Effects of Coexisting Anions on the Formation of Hematite Nanoparticles in a Hydrothermal Process with Urea Hydrolysis and the Congo Red Dye Adsorption Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Hydrothermal Synthesis and Characterization of α-Fe2O3 Nanoparticles
2.2. Batch Adsorption Studies for Congo Red
3. Results and Discussion
3.1. Hydrothermal Synthesis of α-Fe2O3 Nanoparticles with Urea Hydrolysis
3.1.1. Effects of the Reactants on the α-Fe2O3 Formation
3.1.2. Change in the Crystallite Size and Particle Diameter with Coexisting Anion
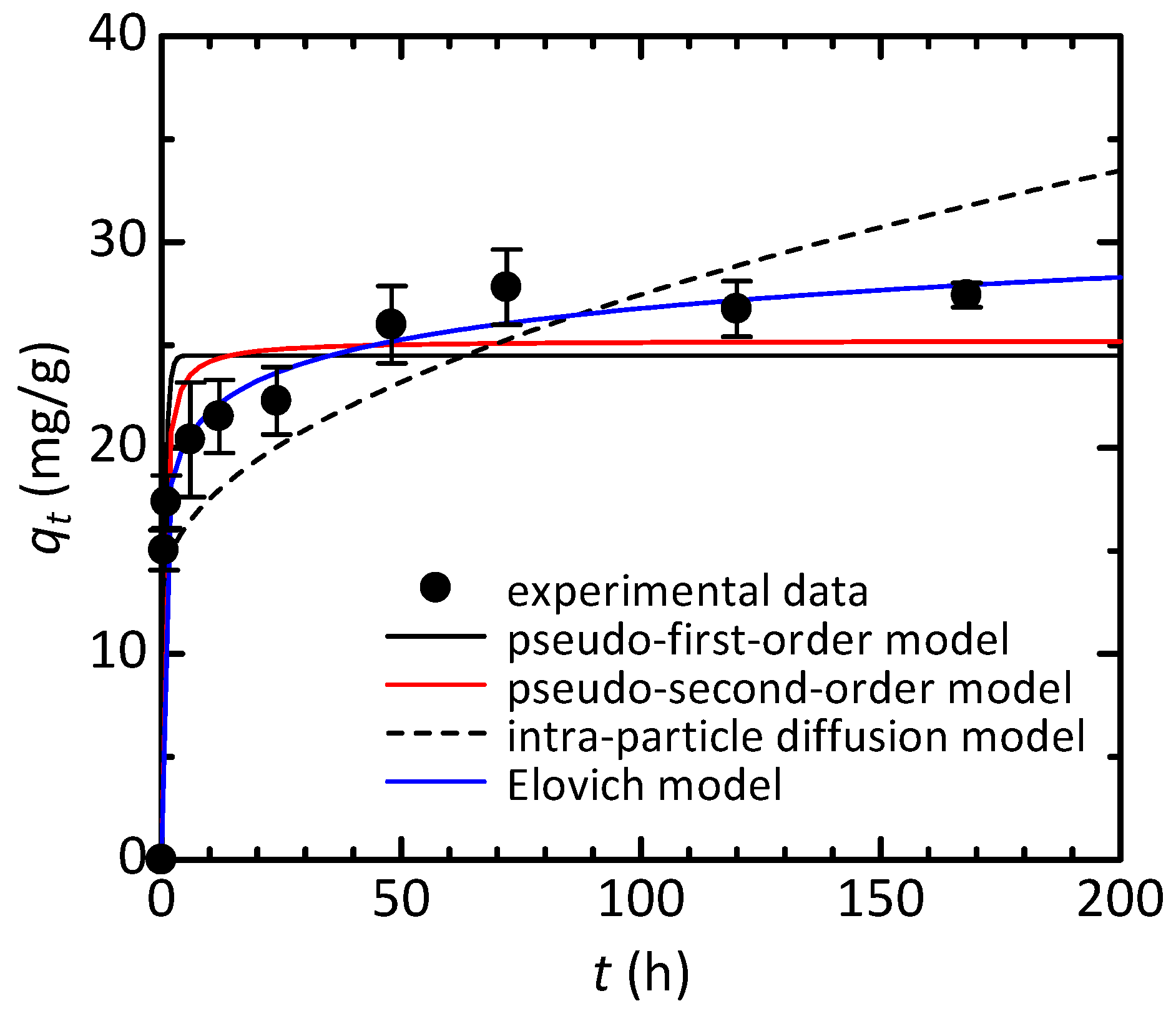
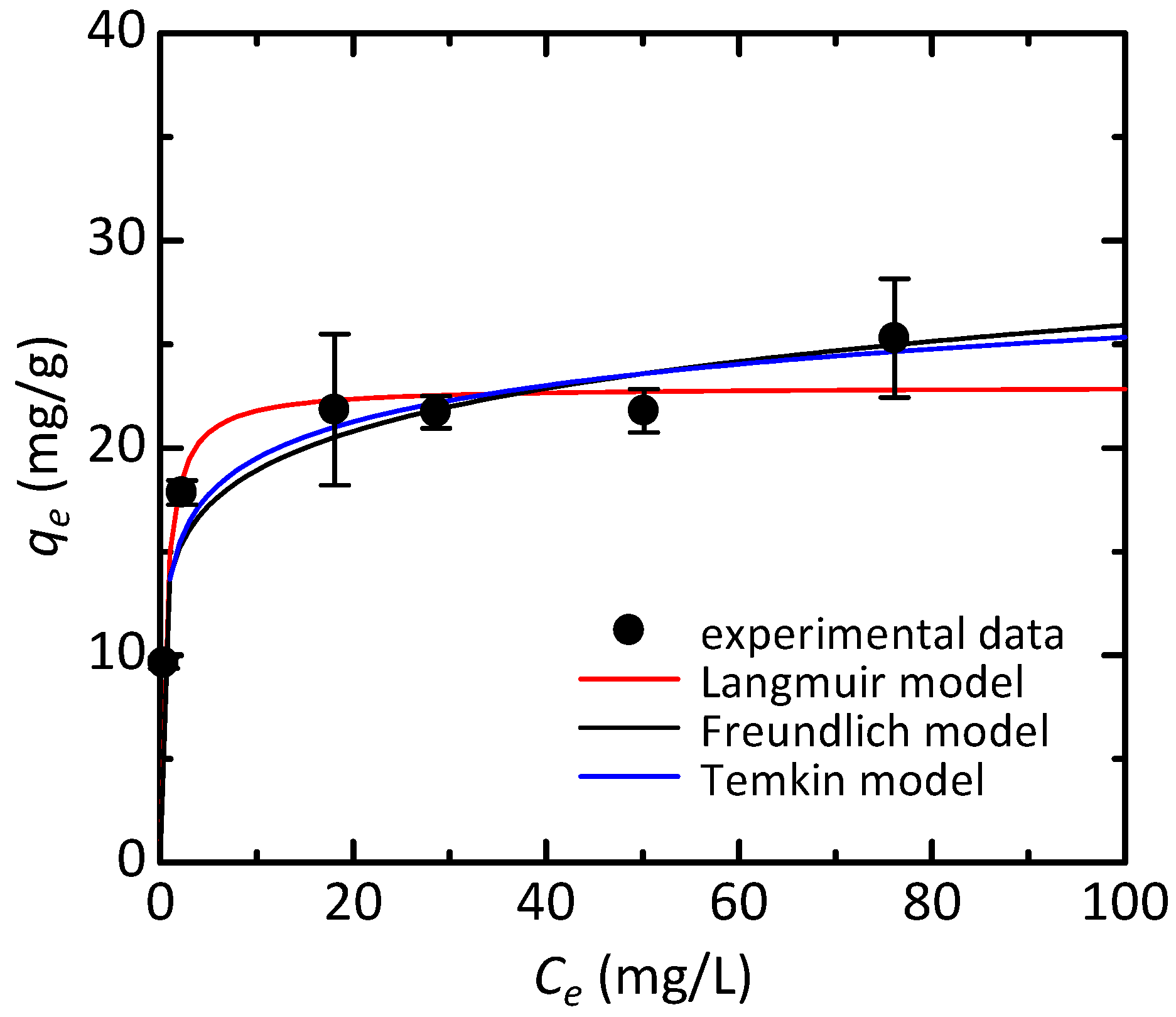
3.2. Adsorption Studies of Congo Red
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davis, J.R.; Mills, K.M.; Lampman, S.R. Properties and Selection: Irons, Steels, and High-Performance Alloys. In ASM Handbook, 10th ed.; ASM International: Materials Park, OH, USA, 1990; Volume 1, p. 1063. [Google Scholar]
- Cornell, R.M.; Schwertman, U. The Iron Oxides: Structure, Properties, Reactions, Occurrence and Uses, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Faivre, D. Iron Oxides: From Nature to Applications; Wiley-VCH: Weinheim, Germany, 2016. [Google Scholar]
- Wang, L.; Ding, K.; Wei, B.; Li, C.; Shi, X.; Zhang, Y.; He, X. Leaf-like α-Fe2O3 micron-particle: Preparation and its usage as anode materials for lithium ion batteries. Mater. Chem. Phys. 2018, 207, 58–66. [Google Scholar] [CrossRef]
- Pan, Y.; Yin, L.; Li, M. Submicron-sized α-Fe2O3 single crystals as anodes for high-performance lithium-ion batteries. Ceram. Int. 2019, 45, 12072–12079. [Google Scholar] [CrossRef]
- Balasingam, S.K.; Kundu, M.; Balakrishnan, B.; Kim, H.-J.; Svensson, A.M.; Jayasayee, K. Hematite microdisks as an alternative anode material for lithium-ion batteries. Mater. Lett. 2019, 247, 163–166. [Google Scholar] [CrossRef]
- Yan, Z.; Sun, Z.; Li, A.; Liu, H. Three-dimensional porous flower-like S-doped Fe2O3 for superior lithium storage. Adv. Compos. Hybrid Mater. 2021, 4, 716–724. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, L.; Qiao, W.; Liu, F.; Zhang, Y.; Tan, W.; Qiu, G. Facile crystal-structure-controlled synthesis of iron oxides for adsorbents and anode materials of lithium batteries. Mater. Chem. Phys. 2016, 170, 239–245. [Google Scholar] [CrossRef]
