Abstract
A novel method has been proposed to induce rapid upward movement of colloidal particles with a density lower than water by applying an electric field of several V/mm in water. This phenomenon, known as the Electrically Induced Rapid Sedimentation (ERS) effect, marks the first occurrence of ‘rapid upward movement of colloidal particles’ within the scope of this phenomenon. Focusing on hollow particles, an investigation of the ERS effect was conducted through transmittance measurement. The hollow particles in water showed a drastic increase in ascending velocity through the application of an electric field. The ascending velocity raised when increasing the electric field strength. Utilizing a quasi-DC electric field (an extremely low-frequency AC electric field), aggregate structures were captured for the first time.
1. Introduction
Control of the dispersion state of colloidal particles in water is very important in industrial applications and from the academic point of view. The control technique of the dispersion state, fibrillation, and alignment of dispersed materials using an electric field has received increasing attention in recent years. In colloidal dispersions using an insulating liquid as the dispersing medium, it is commonly observed that under an electric field exceeding several hundred V/mm, chain-like structures of particles form between electrodes [1]. When a strong electric field is applied, there is a strong electrostatic attraction between particles in the direction of the electric field. However, in the direction perpendicular to the electric field, there is a strong repulsion between particles, resulting in the formation of chain-like aggregates. Kimura et al. have previously investigated the yielding behavior of this chain structure during flow deformation [2]. During their investigation, they frequently observed the phenomenon of particle aggregate settling, instead of forming the chain structure, when a smaller electric field was applied. Furthermore, in blends of non-aqueous immiscible liquids, many researchers observed that droplets with a higher dielectric constant than the matrix underwent elongation in the direction of the electric field, and they subsequently combined to form bridging structures between the electrodes [3,4,5,6]. Under high electric fields of several kV/mm, this formation occurs due to the combined effects of electrostatic attractive forces in the electric field direction and Maxwell stress. Under lower electric fields, chain structures composed of spherical and elongated droplets were formed [7], while under even lower electric fields, instead of forming chain structures, aggregation/settling of droplets was observed. As mentioned above, in non-aqueous systems, dispersed particles or liquid droplets, tend to exhibit a strong tendency to aggregate when subjected to electric fields below several tens of V/mm.
Colloid particles in polar liquids such as water tend to exhibit increased dispersion stability due to the formation of an electrical double layer [8,9] on their particle surfaces. The electric double layer surrounding particles plays a significant role in altering the hydrodynamic size of these particles. Additionally, the emergence of electrostatic repulsion between particles due to the presence of the electric double layer leads to the formation of colloidal crystals [10,11,12,13,14,15,16,17,18], resulting in the exhibition of iridescent colors [17,18]. In prior research conducted by the group to which the author belongs, it was discovered that the sedimentation velocity of colloidal particles (poly(methyl methacrylate) (PMMA) spheres and montmorillonite (Mt) particles) in water increased drastically under a small DC electric field of a few V/mm or less [19,20]. This is referred to as the electrically induced rapid separation (ERS) effect by the author. Regarding the mechanism of ERS effect, there is a possibility that small electric fields of several V/mm or lower destabilize the electrical double layer, leading to aggregation of particles and the formation of network structures among them. If the particles gather with each other, the floc would have a large difference in density from water, which leads to rapid sedimentation/buoyancy. In this study, the focus was on hollow particles with lower density than water, and the ERS effect was investigated through transmittance measurement. The results of this study have revealed that hollow particles rapidly ascend when subjected to an electric field.
2. Materials and Methods
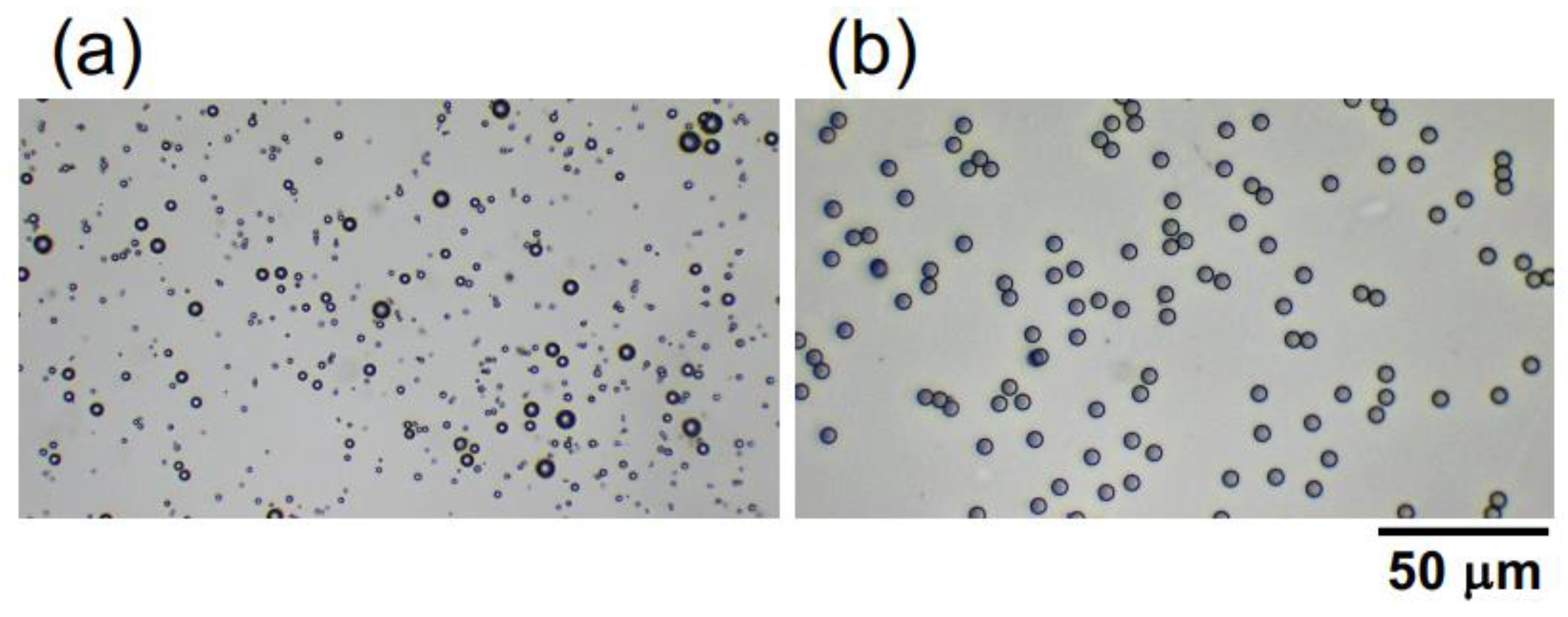
Hollow particles of inorganic oxide were a gift from Taiheiyo Cement Co. (Tokyo, Japan). The particles are relatively polydisperse (Figure 1a), ranging from 1.0 μm to 8.0 μm, with an average particle diameter of approximately 2.6 μm. This value was determined based on the diameters of 200 randomly selected particles in images obtained using a digital microscope (BA81-6T-1080M, Shimadzu RIKA Co.) (Tokyo, Japan). The provided hollow particle shell is composed of alumina silicate glass. Further elaboration will be provided in the subsequent sections; however, the porosity and specific gravity of the hollow particles were estimated as 65% and 0.91, respectively, based on their buoyancy velocity in water. Monodisperse poly(methyl methacrylate) (PMMA) spheres (Figure 1b) were purchased from Sekisui Plastics Co., Ltd. (Osaka, Japan). The sphere diameter is 5.0 ± 0.2 μm, and the specific gravity is 1.2 [20]. The hollow particles and PMMA spheres were carefully dispersed into purified water (Milli-Q Advantage A10, Millipore Co. (Darmstadt, Germany).) using a magnetic stirrer and they were deionized with the coexistence of ion-exchange resins (AG501-X8 (D), 20–50 mesh, Bio-Rad Laboratories, Inc. (Hercules, CA, USA)) for more than 3 months. The zeta potential both of the hollow particles and PMMA spheres was approximately—40 mV, and the measurement was conducted using an electrophoretic spectrophotometer (Zetasizer Nano ZS, Malvern Instruments Ltd.) (Malvern, UK). The sample suspensions of the hollow particles and PMMA spheres were each prepared to have a volume fraction ϕ of 0.0005. The pH of the deionized hollow particles and the PMMA sphere aqueous dispersion was measured at 7.1 and 6.9, respectively, using a pH and electrical conductivity meter (D-74, Horiba, Ltd.) (Kyoto, Japan). When observing the sedimentation of PMMA spheres under an electric field using an optical microscope, the ϕ-value was reduced to 0.0001. Sample preparation was carried out at 25 °C.
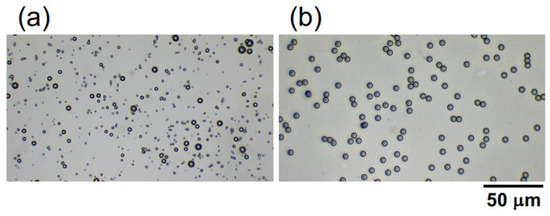
Figure 1.
