32 | 33 | 1 | 20 | 45 | 16 | 22 | 9 | 22 | 14 | |
| % | 71 | 74 | 2 | 44 | 100 | 37 | 49 | 20 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neal, K.; Al Nakeeb, F.; Lambert, K. Nutritional Adequacy and Patient Perceptions of the Hospital Inpatient Haemodialysis Menu: A Mixed Methods Case Series. Dietetics 2023, 2, 203-214. https://doi.org/10.3390/dietetics2030016
Neal K, Al Nakeeb F, Lambert K. Nutritional Adequacy and Patient Perceptions of the Hospital Inpatient Haemodialysis Menu: A Mixed Methods Case Series. Dietetics. 2023; 2(3):203-214. https://doi.org/10.3390/dietetics2030016
Chicago/Turabian StyleNeal, Kate, Fatima Al Nakeeb, and Kelly Lambert. 2023. "Nutritional Adequacy and Patient Perceptions of the Hospital Inpatient Haemodialysis Menu: A Mixed Methods Case Series" Dietetics 2, no. 3: 203-214. https://doi.org/10.3390/dietetics2030016
APA StyleNeal, K., Al Nakeeb, F., & Lambert, K. (2023). Nutritional Adequacy and Patient Perceptions of the Hospital Inpatient Haemodialysis Menu: A Mixed Methods Case Series. Dietetics, 2(3), 203-214. https://doi.org/10.3390/dietetics2030016