- Abd El Aal, S.A.; Abdelhady, A.M.; Mansour, N.A.; Hassan, N.M.; Elbaz, F.; Elmaghraby, E.K. Physical and chemical characteristics of hematite nanoparticles prepared using microwave-assisted synthesis and its application as adsorbent for Cu, Ni, Co, Cd and Pb from aqueous solution. Mater. Chem. Phys. 2019, 235, 121771. [Google Scholar] [CrossRef]
- Dehbi, A.; Dehmani, Y.; Omari, H.; Lammini, A.; Elazhari, K.; Abdallaoui, A. Hematite iron oxide nanoparticles (α-Fe2O3): Synthesis and modelling adsorption of malachite green. J. Environ. Chem. Eng. 2020, 8, 103394. [Google Scholar] [CrossRef]
- Panasenko, A.; Pirogovskaya, P.; Tkachenko, I.; Ivannikov, S.; Arefieva, O.; Marchenko, Y. Synthesis and characterization of magnetic silica/iron oxide composite as a sorbent for the removal of methylene blue. Mater. Chem. Phys. 2020, 245, 122759. [Google Scholar] [CrossRef]
- Mpelane, S.; Mketo, N.; Bingwa, N.; Nomngongo, P.N. Synthesis of mesoporous iron oxide nanoparticles for adsorptive removal of levofloxacin from aqueous solutions: Kinetics, isotherms, thermodynamics and mechanism. Alex. Eng. J. 2022, 61, 8457–8468. [Google Scholar] [CrossRef]
- Hjiri, M. Highly sensitive NO2 gas sensor based on hematite nanoparticles synthesized by sol-gel technique. J. Mater. Sci. Mater. El. 2020, 31, 5025–5031. [Google Scholar] [CrossRef]
- Alizadeh, T.; Zargr, F. Highly selective and sensitive iodide sensor based on carbon paste electrode modified with nanosized sulfate-doped α-Fe2O3. Mater. Chem. Phys. 2020, 240, 122118. [Google Scholar] [CrossRef]
- Sangale, S.S.; Jadhav, V.V.; Shaikh, S.F.; Shinde, P.V.; Ghule, B.G.; Raut, S.D.; Tamboli, M.S.; Al-Enizi, A.M.; Mane, R.S. Facile one-step hydrothermal synthesis and room-temperature NO2 sensing application of α-Fe2O3 sensor. Mater. Chem. Phys. 2020, 246, 122799. [Google Scholar] [CrossRef]
- Deshmanea, V.V.; Patil, A.V. Cobalt oxide doped hematite as a petrol vapor sensor. Mater. Chem. Phys. 2020, 246, 122813. [Google Scholar] [CrossRef]
- Li, G.; Ma, Z.; Hu, Q.; Zhang, D.; Fan, Y.; Wang, X.; Chu, X.; Xu, J. PdPt nanoparticle-functionalized α-Fe2O3 hollow nanorods for triethylamine sensing. ACS Appl. Nano Mater. 2021, 4, 10921–10930. [Google Scholar] [CrossRef]
- Tong, G.; Guan, J.; Zhang, Q. Goethite hierarchical nanostructures: Glucose-assisted synthesis, chemical conversion into hematite with excellent photocatalytic properties. Mater. Chem. Phys. 2011, 127, 371–378. [Google Scholar] [CrossRef]