Microscopic photographs of (a) hollow and (b) poly(methyl methacrylate) (PMMA) spheres in water.
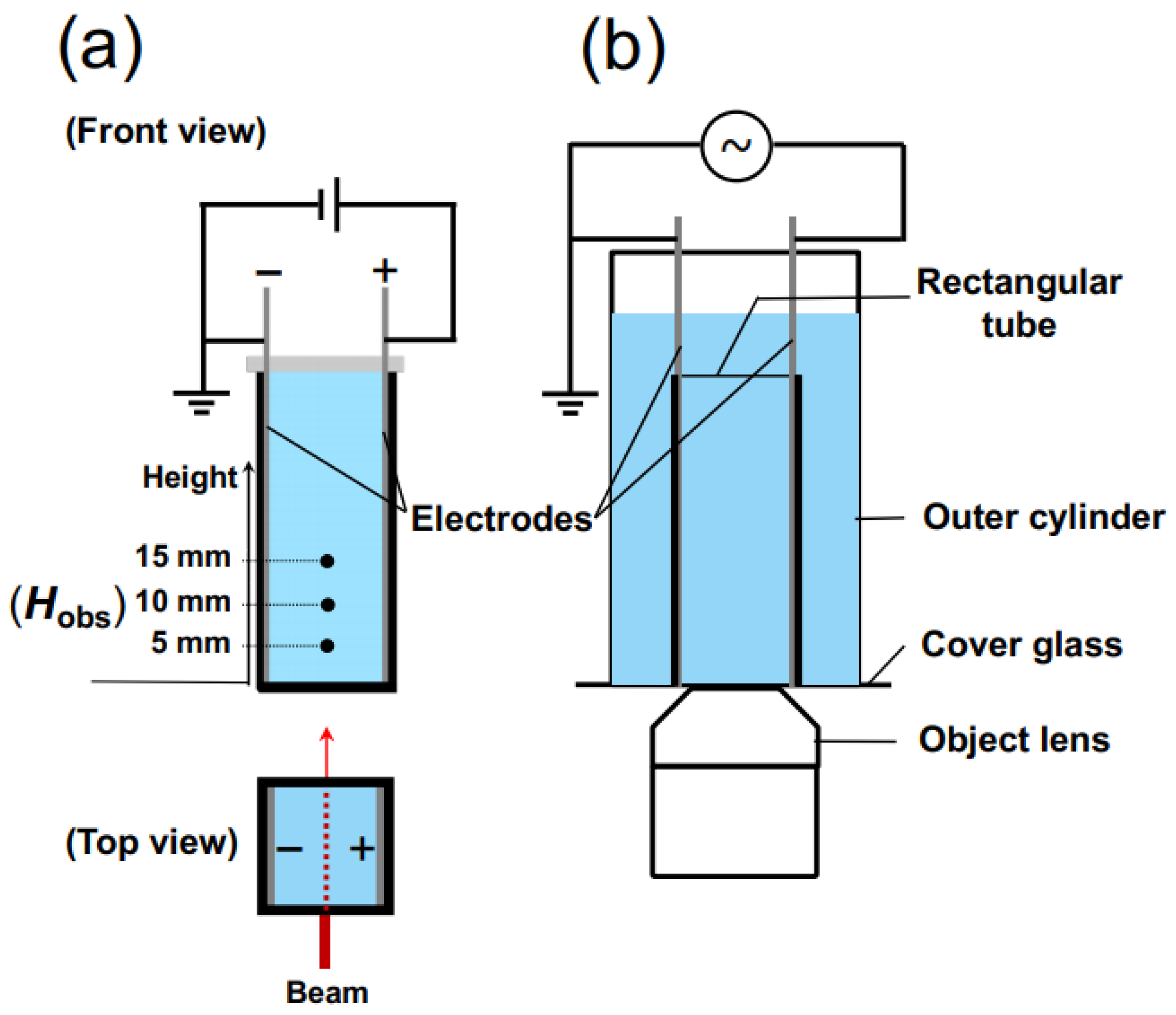
For the measurement of transmittance, a rectangular transparent cell with inner dimensions of 10 mm × 10 mm × 45 mm was utilized. On the inner walls of the cell, thin stainless steel plates (SUS304), positioned in parallel to each other and functioning as electrodes, were installed (Figure 2a). The distance between the electrodes was 9.8 mm. Once the observation cell was filled with the sample suspension, it was sealed with a rubber cap to prevent air contamination and water evaporation. A laser beam with a wavelength of 632.8 nm and a beam diameter of 0.5 mm was directed perpendicular to the cell wall. The observation points, Hobs, were located at heights of 5.0 mm, 10 mm, and 15 mm from the bottom of the measuring cell. A DC electric field was applied to the sample suspension using a synthesized function generator (FG110, Yokogawa Meters & Instruments Corp.) (Tokyo, Japan). The electric field strength, E, ranged from 0.1 V/mm DC to 0.5 V/mm DC.
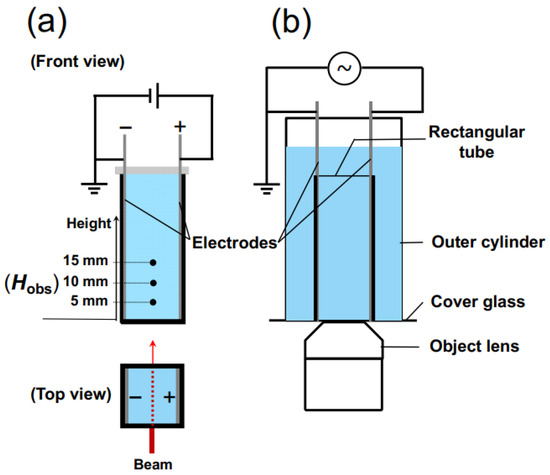
Figure 2.
Diagrams of the measurement cells. (a) Transmittance measurement. The observation positions, Hobs, were in the lower region of the cell, at heights of 5 mm, 10 mm, and 15 mm from the bottom of the cell. The laser light was incident perpendicular to the cell wall. (b) Macroscopic observation of sedimentation of PMMA spheres in water.
The sedimentation velocity of colloidal spheres in water can be determined by calculating the rate of movement of the boundary between the water phase and the colloid-rich phase, which is obtained from the time-dependent changes in transmittance at various observation heights [20]. This calculation is applicable when the colloidal spheres are uniformly sized and fall within an appropriate range of sphere concentrations. Under the measuring conditions of this study, the settling velocity without an electric field can be assumed to have reached a terminal velocity immediately. All the measurements were performed at 25 °C.
In order to observe the sedimentation state of PMMA spheres under an electric field using an optical microscope, a cover glass was adhered to the bottom surface of a cylindrical container of a size capable of accommodating the rectangular experimental cell. A modified version of the rectangular cell, with its bottom surface cut, was inserted into the cylindrical container (Figure 2b). The aqueous suspension of PMMA spheres was placed in both a cylindrical cell and a rectangular cell. The observation of precipitated PMMA spheres was carried out using an inverted reflected-light microscope (Axiovert 25 CA, Carl Zeiss, Inc.) (Oberkochen, Germany). To minimize electrophoresis of PMMA spheres under an electric field, a 0.001 Hz altering current field (sinusoidal wave) was applied. An observation was conducted for 1200 s, corresponding to 1.2 cycles of the electric field waveform. Due to the extremely low field frequency, it can be effectively considered as a direct current field in practice. The electric field intensity was set to an effective value Eeff of 0.1 V/mm. The observation focused on the central region between the electrodes. All the observations were performed at 25 °C.
