Comparing Self-Reported Dietary Intake to Provided Diet during a Randomized Controlled Feeding Intervention: A Pilot Study
Abstract
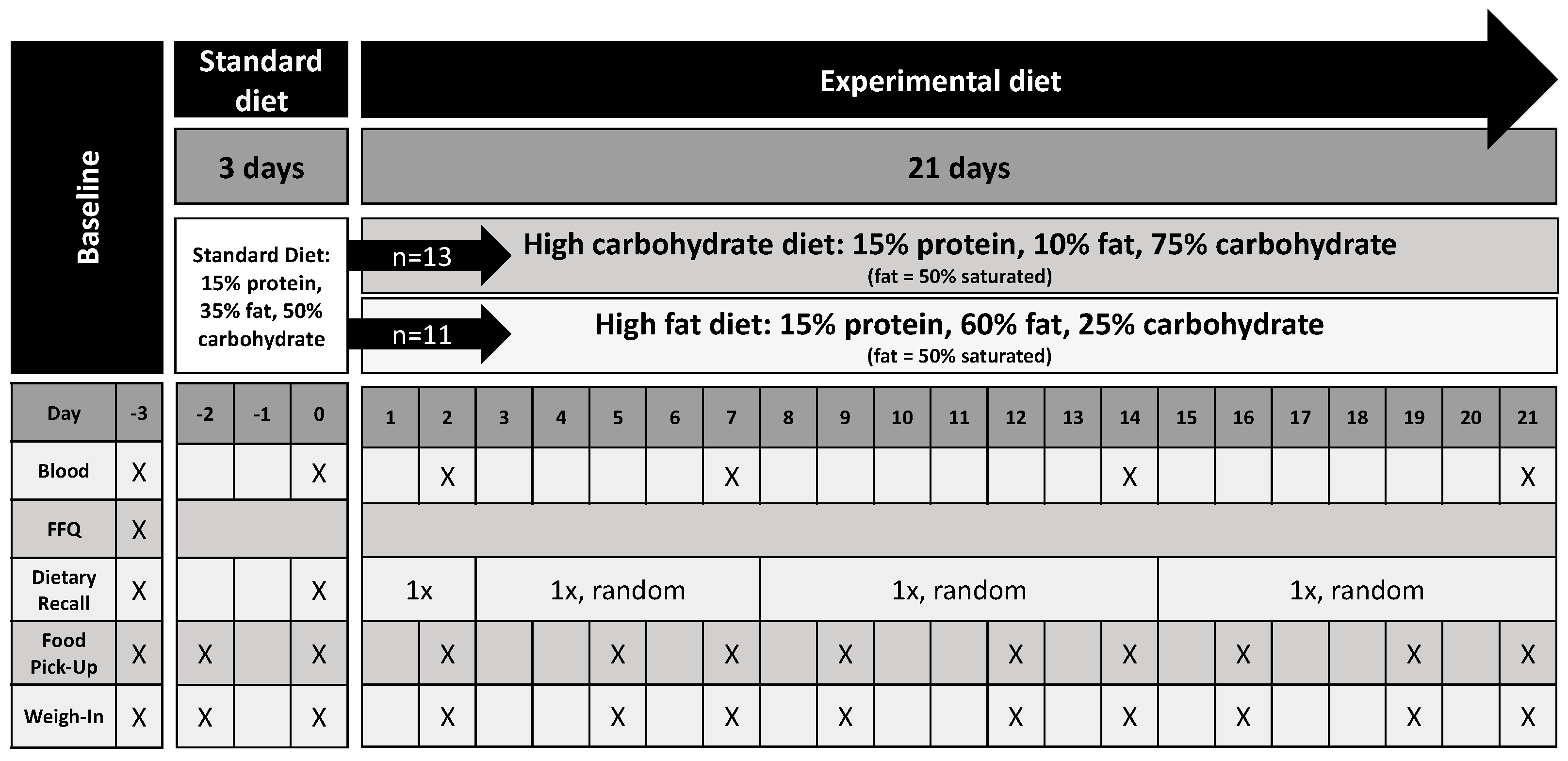
:1. Introduction
2. Materials and Methods
3. Results
3.1. Description of Dietary Comparisons
3.2. Discrepancies in Energy and Macronutrient Intake between Dietary Recall vs. Provided Meals
3.3. Discrepancies in Servings of Food Groups between Dietary Recall vs. Provided Meals
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Archer, E.; Marlow, M.L.; Lavie, C.J. Controversy and debate: Memory-Based Methods Paper 1: The fatal flaws of food frequency questionnaires and other memory-based dietary assessment methods. J. Clin. Epidemiol. 2018, 104, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Archer, E.; Pavela, G.; Lavie, C.J. The Inadmissibility of What We Eat in America and NHANES Dietary Data in Nutrition and Obesity Research and the Scientific Formulation of National Dietary Guidelines. Mayo Clin. Proc. 2015, 90, 911–926. [Google Scholar] [CrossRef] [PubMed]
- Macdiarmid, J.; Blundell, J. Assessing dietary intake: Who, what and why of under-reporting. Nutr. Res. Rev. 1998, 11, 231–253. [Google Scholar] [CrossRef]
- Chi, S.A.; Lee, H.; Lee, J.E.; Lee, H.S.; Kim, K.; Yeo, I.K. An ensemble method based on marginal-effect models (EMM) for estimating usual food intake from single-day dietary data and internal/external two-day dietary data. Eur. J. Clin. Nutr. 2023, 77, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Palaniappan, U.; Cue, R.I.; Payette, H.; Gray-Donald, K. Implications of day-to-day variability on measurements of usual food and nutrient intakes. J. Nutr. 2003, 133, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Young, L.R.; Nestle, M.S. Portion sizes in dietary assessment: Issues and policy implications. Nutr. Rev. 1995, 53, 149–158. [Google Scholar] [CrossRef]
- Tomoyasu, N.J.; Toth, M.J.; Poehlman, E.T. Misreporting of total energy intake in older men and women. J. Am. Geriatr. Soc. 1999, 47, 710–715. [Google Scholar] [CrossRef]
- Tomoyasu, N.J.; Toth, M.J.; Poehlman, E.T. Misreporting of total energy intake in older African Americans. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 20–26. [Google Scholar] [CrossRef]
- Taren, D.L.; Tobar, M.; Hill, A.; Howell, W.; Shisslak, C.; Bell, I.; Ritenbaugh, C. The association of energy intake bias with psychological scores of women. Eur. J. Clin. Nutr. 1999, 53, 570–578. [Google Scholar] [CrossRef]