- Alharbi, A.; Abdelrahman, E.A. Efficient photocatalytic degradation of malachite green dye using facilely synthesized hematite nanoparticles from Egyptian insecticide cans. Spectrochim. Acta A 2020, 226, 117612. [Google Scholar] [CrossRef]
- Haider, S.; Shar, S.S.; Shakir, I.; Agboola, P.O. Visible light active Cu-doped iron oxide for photocatalytic treatment of methylene blue. Ceram. Int. 2022, 48, 7605–7612. [Google Scholar] [CrossRef]
- Harijan, D.K.L.; Gupta, S.; Ben, S.K.; Srivastava, A.; Singh, J.; Chandra, V. High photocatalytic efficiency of α-Fe2O3-ZnO composite using solar energy for methylene blue degradation. Phys. B Condens. Matter. 2022, 627, 413567. [Google Scholar] [CrossRef]
- Ruan, C.; Wang, J.; Gao, M.; Zhao, G. The influence of structural size on thermal stability in single crystalline hematite uniform nano/micro-cubes. Mater. Chem. Phys. 2016, 183, 158–164. [Google Scholar] [CrossRef]
- Fan, H.; Song, B.; Liu, J.; Yang, Z.; Li, Q. Thermal formation mechanism and size control of spherical hematite nanoparticles. Mater. Chem. Phys. 2005, 89, 321–325. [Google Scholar] [CrossRef]
- Tadic, M.; Panjan, M.; Tadic, B.V.; Lazovic, J.; Damnjanovic, V.; Kopani, M.; Kopanja, L. Magnetic properties of hematite (α-Fe2O3) nanoparticles synthesized by sol-gel synthesis method: The influence of particle size and particle size distribution. J. Electr. Eng. 2019, 70, 71–76. [Google Scholar] [CrossRef]
- Katsuki, H.; Choi, E.-K.; Lee, W.-J.; Hwang, K.-T.; Cho, W.-S.; Huang, W.; Komarneni, S. Ultrafast microwave-hydrothermal synthesis of hexagonal plates of hematite. Mater. Chem. Phys. 2018, 205, 210–216. [Google Scholar] [CrossRef]
- Yang, P.; Yu, J.; Wang, Z.; Liu, Q.; Wu, T. Urea method for the synthesis of hydrotalcites. React. Kinet. Catal. Lett. 2004, 83, 275–282. [Google Scholar] [CrossRef]
- Zeng, H.-Y.; Deng, X.; Wang, Y.-J.; Liao, K.-B. Preparation of Mg-Al hydrotalcite by urea method and its catalytic activity for transesterification. AIChE J. 2009, 55, 1229–1235. [Google Scholar] [CrossRef]