3. Results
3.1. Aqueous Suspension of Hollow Particles
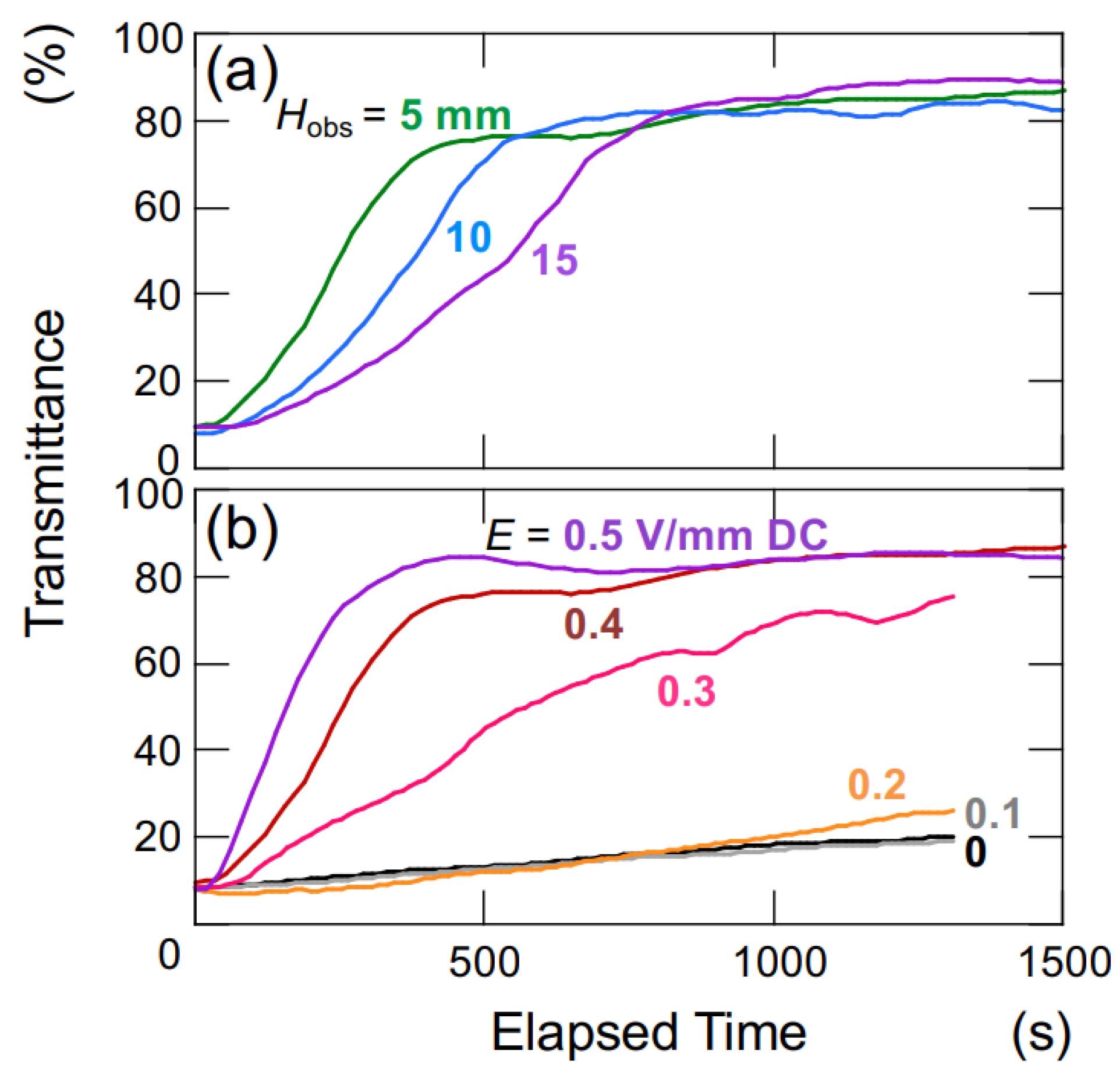
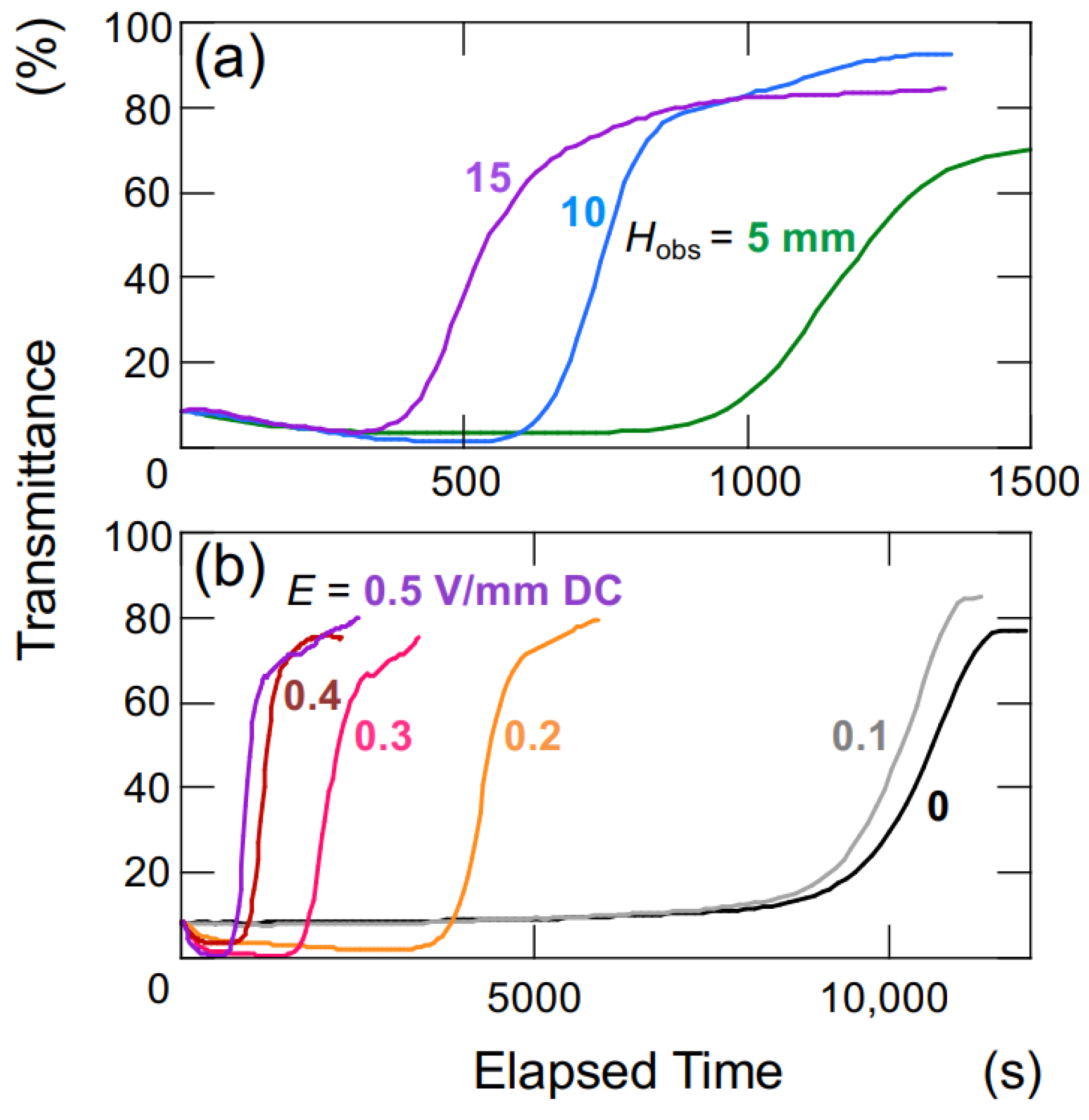
The changes in transmittance for the suspension of hollow particles are depicted in Figure 3. Under a DC electric field of 0.4 V/mm (Figure 3a), the suspension of hollow particles exhibited the earliest increase in transmittance at an Hobs of 5 mm, followed by 10 mm and 15 mm sequentially. The increase in transmittance was considerably more gradual compared to that of the later-described PMMA sphere aqueous suspension. Additionally, the increase occurred almost simultaneously from the moment of settling, regardless of the Hobs-value. Figure 3b illustrates the dependence of electric field strength on changes in transmittance for an observation point at a height of 5 mm. At zero electric field and electric field strengths below 0.2 V/mm DC, the transmittance remained nearly unchanged. On the other hand, for electric field strength equal or above 0.3 V/mm DC, the rate of increase in transmittance became more significant with an increase in electric field strength. As mentioned later, hollow particles in water exhibit an extremely slow rise in the absence of an electric field. When a sufficiently strong electric field is applied (E 0.3 V/mm DC), the ascending velocity becomes extremely high.
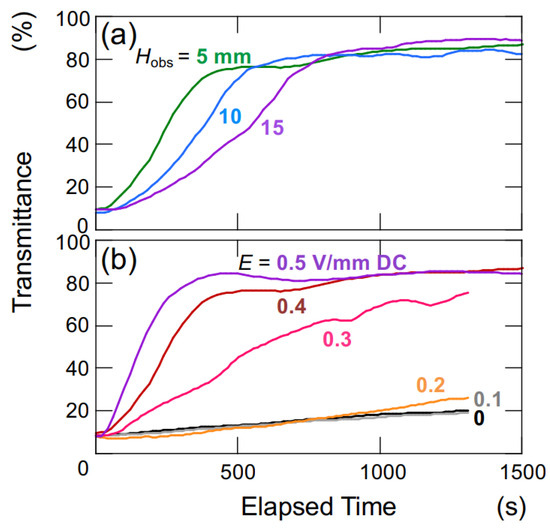
Figure 3.
Change in the transmittance of aqueous suspensions of hollow particles at 25 °C. ϕ = 0.0005. (a) Height dependence of observation position, E = 0.4 V/mm DC. (b) Dependence of electric field strength, Hobs = 5 mm.