- Poppitt, S.D.; Swann, D.; Black, A.E.; Prentice, A.M. Assessment of selective under-reporting of food intake by both obese and non-obese women in a metabolic facility. Int. J. Obes. Relat. Metab. Disord. 1998, 22, 303–311. [Google Scholar] [CrossRef]
- Heitmann, B.L.; Lissner, L.; Osler, M. Do we eat less fat, or just report so? Int. J. Obes. Relat. Metab. Disord. 2000, 24, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Garden, L.; Clark, H.; Whybrow, S.; Stubbs, R.J. Is misreporting of dietary intake by weighed food records or 24-h recalls food specific? Eur. J. Clin. Nutr. 2018, 72, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
- Gemming, L.; Ni Mhurchu, C. Dietary under-reporting: What foods and which meals are typically under-reported? Eur. J. Clin. Nutr. 2016, 70, 640–641. [Google Scholar] [CrossRef] [PubMed]
- Most, M.M.; Ershow, A.G.; Clevidence, B.A. An overview of methodologies, proficiencies, and training resources for controlled feeding studies. J. Am. Diet. Assoc. 2003, 103, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, R.J.; O’Reilly, L.M.; Whybrow, S.; Fuller, Z.; Johnstone, A.M.; Livingstone, M.B.; Ritz, P.; Horgan, G.W. Measuring the difference between actual and reported food intakes in the context of energy balance under laboratory conditions. Br. J. Nutr. 2014, 111, 2032–2043. [Google Scholar] [CrossRef]
- Whybrow, S.; Stubbs, R.J.; Johnstone, A.M.; O’Reilly, L.M.; Fuller, Z.; Livingstone, M.B.; Horgan, G.W. Plausible self-reported dietary intakes in a residential facility are not necessarily reliable. Eur. J. Clin. Nutr. 2016, 70, 130–135. [Google Scholar] [CrossRef]
- Schebendach, J.E.; Porter, K.J.; Wolper, C.; Walsh, B.T.; Mayer, L.E. Accuracy of self-reported energy intake in weight-restored patients with anorexia nervosa compared with obese and normal weight individuals. Int. J. Eat. Disord. 2012, 45, 570–574. [Google Scholar] [CrossRef]
- Westerterp, K.R.; Goris, A.H. Validity of the assessment of dietary intake: Problems of misreporting. Curr. Opin. Clin. Nutr. Metab. Care 2002, 5, 489–493. [Google Scholar] [CrossRef]
- Saksena, M.J.; Okrent, A.M.; Anekwe, T.D.; Cho, C.; Dicken, C.; Effland, A.; Elitzak, H.; Guthrie, J.; Hamrick, K.S.; Hyman, J.; et al. America’s Eating Habits: Food Away from Home; United States Department of Agriculture, Economic Research Service: Washington, DC, USA, 2018.