- Wang, J.; Li, D.; Yu, X.; Zhang, M.; Jing, X. Fabrication of layered double hydroxide spheres through urea hydrolysis and mechanisms involved in the formation. Colloid Polym. Sci. 2010, 288, 1411–1418. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, R.; Dong, Y.; Li, W.; Zhou, W. Preparation of α-Fe2O3 hollow spheres, nanotubes, nanoplates and nanorings as highly efficient Cr(VI) adsorbents. RSC Adv. 2016, 6, 82854. [Google Scholar] [CrossRef]
- Tadic, M.; Trpkov, D.; Kopanja, L.; Vojnovic, S.; Panjan, M. Hydrothermal synthesis of hematite (α-Fe2O3) nanoparticle forms: Synthesis conditions, structure, particle shape analysis, cytotoxicity and magnetic properties. J. Alloys Compd. 2019, 792, 599–609. [Google Scholar] [CrossRef]
- Li, D.; Xu, R.; Jia, Y.; Ning, P.; Li, K. Controlled synthesis of α-Fe2O3 hollows from β-FeOOH rods. Chem. Phys. Lett. 2019, 731, 136623. [Google Scholar] [CrossRef]
- Mizutani, N.; Iwasaki, T.; Watano, S.; Yanagida, T.; Kawai, T. Size control of magnetite nanoparticles in hydrothermal synthesis by coexistence of lactate and sulfate ions. Curr. Appl. Phys. 2010, 10, 801–806. [Google Scholar] [CrossRef]
- Iwasaki, T.; Mizutani, N.; Watano, S.; Yanagida, T.; Kawai, T. Size control of magnetite nanoparticles by organic solvent-free chemical coprecipitation at room temperature. J. Exp. Nanosci. 2010, 5, 251–262. [Google Scholar] [CrossRef]
- Tan, W.; Liang, Y.; Xu, Y.; Wang, M. Structural-controlled formation of nano-particle hematite and their removal performance for heavy metal ions: A review. Chemosphere 2022, 306, 135540. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, R.; Pai, S.; Vinayagam, R.; Varadavenkatesan, T.; Kumar, P.S.; Duc, P.A.; Rangasamy, G. A recent update on green synthesized iron and iron oxide nanoparticles for environmental applications. Chemosphere 2022, 308, 136331. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, D.M.S.N.; Mantilaka, M.M.M.G.P.G.; Palihawadana, T.C.; Chandrakumara, G.T.D.; De Silva, R.T.; Pitawala, H.M.T.G.A.; Nalin De Silva, K.M.; Amaratunga, G.A.J. Facile and low-cost synthesis of pure hematite (α-Fe2O3) nanoparticles from naturally occurring laterites and their superior adsorption capability towards acid-dyes. RSC Adv. 2019, 9, 21249–21257. [Google Scholar] [CrossRef] [PubMed]
- Dehbi, A.; Dehmani, Y.; Omari, H.; Lammini, A.; Elazhari, K.; Abouarnadasse, S.; Abdallaoui, A. Comparative study of malachite green and phenol adsorption on synthetic hematite iron oxide nanoparticles (α-Fe2O3). Surf. Interfaces 2020, 21, 100637. [Google Scholar] [CrossRef]