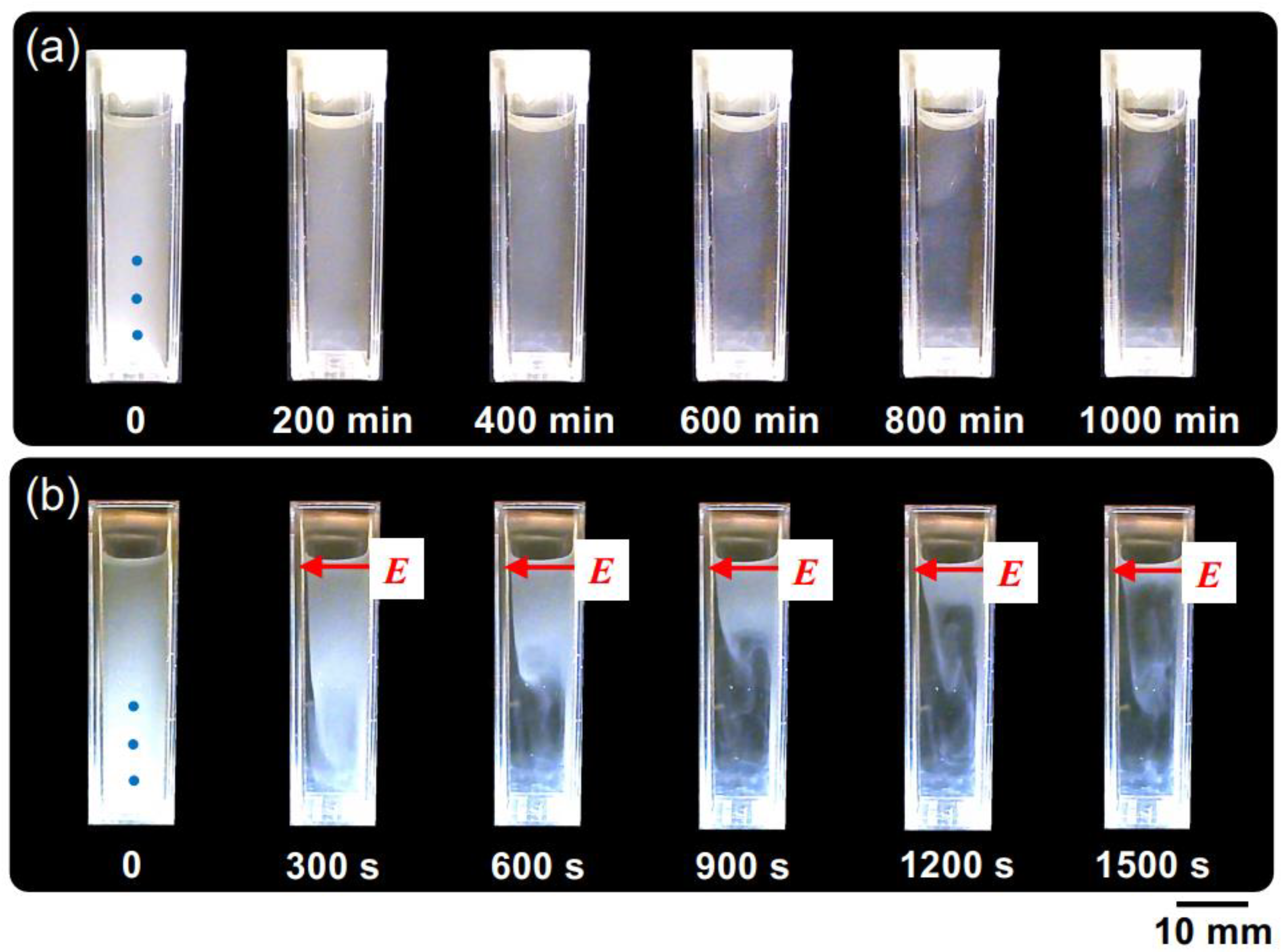
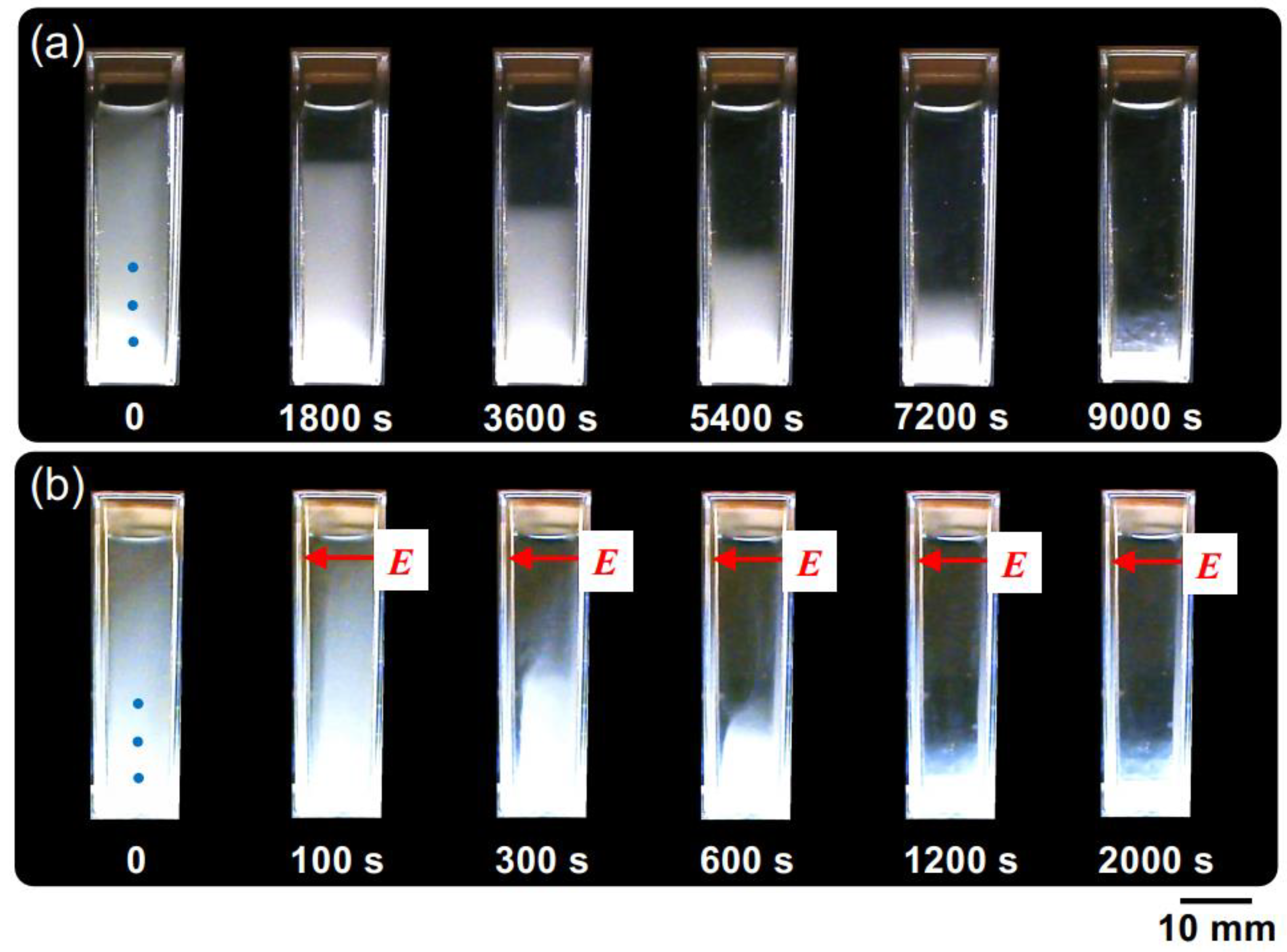
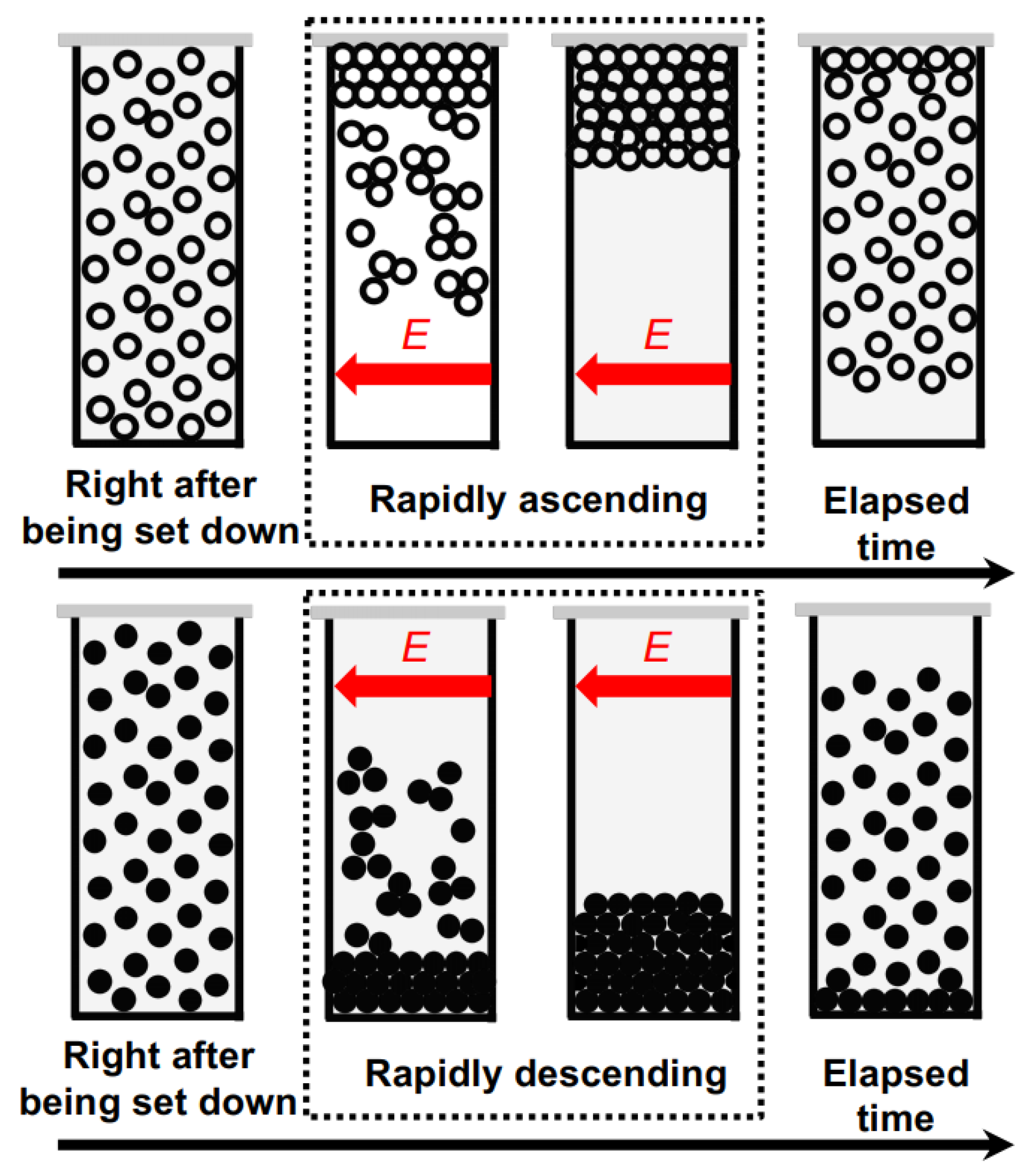
Figure 4 shows the changes in the dispersion state of hollow particles in water over time, both in the absence of an electric field (Figure 4a) and under the application of an electric field (Figure 4b). In the absence of an electric field, the changes in dispersion state were extremely slow, taking approximately 1000 min to reach a quasi-stable state. Additionally, no formation of a boundary line accompanying the buoyancy of individual hollow particles was observed by the naked eye, suggesting non-uniformity in the buoyancy velocities of the individual hollow particles. On the other hand, at E = 0.5 V/mm DC, the ascending velocity of the hollow particles clearly increased. Additionally, the formation of boundary line accompanying the buoyancy of the hollow particles was observed. It took approximately 1000 s to reach a quasi-stable state; then, their convective motion between the electrodes was observed. This suggests the influence of electrohydrodynamic (EHD) convection under the direct current electric field. The phenomenon of an increase in the rising velocity of particles suspended in water due to the application of an electric field of several V/mm or lower has been elucidated for the first time in this research paper.
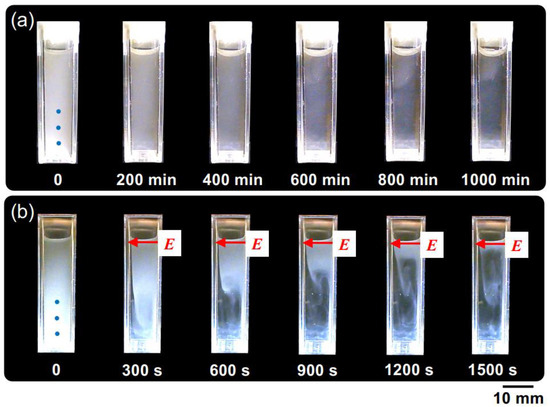
Figure 4.
The temporal evolution of the dispersion state of hollow particle aqueous suspension at 25 °C. ϕ = 0.0005. (a) Without an electric field; (b) E = 0.5 V/mm DC. The electrodes adhered to the inside right (positive) and left (negative) cell walls. The markers of the three blue circles in the image immediately after settling represent observation points for transmittance.
3.2. Aqueous Suspension of PMMA Spheres
Figure 5 illustrates the temporal variation of the transmittance in a water suspension of PMMA spheres, under a DC electric field of 0.4 V/mm (Figure 5a). The earliest change in transmittance occurred at the highest Hobs, which was 15 mm, followed by 10 mm and then 5 mm. The sequence corresponds to the sedimentation of PMMA spheres over time. Compared to hollow particles, the change in transmittance was more abrupt and the timing of the increase clearly diverged depending on the difference in Hobs value. This is due to the high uniformity in size of the