- Lachat, C.; Nago, E.; Verstraeten, R.; Roberfroid, D.; Van Camp, J.; Kolsteren, P. Eating out of home and its association with dietary intake: A systematic review of the evidence. Obes. Rev. 2012, 13, 329–346. [Google Scholar] [CrossRef]
- Institute of Medicine (IOM) of the National Academies. Dietary Reference Intakes: Research Synthesis Workshop Summary; The National Academies Press: Washington, DC, USA, 2007. [Google Scholar]
- van Horn, L.V.; Stumbo, P.; Moag-Stahlberg, A.; Obarzanek, E.; Hartmuller, V.W.; Farris, R.P.; Kimm, S.Y.; Frederick, M.; Snetselaar, L.; Liu, K. The Dietary Intervention Study in Children (DISC): Dietary assessment methods for 8- to 10-year-olds. J. Am. Diet. Assoc. 1993, 93, 1396–1403. [Google Scholar] [CrossRef]
- Feskanich, D.; Sielaff, B.H.; Chong, K.; Buzzard, I.M. Computerized collection and analysis of dietary intake information. Comput. Methods Programs Biomed. 1989, 30, 47–57. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Stewart, C.; Bianchi, F.; Frie, K.; Jebb, S.A. Comparison of Three Dietary Assessment Methods to Estimate Meat Intake as Part of a Meat Reduction Intervention among Adults in the UK. Nutrients 2022, 14, 411. [Google Scholar] [CrossRef] [PubMed]
- McNaughton, S.A.; Mishra, G.D.; Bramwell, G.; Paul, A.A.; Wadsworth, M.E. Comparability of dietary patterns assessed by multiple dietary assessment methods: Results from the 1946 British Birth Cohort. Eur. J. Clin. Nutr. 2005, 59, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, S.I.; Potischman, N.; Dodd, K.W.; Douglass, D.; Zimmerman, T.P.; Kahle, L.L.; Thompson, F.E.; George, S.M.; Subar, A.F. The Use of Digital Images in 24-Hour Recalls May Lead to Less Misestimation of Portion Size Compared with Traditional Interviewer-Administered Recalls. J. Nutr. 2016, 146, 2567–2573. [Google Scholar] [CrossRef] [PubMed]
- Moshfegh, A.J.; Rhodes, D.G.; Baer, D.J.; Murayi, T.; Clemens, J.C.; Rumpler, W.V.; Paul, D.R.; Sebastian, R.S.; Kuczynski, K.J.; Ingwersen, L.A.; et al. The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am. J. Clin. Nutr. 2008, 88, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Lucassen, D.A.; Willemsen, R.F.; Geelen, A.; Brouwer-Brolsma, E.M.; Feskens, E.J.M. The accuracy of portion size estimation using food images and textual descriptions of portion sizes: An evaluation study. J. Hum. Nutr. Diet. 2021, 34, 945–952. [Google Scholar] [CrossRef]
- Naghshi, S.; Sadeghi, O.; Willett, W.C.; Esmaillzadeh, A. Dietary intake of total, animal, and plant proteins and risk of all cause, cardiovascular, and cancer mortality: Systematic review and dose-response meta-analysis of prospective cohort studies. BMJ 2020, 370, m2412. [Google Scholar] [CrossRef]
- Limketkai, B.N.; Mauldin, K.; Manitius, N.; Jalilian, L.; Salonen, B.R. The Age of Artificial Intelligence: Use of Digital Technology in Clinical Nutrition. Curr. Surg. Rep. 2021, 9, 20. [Google Scholar] [CrossRef]