- Onizuka, T.; Iwasaki, T. Low-temperature solvent-free synthesis of polycrystalline hematite nanoparticles via mechanochemical activation and their adsorption properties for Congo red. Solid State Sci. 2022, 129, 106917. [Google Scholar] [CrossRef]
- Oladoye, P.O.; Bamigboye, M.O.; Ogunbiyi, O.D.; Akano, M.T. Toxicity and decontamination strategies of Congo red dye. Groundw. Sustain. Dev. 2022, 19, 100844. [Google Scholar] [CrossRef]
- Chattopadhyay, D.P. Chemistry of Dyeing. In Handbook of Textile and Industrial Dyeing Principle, Processes and Types of Dyes, 1st ed.; Clark, M., Ed.; Woodhead Publishing: Cambridge, UK, 2011; Volume 1, pp. 150–183. [Google Scholar]
- Maruthupandy, M.; Muneeswaran, T.; Vennila, T.; Anand, M.; Cho, W.-S.; Quero, F. Development of chitosan decorated Fe3O4 nanospheres for potential enhancement of photocatalytic degradation of Congo red dye molecules. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 267, 120511. [Google Scholar] [CrossRef]
- Hitkari, G.; Chowdhary, P.; Kumar, V.; Singh, S.; Motghare, A. Potential of copper-zinc oxide nanocomposite for photocatalytic degradation of Congo red dye. Clean. Chem. Eng. 2022, 1, 100003. [Google Scholar] [CrossRef]
- Chen, H.; Wang, S.; Huang, L.; Zhang, L.; Han, J.; Ren, W.; Pan, J.; Li, J. Core-shell hierarchical Fe/Cu bimetallic Fenton catalyst with improved adsorption and catalytic performance for Congo red degradation. Catalysts 2022, 12, 1363. [Google Scholar] [CrossRef]
- Bencheqroun, Z.; Sahin, N.E.; Soares, O.S.G.P.; Pereira, M.F.R.; Zaitan, H.; Nawdali, M.; Rombi, E.; Fonseca, A.M.; Parpot, P.; Neves, I.C. Fe(III)-exchanged zeolites as efficient electrocatalysts for Fenton-like oxidation of dyes in aqueous phase. J. Environ. Chem. Eng. 2022, 10, 107891. [Google Scholar] [CrossRef]
- Han, G.; Du, Y.; Huang, Y.; Wang, W.; Su, S.; Liu, B. Study on the removal of hazardous Congo red from aqueous solutions by chelation flocculation and precipitation flotation process. Chemosphere 2022, 289, 133109. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-F.; He, Y.-H.; Liang, J.-B.; Jin, Q.; Ou, Y.-C.; Wu, J.-Z. First cadmium coordination compound as an efficient flocculant for Congo red. Inorg. Chem. Commun. 2022, 141, 109544. [Google Scholar] [CrossRef]