PMMA spheres. Figure 5b illustrates the influence of electric field intensity on the temporal variation of transmittance at an Hobs of 5 mm. Since the observation points are located in the lower region of the cell, the boundary between the colloid-rich region and water region, which descends with the sedimentation of PMMA spheres, took a significantly long time to reach the observation point. At E = 0.1 V/mm DC, it exhibited a transmittance change almost identical to that under no electric field. However, at E = 0.2 V/mm DC and higher, compared to the zero-field condition, the rapid increase in transmittance occurred at an earlier stage with the increase in electric field strength. The degree to which the timing of rapid increase in transmittance is accelerated by the application of an electric field appeared to saturate around 0.5 V/mm DC.
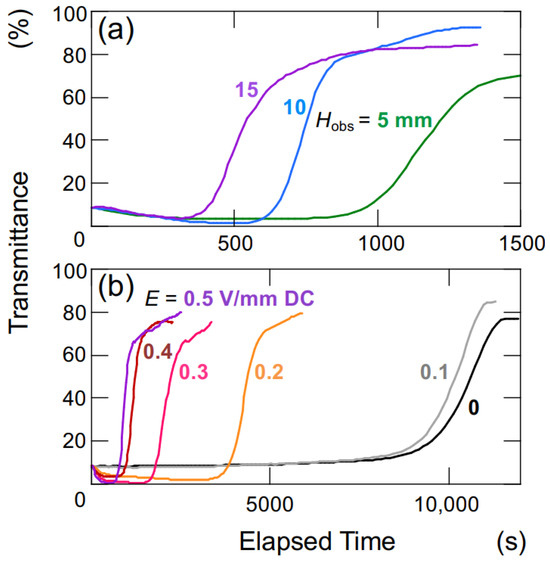
Figure 5.
Change in the transmittance of aqueous suspensions of PMMA spheres at 25 °C. ϕ = 0.0005. (a) Height dependence of observation position, E = 0.4 V/mm DC. (b) Dependence of electric field strength, Hobs = 5 mm.
Figure 6 illustrates the temporal variation of the dispersion state of PMMA spheres in water. In the absence of an electric field, the boundary line between the colloid-rich region and water region was observed very clearly. This observation aligns with the fact that the transmittance at the observation point undergoes a rapid change. At E = 0.5 V/mm DC, it can be observed that the descent speed of the boundary line is significantly greater compared to the zero-field condition. From the results, it is evident that the application of an electric field increased the sedimentation rate of PMMA spheres [19,20]. When an electric field was applied for an extended period, EHD convection was observed in the suspension of hollow particles, but it was not visually confirmed in the PMMA sphere suspension. The reason for this remains unclear at this point.
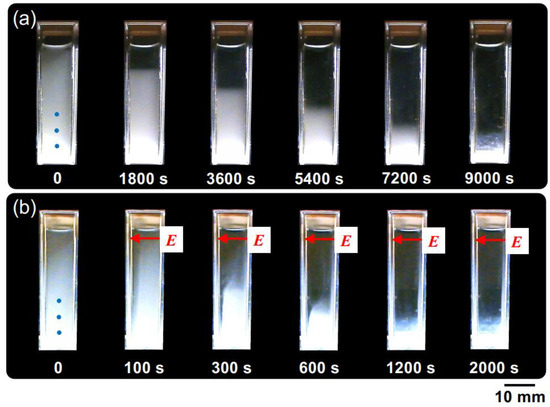
Figure 6.
The temporal evolution of the dispersion state of PMMA sphere aqueous suspension at 25 °C. ϕ = 0.0005. (a) Without an electric field; (b) E = 0.5 V/mm DC. The electrodes adhered to the inside right (positive) and left (negative) cell walls. The markers of the three blue circles in the image immediately after settling represent observation points for transmittance.