- Doulah, A.; Ghosh, T.; Hossain, D.; Marden, T.; Parton, J.M.; Higgins, J.A.; McCrory, M.A.; Sazonov, E. Energy intake estimation using a novel wearable sensor and food images in a laboratory (pseudo-free-living) meal setting: Quantification and contribution of sources of error. Int. J. Obes. 2022, 46, 2050–2057. [Google Scholar] [CrossRef]
- Das, S.K.; Miki, A.J.; Blanchard, C.M.; Sazonov, E.; Gilhooly, C.H.; Dey, S.; Wolk, C.B.; Khoo, C.S.H.; Hill, J.O.; Shook, R.P. Perspective: Opportunities and Challenges of Technology Tools in Dietary and Activity Assessment: Bridging Stakeholder Viewpoints. Adv. Nutr. 2022, 13, 1–15. [Google Scholar] [CrossRef]
- Wehling, H.; Lusher, J. People with a body mass index ≥30 under-report their dietary intake: A systematic review. J. Health Psychol. 2019, 24, 2042–2059. [Google Scholar] [CrossRef] [PubMed]
- Bartholome, L.T.; Peterson, R.E.; Raatz, S.K.; Raymond, N.C. A comparison of the accuracy of self-reported intake with measured intake of a laboratory overeating episode in overweight and obese women with and without binge eating disorder. Eur. J. Nutr. 2013, 52, 193–202. [Google Scholar] [CrossRef] [PubMed]


| Provided Intake | Reported Intake | p-Value | |
|---|---|---|---|
| standard diet | |||
| calories (kcal) | 2676 ± 498 | 2726 ± 668 | 0.82 |
| carbohydrates (g) | 328 ± 70 | 322 ± 88 | 0.86 |
| fat (g) | 115 ± 35 | 109 ± 35 | 0.68 |
| protein (g) | 95 ± 19 | 123 ± 41 | 0.03 |
| high-carbohydrate diet | |||
| calories (kcal) | 2777 ± 495 | 2823 ± 991 | 0.88 |
| carbohydrates (g) | 535 ± 97 | 479 ± 171 | 0.32 |
| fat (g) | 34 ± 9 | 55 ± 21 | 0.003 |
| protein (g) | 103 ± 19 | 119 ± 41 | 0.23 |
| high-fat diet | |||
| calories (kcal) | 2722 ± 496 | 2791 ± 565 | 0.77 |
| carbohydrates (g) | 173 ± 33 | 205 ± 55 | 0.12 |
| fat (g) | 185 ± 34 | 171 ± 37 | 0.36 |
| protein (g) | 101 ± 18 | 116 ± 28 | 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casey, J.L.; Meijer, J.L.; IglayReger, H.B.; Ball, S.C.; Han-Markey, T.L.; Braun, T.M.; Burant, C.F.; Peterson, K.E. Comparing Self-Reported Dietary Intake to Provided Diet during a Randomized Controlled Feeding Intervention: A Pilot Study. Dietetics 2023, 2, 334-343. https://doi.org/10.3390/dietetics2040024
Casey JL, Meijer JL, IglayReger HB, Ball SC, Han-Markey TL, Braun TM, Burant CF, Peterson KE. Comparing Self-Reported Dietary Intake to Provided Diet during a Randomized Controlled Feeding Intervention: A Pilot Study. Dietetics. 2023; 2(4):334-343. https://doi.org/10.3390/dietetics2040024
Chicago/Turabian StyleCasey, James L., Jennifer L. Meijer, Heidi B. IglayReger, Sarah C. Ball, Theresa L. Han-Markey, Thomas M. Braun, Charles F. Burant, and Karen E. Peterson. 2023. "Comparing Self-Reported Dietary Intake to Provided Diet during a Randomized Controlled Feeding Intervention: A Pilot Study" Dietetics 2, no. 4: 334-343. https://doi.org/10.3390/dietetics2040024
APA StyleCasey, J. L., Meijer, J. L., IglayReger, H. B., Ball, S. C., Han-Markey, T. L., Braun, T. M., Burant, C. F., & Peterson, K. E. (2023). Comparing Self-Reported Dietary Intake to Provided Diet during a Randomized Controlled Feeding Intervention: A Pilot Study. Dietetics, 2(4), 334-343. https://doi.org/10.3390/dietetics2040024