- Liu, Y.; Qiu, G.; Liu, Y.; Niu, Y.; Qu, R.; Ji, C.; Wang, Y.; Zhang, Y.; Sun, C. Fabrication of CoFe-MOF materials by different methods and adsorption properties for Congo red. J. Mol. Liq. 2022, 360, 119405. [Google Scholar] [CrossRef]
- Wang, L.; Tao, Y.; Wang, J.; Tian, M.; Liu, S.; Quan, T.; Yang, L.; Wang, D.; Li, X.; Gao, D. A novel hydroxyl-riched covalent organic framework as an advanced adsorbent for the adsorption of anionic azo dyes. Anal. Chim. Acta 2022, 1227, 340329. [Google Scholar] [CrossRef]
- Tian, P.; Sun, C.; Zhu, P.; Pang, H.; Gong, W.; Ye, J.; Ning, G. Template-engaged synthesis of macroporous tubular hierarchical α-Fe2O3 and its ultrafast transfer performance. Chem. Phys. Lett. 2018, 706, 261–265. [Google Scholar] [CrossRef]
- Prajapati, A.K.; Mondal, M.K. Development of CTAB modified ternary phase α-Fe2O3-Mn2O3-Mn3O4 nanocomposite as innovative super-adsorbent for Congo red dye adsorption. J. Environ. Chem. Eng. 2021, 9, 104827. [Google Scholar] [CrossRef]
- Maiti, D.; Mukhopadhyay, S.; Devi, P.S. Evaluation of mechanism on selective, rapid, and superior adsorption of Congo red by reusable mesoporous α-Fe2O3 nanorods. ACS Sustain. Chem. Eng. 2017, 5, 11255–11267. [Google Scholar] [CrossRef]
- Lima, E.C.; Hosseini-Bandegharaei, A.; Moreno-Piraján, J.C.; Anastopoulos, I. A critical review of the estimation of the thermodynamic parameters on adsorption equilibria. Wrong use of equilibrium constant in the Van’t Hoof equation for calculation of thermodynamic parameters of adsorption. J. Mol. Liq. 2019, 273, 425–434. [Google Scholar] [CrossRef]
- Song, Y.; Bac, B.H.; Lee, Y.-B.; Kim, M.H.; Yoon, W.-S.; Kim, J.H.; Moon, H.-S. Ge-incorporation into 6-line ferrihydrite nanocrystals. CrystEngComm 2010, 12, 1997–2000. [Google Scholar] [CrossRef]
- Xu, H.; Zhu, S.; Lu, K.; Jia, H.; Xia, M.; Wang, F. Preparation of hierarchically floral ZIF-8 derived carbon@polyaniline@Ni/Al layered double hydroxides composite with outstanding removal phenomenon for saccharin. Chem. Eng. J. 2022, 450, 138127. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption kinetic models: Physical meanings, applications, and solving methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Maimaitiniyazi, R.; Wang, Y. Some consideration triggered by misquotation of Temkin model and the derivation of its correct form. Arab. J. Chem. 2022, 15, 104267. [Google Scholar] [CrossRef]