3.3. Aggregation Behavior of Hollow Particles and PMMA Spheres under an Electric Field
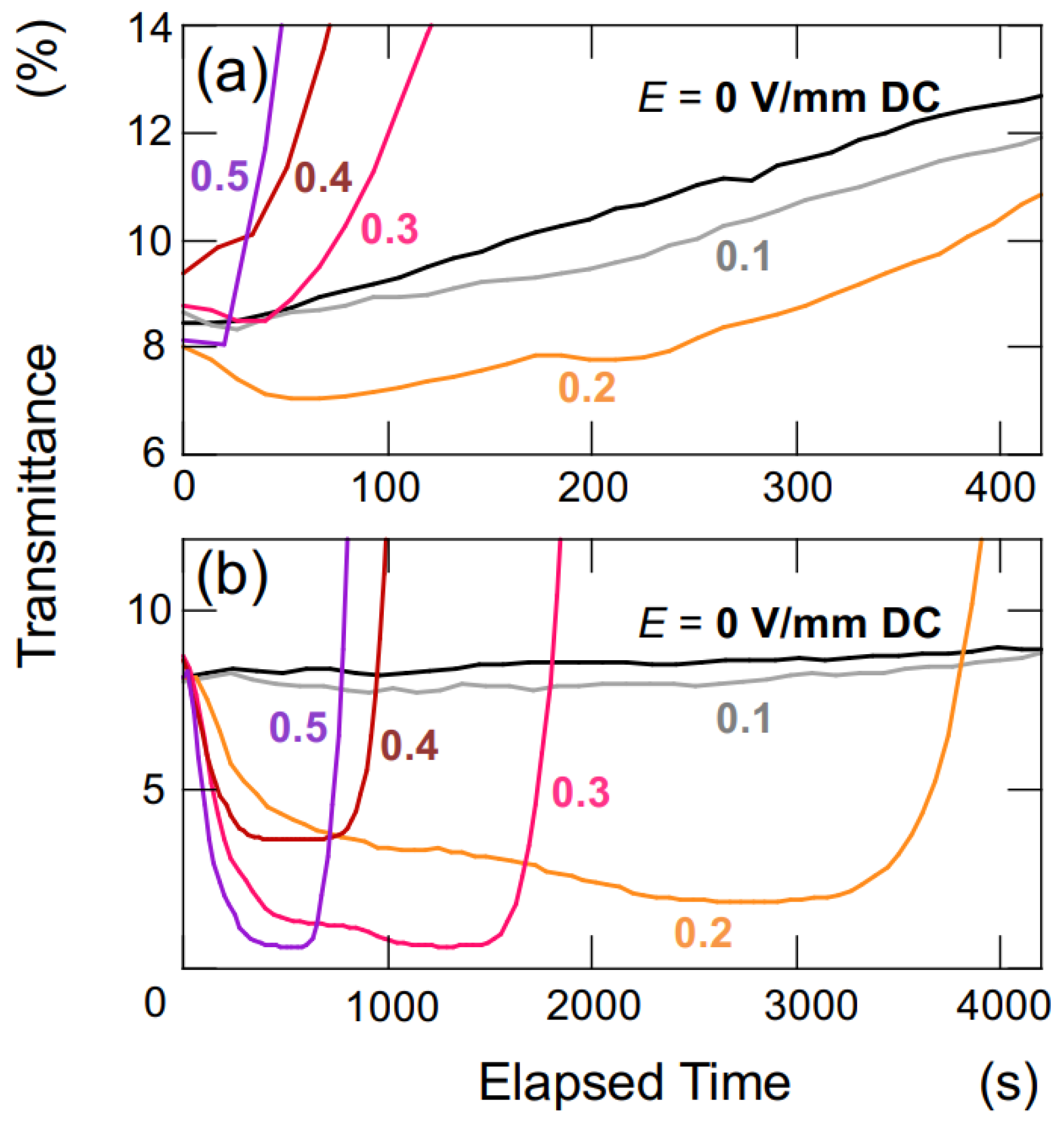
Based on the results obtained so far, it has been demonstrated that under a certain threshold of electric field strength, hollow particles and PMMA spheres tend to form aggregates of certain shapes and sizes. The water suspension of both the hollow particles and PMMA spheres used in this experiment appeared cloudy and white due to Mie scattering, regardless of the presence of an applied electric field. The intensity of Mie scattering is highly dependent on the size of the dispersed particles, ranging from a few μm to several hundred μm. Therefore, changes in aggregate size are expected to impact transmittance. Figure 7 presents enlarged views of the initial stages of the time-dependent transmittance for the aqueous suspensions of both hollow particles (Figure 3b) and PMMA spheres (Figure 5b). Immediately after the application of the electric field, the transmittance decreased in both water suspensions, and this reduction became more pronounced with increasing electric field intensity. After a significant decrease in transmittance, there was a rapid increase in transmittance due to the buoyancy/sedimentation of hollow particles and PMMA spheres. The decrease in transmittance, indicating an increase in Mie scattering intensity, strongly suggests that both hollow particles and PMMA spheres agglomerated and increased in size under the applied electric field.
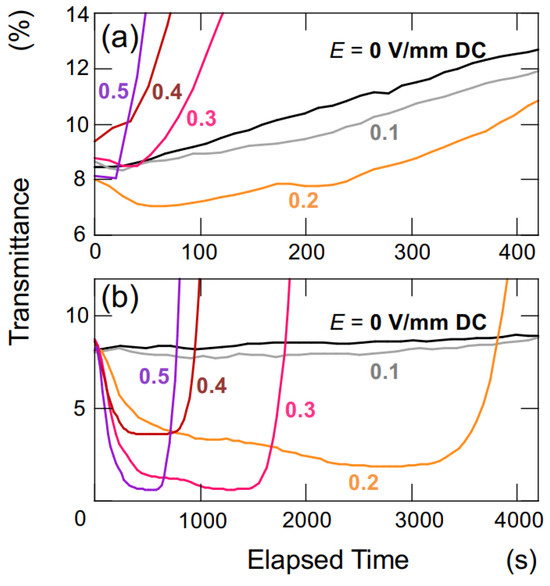
Figure 7.
Change in the transmittance of aqueous suspensions under various electric field strength at 25 °C. ϕ = 0.0005. Hobs = 5 mm. (a) Hollow particles; (b) PMMA spheres. (a) A magnified view of the initial stage depicted in Figure 3b; (b) a magnified view of the initial stage shown in Figure 5b.
The changes in the sedimentation state of PMMA spheres over time are illustrated in Figure 8. In the absence of an electric field (Figure 8a), the PMMA spheres settled at the bottom of the cell, and their number increased as time passed. The majority of PMMA spheres settled individually and remained well dispersed without forming aggregates. During the application of an electric field (Figure 8b), up to 600 s after the field was applied, a dispersed state similar to that in the absence of an electric field was observed. However, starting from 900 s onwards, aggregated PMMA spheres were observed. It is believed that the aggregates formed in water sedimented on the cover glass at the bottom of the cell. The sedimented aggregates were not undergoing electrophoretic motion and remained attached to the cover glass. The aggregates exhibited irregular shapes that were independent of the direction of the electric field. The formation of aggregates under a DC electric field has remained elusive until now. However, through the application of a quasi-DC field (an extremely low-frequency AC field), the formation of aggregate structures was captured for the first time.

Figure 8.
The sedimentation of PMMA spheres in water under no-field and electric field conditions. (a) No field; (b) Eeff = 1.0 V/mm, electric field frequency was 0.001 Hz. The direction of the electric field at each moment is indicated in the figure. For 300 s and 1200 s: Eeff = 0.95 V/mm; 600 s and 900 s: Eeff = 0.59 V/mm.
Sakai et al. observed that polystyrene particles (5 μm in diameter) in water formed a chain-like structure parallel to an AC electric field (7.5 V/mm in Eeff, 100 Hz to 2 MHz) between two plate electrodes [21]. The observation of such chain-like structures is believed to be due to the application of an AC electric field, particularly under conditions of higher electric field strength. If a chain-like structure of sufficient length that does not bridge between the electrodes is formed, it is expected that the sedimentation rate will naturally increase. Therefore, it is highly likely that the ERS effect will occur under the aforementioned experimental conditions.