- Chu, K.H. Revisiting the Temkin isotherm: Dimensional inconsistency and approximate forms. Ind. Eng. Chem. Res. 2021, 60, 13140–13147. [Google Scholar] [CrossRef]
- Wo, R.; Li, Q.-L.; Zhu, C.; Zhang, Y.; Qiao, G.; Lei, K.; Du, P.; Jiang, W. Preparation and characterization of functionalized metal–organic frameworks with core/shell magnetic particles (Fe3O4@SiO2@MOFs) for removal of Congo red and methylene blue from water solution. J. Chem. Eng. Data 2019, 64, 2455–2463. [Google Scholar] [CrossRef]
- Tabti, H.A.; Medjahed, B.; Boudinar, M.; Kadeche, A.; Bouchikhi, N.; Ramdani, A.; Taleb, S.; Adjdir, M. Enhancement of Congo red dye removal efficiency using Mg-Fe-layered double hydroxide. Res. Chem. Intermed. 2022, 48, 2683–2703. [Google Scholar] [CrossRef]
- Rathee, G.; Awasthi, A.; Sood, D.; Tomar, R.; Tomar, V.; Chandra, R. A new biocompatible ternary layered double hydroxide adsorbent for ultrafast removal of anionic organic dyes. Sci. Rep. 2019, 9, 16225. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, Y.; Liu, X.; Jiang, X.; Zhang, Z.; Zhang, T.; Zhang, L. The investigation of synergistic and competitive interaction between dye Congo red and methyl blue on magnetic MnFe2O4. Chem. Eng. J. 2014, 246, 88–96. [Google Scholar] [CrossRef]
- Gautam, R.K.; Rawat, V.; Banerjee, S.; Sanroman, M.A.; Soni, S.; Singh, S.K.; Chattopadhyaya, M.C. Synthesis of bimetallic Fe–Zn nanoparticles and its application towards adsorptive removal of carcinogenic dye malachite green and Congo red in water. J. Mol. Liq. 2015, 212, 227–236. [Google Scholar] [CrossRef]
- Wang, J.; Sun, P.; Xue, H.; Chen, J.; Zhang, H.; Zhu, W. Red mud derived facile hydrothermal synthesis of hierarchical porous α-Fe2O3 microspheres as efficient adsorbents for removal of Congo red. J. Phys. Chem. Solids 2020, 140, 109379. [Google Scholar] [CrossRef]




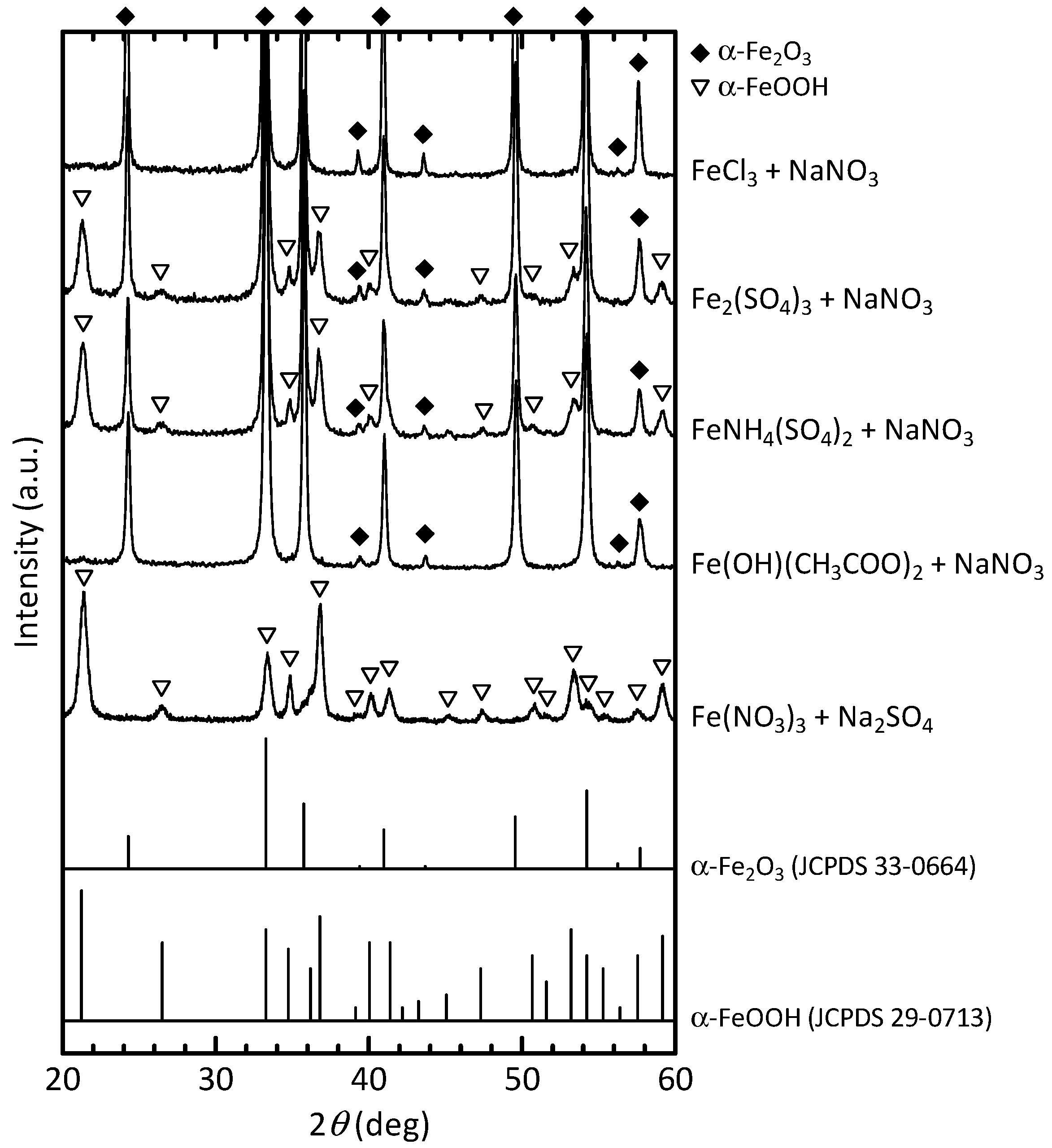
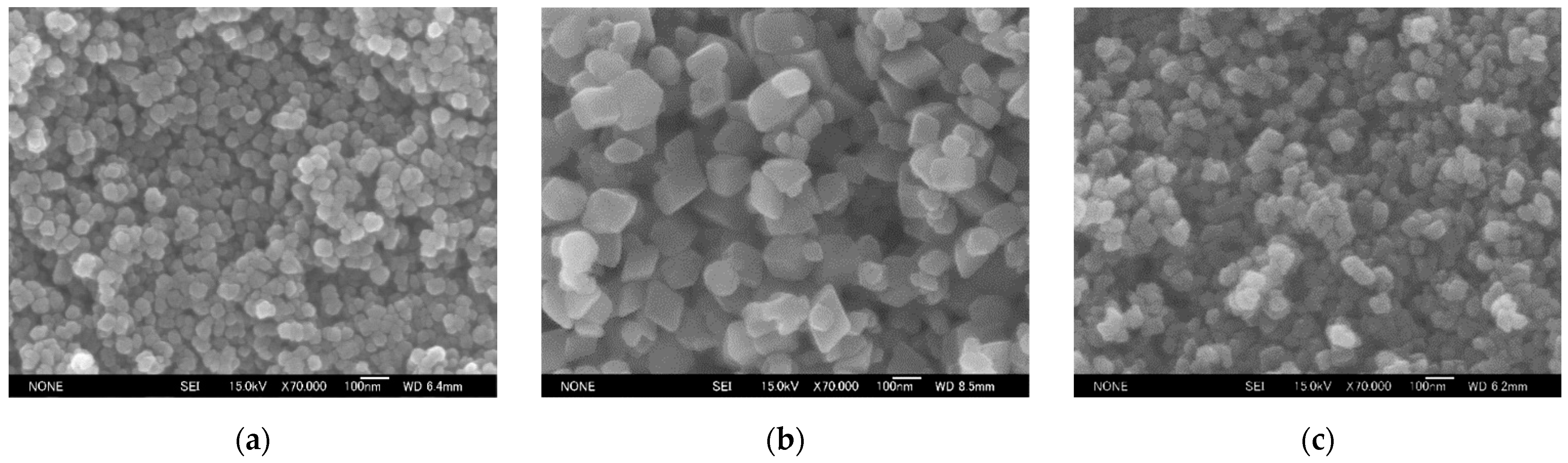


| Starting Material | Average Crystallite Size (nm) | Crystallinity Index (%) | Median Diameter (nm) | |
|---|---|---|---|---|
| Iron Source | Additive | |||
| Fe(NO3)3 | – | 24.1 | 98.1 | 53.2 |
| FeCl3 | NaNO3 | 46.8 | 99.0 | 125.4 |
| Fe(OH)(CH3COO)2 | NaNO3 | 30.8 | 98.6 | 55.5 |
| Kinetic Model | Parameter | Value |