4. Discussion
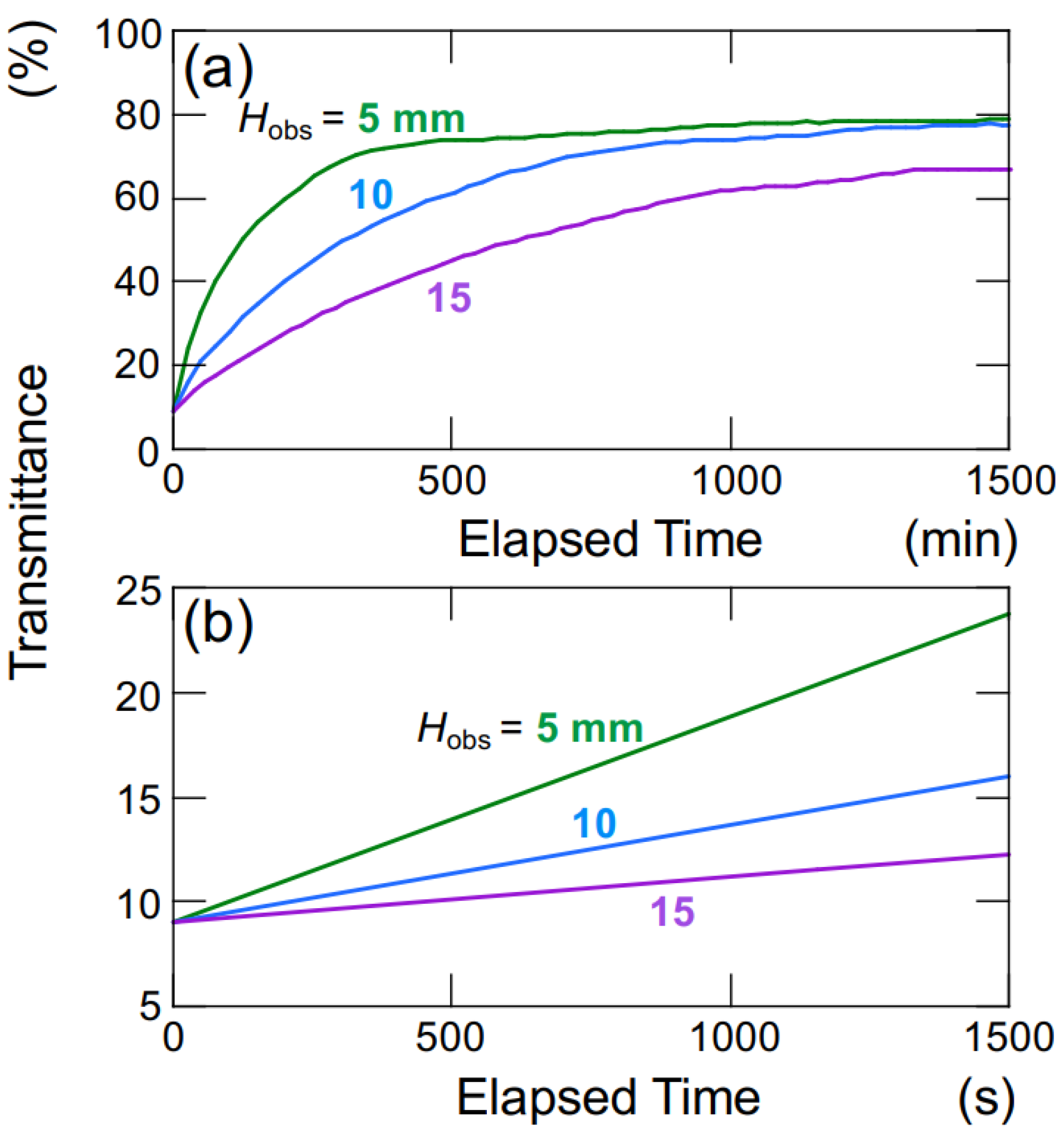
The hollow particles used in this study, to maintain a lower density than water, generate buoyancy in water. Figure 9 illustrates the time-dependent changes in the transmittance of the aqueous hollow particle suspension in the absence of an electric field. The upward velocity of hollow particles was evaluated to be 0.36 μm/s based on the time difference at which the transmittance reached 50% at observation heights of Hobs = 5 mm and 10 mm (Figure 9a). Within this range of observation heights, it was assumed that the hollow particles were ascending at a constant velocity. Hollow particles ascending in water experience buoyancy, gravity, and viscous drag forces. During steady ascent at a constant velocity, these forces are in equilibrium. Assuming a diameter of 2.6 μm for the hollow particles, the individual particle’s filling fraction is calculated to be 34% (void fraction 66%), resulting in a density of 0.87. When an electric field (E = 0.4 V/mm DC) was applied, as calculated similarly to the above, the upward velocity of the hollow particles was found to be 27.8 μm/s. This represents an approximately 60-fold increase in ascent velocity compared to the case without an electric field. The settling/rising velocity of aggregates is influenced by factors such as their size, porosity, fractal dimension, and others [22]. Therefore, a rigorous discussion regarding settling/rising velocity cannot be conducted here.
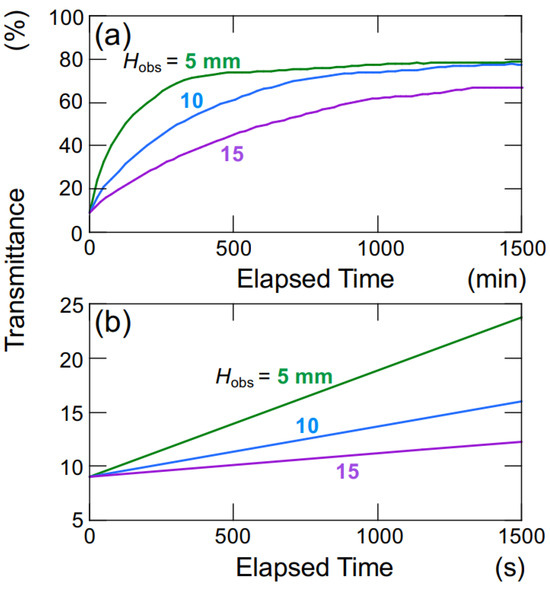
Figure 9.
Change in the transmittance of aqueous suspensions of hollow particles in the absence of an electric field at 25 °C. ϕ = 0.0005. (a) Height dependence of observation position; (b) a magnified view of the initial stage depicted in Figure 9a.
During the initial stages of the transmittance change in the aqueous hollow particle suspension in the absence of an electric field (Figure 9b), it did not exhibit the temporary decrease in transmittance seen when an electric field was applied. Instead, it showed a very slow and gradual increase. This observation once again suggests that both the hollow particles and PMMA spheres in water tend to aggregate when subjected to an applied electric field.
Figure 10 shows a schematic diagram illustrating the dispersion state of hollow particles and PMMA spheres in water. In this study, it has been discovered that particles in water can form aggregates when subjected to a DC electric field of approximately several V/mm. These aggregates then settle or rise at a speed corresponding to their specific gravity. The observed chain-like structures in non-aqueous suspensions under the application of an electric field [1] can be attributed to the generation of attractive forces in the electric field direction, which is clearly due to the induced dipole moments. This phenomenon becomes evident when applying electric fields in the order of several kV/mm. On the contrary, as evident from the results in this study, when small external electric fields of the order of a few V/mm or lower are applied to aqueous suspensions, the observation does not consist of chain-like aggregates but rather non-directional agglomerates. When an external electric field is applied to particles in water, it causes changes in the charge distribution around the particles. This leads to the destabilization of the electrical double layer and brings particles in close proximity together through van der Waals forces. Therefore, it is inferred that both hollow particles and PMMA spheres form aggregates under the influence of these forces. In the case of particles in water as well, if a higher electric field is applied, there is a possibility that chain-like aggregates, similar to those observed in non-aqueous suspensions, may be observed.
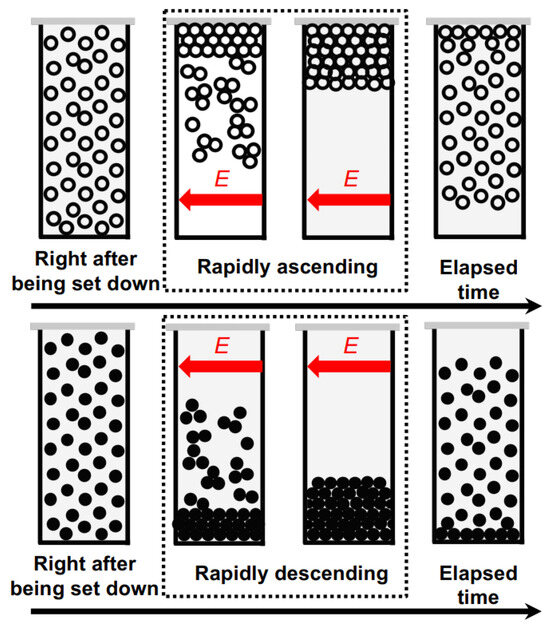
Figure 10.
Scheme of hollow particles and PMMA spheres in water without and under an electric field. Open circle: hollow particles; closed circle: PMMA spheres.
Mori et al. conducted a study on the influence of pH on the settling velocity of nanoparticles in water under the influence of an electric field [23]. They have concluded that induced dipole moments occur due to the deformation of the electrical double layer in the direction of the electric field, resulting in particle aggregation. This aggregation mechanism is fundamentally similar to particle aggregation induced by the application of an electric field in non-aqueous particle suspensions [24,25,26,27]. It is well known that the presence of an electrical double layer due to adsorbed water on the surfaces of particles in oil enhances the electrostatic attraction between particles in the direction of the electric field when an electric field is applied. When applying electric fields of the order of several hundred V/mm to non-aqueous particle dispersions, chain-like aggregates are often observed, which are distinct from the irregular aggregates. This difference may be attributed to variations in the applied electric field strength. The detailed mechanism of aggregation remains incompletely understood. It can be argued that the influence of the electrophoresis of particles and the electrolysis of water can be disregarded under relatively high-frequency AC electric fields. Consequently, conducting investigations under such conditions is believed to facilitate a more thorough examination and analysis.