|---|---|---|
| Pseudo-first-order | qe (mg/g) | 24.5 |
| k1 (h−1) | 1.55 | |
| R2 | 0.902 | |
| Pseudo-second-order | qe (mg/g) | 25.2 |
| k2 (g/(mg·h)) | 0.0928 | |
| R2 | 0.934 | |
| Intra-particle diffusion | c (mg/g) | 12.9 |
| ki (mg/(g·h0.5)) | 1.45 | |
| R2 | 0.607 | |
| Elovich | α (mg/(g·h)) | 4.55 × 103 |
| β (g/mg) | 0.457 | |
| R2 | 0.959 |
| Isotherm Model | Parameter | Value |
|---|---|---|
| Langmuir | qm (mg/g) | 23.0 |
| KL (L/mg) | 1.86 | |
| R2 | 0.944 | |
| Freundlich | KF (mg/(g·(mg/L)1/n)) | 13.8 |
| n (−) | 7.32 | |
| R2 | 0.885 | |
| Temkin | AT (L/mg) | 221 |
| qT (mg/g) | 2.53 | |
| R2 | 0.926 |
| Adsorbent | qm (mg/g) | Reference |
|---|---|---|
| Porous α-Fe2O3 nanorod | 57.2 | [51] |
| Fe3O4@SiO2@Zn–TDPAT | 17.7 | [58] |
| MgFeAl LDHs | 14.8 | [59] |
| NiFeTi LDHs | 30.0 | [60] |
| MnFe2O4 | 25.8 | [61] |
| Fe–Zn bimetallic nanoparticles | 28.6 | [62] |
| α-Fe2O3 nanoparticles | 23.0 | This work |
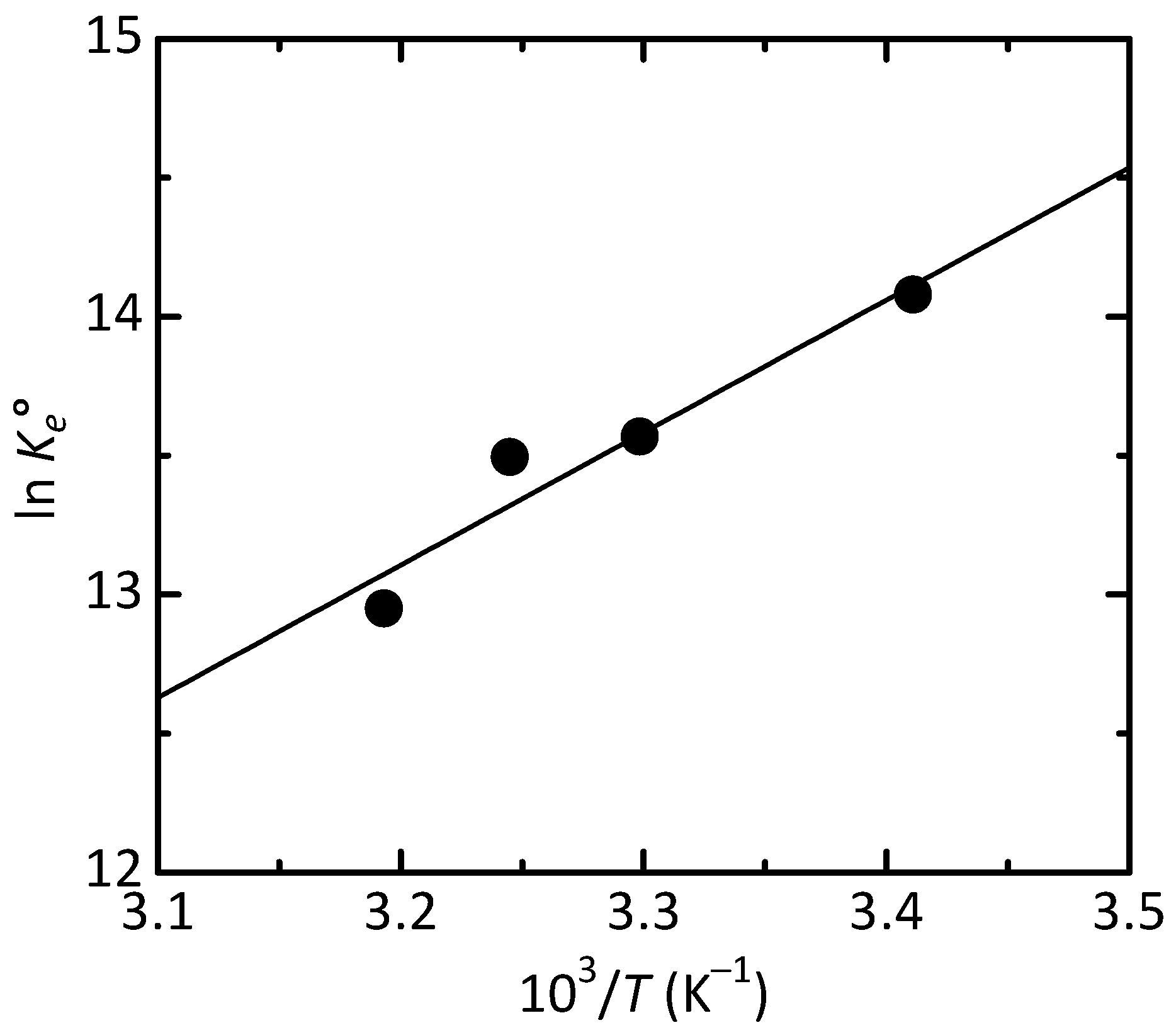
| Temperature (K) | ΔG° (kJ/mol) | ΔS° (J/(mol·K)) | ΔH° (kJ/mol) |
|---|---|---|---|
| 293 | −34.40 | −18.0 | −39.66 |
| 303 | −34.22 | ||
| 308 | −34.13 | ||
| 313 | −34.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onizuka, T.; Fukuda, M.; Iwasaki, T. Effects of Coexisting Anions on the Formation of Hematite Nanoparticles in a Hydrothermal Process with Urea Hydrolysis and the Congo Red Dye Adsorption Properties. Powders 2023, 2, 338-352. https://doi.org/10.3390/powders2020020
Onizuka T, Fukuda M, Iwasaki T. Effects of Coexisting Anions on the Formation of Hematite Nanoparticles in a Hydrothermal Process with Urea Hydrolysis and the Congo Red Dye Adsorption Properties. Powders. 2023; 2(2):338-352. https://doi.org/10.3390/powders2020020
Chicago/Turabian StyleOnizuka, Takahiro, Mikihisa Fukuda, and Tomohiro Iwasaki. 2023. "Effects of Coexisting Anions on the Formation of Hematite Nanoparticles in a Hydrothermal Process with Urea Hydrolysis and the Congo Red Dye Adsorption Properties" Powders 2, no. 2: 338-352. https://doi.org/10.3390/powders2020020
APA StyleOnizuka, T., Fukuda, M., & Iwasaki, T. (2023). Effects of Coexisting Anions on the Formation of Hematite Nanoparticles in a Hydrothermal Process with Urea Hydrolysis and the Congo Red Dye Adsorption Properties. Powders, 2(2), 338-352. https://doi.org/10.3390/powders2020020