When considering the mechanisms underlying the rapid upward movement of hollow particles and the quick sedimentation of PMMA spheres caused by an applied electric field, it becomes essential to take into account the incorporation of ions from the electrodes. In conclusion, the reduction in the electrical double layer due to the dissolution of metal ions from stainless-steel plates (SUS304) used as electrodes can be neglected. As demonstrated in a previous paper [20], it is evident that the ERS effect manifests only when an electric field is applied and reverts to its behavior under zero-field conditions upon field removal, confirming its reversibility. Therefore, the ERS effect is not related to the aggregation of colloidal particles due to an increase in electrolyte concentration. Based on these observations, it is suggested that the application of an electric field may induce the appearance of the “secondary minimum” in the potential energy curve in the DLVO theory. Consequently, it can be reasonably expected that the ERS effect will only manifest when the electrical double layer is sufficiently developed.
5. Conclusions
A novel phenomenon has been discovered, where the rapid upward movement of colloidal particles with a density lower than water is induced by applying an electric field of several V/mm in water. Utilizing a quasi-DC electric field, aggregate structures were captured for the first time. In a polar liquid, electrical double layers are formed around colloidal particles, and the thickness of this layer determines the dispersion state of the particles. At this stage, it remains unclear how the application of an electric field specifically affects this layer. The pursuit of the investigated ERS effect in this study has the potential to lead to applications such as particle separation and removal in polar liquids like water, as well as a deeper understanding of the behavior of electrical double layers under external electric fields. In the future, there are plans to investigate the ERS effect in an AC electric field and report the findings in a research paper to be published elsewhere.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The author wishes to convey their profound appreciation for Taiheiyo Cement Co.’s contribution of hollow particles. The author would like to express gratitude for the invaluable guidance received from the late Tsuneo Okubo on the topic of electric double layers. Furthermore, the author extends his appreciation to Akira Tsuchida for assistance with the setup of the transmission light measurement apparatus. H.K. would like to acknowledge Yuga Murakami, Moriaki Shibata, and Haruto Ikeshita for their valuable experimental support. This research was partly supported by Instrumental Analysis, Life Science Research Center, Gifu University, for sample characterization.
Conflicts of Interest
The author declares no conflict of interest.
References
- Winslow, W.M. Induced fibration of suspensions. J. Appl. Phys. 1949, 20, 1137–1140. [Google Scholar] [CrossRef]
- Kimura, H.; Minagawa, K.; Koyama, K. Strain dependent hysteresis in the stress response of ER suspensions. Polym. Prepr. (Am. Chem. Soc. Div. Polym. Chem.) 1994, 101, 358–359. [Google Scholar]
- Nishiwaki, T.; Adachi, K.; Kotaka, T. Deformation of viscous droplets in an electric field: Poly(propylene oxide)/poly(dimethylsiloxane) systems. Langmuir 1988, 4, 170–175. [Google Scholar] [CrossRef]
- Venugopal, G.; Krause, S. Development of phase morphologies of poly(methyl methacrylate)-polystyrene-toluene mixtures in electric fields. Macromolecules 1992, 25, 4626–4634. [Google Scholar] [CrossRef]
- Tajiri, K.; Sugaya, M.; Orihara, H.; Nagaya, T.; Ishibashi, Y.; Doi, M.; Inoue, A. Electrorheological effect in immiscible polymer blends. J. Rheol. 1997, 41, 335–341. [Google Scholar] [CrossRef]
- Kimura, H.; Aikawa, K.; Masubuchi, Y.; Takimoto, J.; Koyama, K.; Uemura, T. ‘Positive’ and ‘negative’ electro-rheological effect of liquid blends. J. Non-Newtonian Fluid Mech. 1998, 76, 199–211. [Google Scholar] [CrossRef]
- Kimura, H.; Aikawa, K.; Masubuchi, Y.; Takimoto, J.; Koyama, K.; Minagawa, K. Phase structure change and ER effect in liquid crystalline polymer/dimethylsiloxane blends. Rheol. Acta 1998, 37, 54–60. [Google Scholar] [CrossRef]
- Derjaguin, B.; Landau, L.D. Theory of the stability of strongly charged lyophobic sols and of the adhesion of strongly charged particles in solutions of electrolytes. Acta Physicochim. URSS 1941, 14, 633–662. [Google Scholar] [CrossRef]
- Verwey, E.J.W.; Overbeek, J.T.G. Theory of the Stability of Lyophobic Colloids; Elsevier: Amsterdam, The Netherlands, 1948. [Google Scholar]
- Ottewill, R.H. Dispersed systems-recent developments. Ber. Bunsenges. Phys. Chem. 1985, 89, 517–525. [Google Scholar] [CrossRef]
- Luck, W.; Klier, M.; Wesslau, H. Über Bragg-reflexe mit sichtbarem Licht an monodispersen Kunststofflatices. I. Ber. Bunsenges. Phys. Chem. 1963, 67, 75–83. [Google Scholar] [CrossRef]
- Luck, W.; Klier, M.; Wesslau, H. Über Bragg-reflexe mit sichtbarem Licht an monodispersen Kunststofflatices. II. Ber. Bunsenges. Phys. Chem. 1963, 67, 84–85. [Google Scholar] [CrossRef]
- Kose, A.; Ozaki, M.; Takano, K.; Kobayashi, Y.; Hachisu, S. Direct observation of ordered latex suspension by metallurgical microscope. J. Coll. Interf. Sci. 1973, 44, 330–338. [Google Scholar] [CrossRef]
- Pieranski, P. Colloidal crystals. Contemp. Phys. 1983, 24, 25–73. [Google Scholar] [CrossRef]
- Pusey, P.N.; van Megcn, W. Phase behaviour of concentrated suspensions of nearly hard colloidal spheres. Nature 1986, 320, 340–342. [Google Scholar] [CrossRef]
- Okubo, T. Extraordinary behavior in the structural properties of colloidal macroions in deionized suspension and the importance of the Debye screening length. Acc. Chem. Res. 1988, 21, 281–286. [Google Scholar] [CrossRef]
- Ottewill, R.H. Colloid stability and instability: “order disorder”. Langmuir 1989, 5, 4–11. [Google Scholar] [CrossRef]
- Okubo, T.; Kimura, H.; Tsuchida, K. Phase diagram of colloidal crystals of poly(methyl methacrylate) spheres in the exhaustively deionized dispersion. Coll. Polym. Sci. 2019, 297, 115–124. [Google Scholar] [CrossRef]
- Kimura, H.; Takahashi, S.; Tsuchida, A. Rapid sedimentation of poly(methyl methacrylate) spheres and montmorillonite particles in water upon application of a DC electric field of the order of a few V/mm. Appl. Clay Sci. 2014, 101, 623–625. [Google Scholar] [CrossRef]
- Kimura, H.; Tsuchida, A. Sedimentation behavior of poly(methyl methacrylate) spheres in water upon application of a DC vertical electric field of the order of a few V/mm. Powder Technol. 2017, 320, 362–367. [Google Scholar] [CrossRef]
- Sakai, H.; Orita, M.; Yamazaki, H.; Sakai, T.; Momozawa, N.; Ohkubo, T.; Abe, M. Ordered arrangement of polystyrene latex particles conducted by electrophoresis and dielectrophoresis. J. Oleo Sci. 2004, 53, 355–362. [Google Scholar] [CrossRef]
- Johnson, C.P.; Li, X.; Logan, B.E. Settling velocities of fractal aggregates. Environ. Sci. Technol. 1996, 30, 1911–1918. [Google Scholar] [CrossRef]
- Mori, T.; Nagashima, H.; Ito, Y.; Era, Y.; Tsubaki, J. Agglomeration of fine particles in water upon application of DC electric field. Miner. Eng. 2019, 133, 119–126. [Google Scholar] [CrossRef]
- Block, H.; Kelly, J.P. Electro-rheology. J. Phys. D Appl. Phys. 1988, 21, 1661–1677. [Google Scholar] [CrossRef]
- Jordan, T.C.; Shaw, M.T. Electrorheology. IEEE Trans. Dielectr. Electr. Insul. 1989, 24, 849–878. [Google Scholar] [CrossRef]
- Halsey, T.C. Electrorheological fluids. Science 1992, 258, 761–766. [Google Scholar] [CrossRef]
- Chin, B.D.; Winter, H.H. Field-induced gelation, yield stress, and fragility of an electro-rheological suspension. Rheol. Acta 2002, 41, 265–275. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).