Metformin Lowers Plasma Triacylglycerol Levels in Mice with Impaired Carnitine Biosynthesis and Fatty Liver
Abstract
:1. Introduction
2. Results
2.1. The Effect of Metformin on Weight Development in Mice with Meldonium-Induced Metabolic Disturbanses
2.2. The Effect of Metformin on Meldonium-Induced Fatty Liver
2.3. Plasma Carnitine Levels in Response to Mildronate and Meldonium Treatment
2.4. Metformin Reduced TAG in Mice with Meldonium-Induced Fatty Liver
2.5. Effects of Meldonium and Metformin on Fatty Acid Metabolism in Liver
3. Discussion
4. Materials and Methods
4.1. Animals and Treatment
4.2. Tissue Harvesting
4.3. Plasma Lipids
4.4. Hepatic Enzyme Activities and Hepatic Lipids
4.5. Gene Expression Analysis
4.6. Measurements of Plasma Levels of Carnitine, Its Precursors and Acylcarnitines
4.7. Histology
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- LaBrecque, D.; Abbas, Z.; Anania, F.; Ferenci, P.; Ghafoor Khan, A.; Goh, K.L.; Hamid, S.S.; Isakov, V.; Lizarzabal, M.; Mojica Pernaranda, M.; et al. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. World Gastroenterol. Organ. Glob. Guidel. 2012. Available online: https://www.worldgastroenterology.org/guidelines (accessed on 12 January 2024).
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Grattagliano, I.; de Bari, O.; Bernardo, T.C.; Oliveira, P.J.; Wang, D.Q.-H.; Portincasa, P. Role of mitochondria in nonalcoholic fatty liver disease--from origin to propagation. Clin. Biochem. 2012, 45, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Serviddio, G.; Bellanti, F.; Vendemiale, G.; Altomare, E. Mitochondrial dysfunction in nonalcoholic steatohepatitis. Expert. Rev. Gastroenterol. Hepatol. 2011, 5, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.D. Fatty liver: Role of inflammation and fatty acid nutrition. Prostaglandins Leukot. Essent. Fat. Acids 2010, 82, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Heeren, J.; Scheja, L. Metabolic-associated fatty liver disease and lipoprotein metabolism. Mol. Metab. 2021, 50, 101238. [Google Scholar] [CrossRef] [PubMed]
- Ziolkowska, S.; Binienda, A.; Jabłkowski, M.; Szemraj, J.; Czarny, P. The interplay between insulin resistance, inflammation, oxidative stress, base excision repair and metabolic syndrome in nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2021, 22, 11128. [Google Scholar] [CrossRef]
- Angelico, F.; Del Ben, M.; Conti, R.; Francioso, S.; Feole, K.; Fiorello, S.; Cavallo, M.G.; Zalunardo, B.; Lirussi, F.; Alessandri, C.; et al. Insulin resistance, the metabolic syndrome, and nonalcoholic fatty liver disease. J. Clin. Endocrinol. Metab. 2005, 90, 1578–1582. [Google Scholar] [CrossRef] [PubMed]
- Pais, R.; Charlotte, F.; Fedchuk, L.; Bedossa, P.; Lebray, P.; Poynard, T.; Ratziu, V. A systematic review of follow-up biopsies reveals disease progression in patients with non-alcoholic fatty liver. J. Hepatol. 2013, 59, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Gofton, C.; Upendran, Y.; Zheng, M.H.; George, J. MAFLD: How is it different from NAFLD? Clin. Mol. Hepatol. 2023, 29, S17–S31. [Google Scholar] [CrossRef]
- Szymczak-Pajor, I.; Wenclewska, S.; Śliwińska, A. Metabolic Action of Metformin. Pharmaceuticals 2022, 15, 810. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Patel, D.; E Ayesha, I.; Monson, N.R.; Klair, N.; Patel, U.; Saxena, A.; Hamid, P.; Ayesha, I.E. The Effectiveness of Metformin in Diabetes Prevention: A Systematic Review and Meta-Analysis. Cureus 2023, 15, e46108. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, Y.; Wang, X.; Yan, C.; Li, C.; Zhang, L.; Zhang, L.; Liang, E.; Liu, T.; Mao, J. Effect of metformin on nonalcoholic fatty liver based on meta-analysis and network pharmacology. Medicine 2022, 101, e31437. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bugianesi, E.; Marzocchi, R.; Villanova, N.; Marchesini, G. Non-alcoholic fatty liver disease/non-alcoholic steatohepatitis (NAFLD/NASH): Treatment. Best. Pract. Res. Clin. Gastroenterol. 2004, 18, 1105–1116. [Google Scholar] [CrossRef] [PubMed]
- Pinyopornpanish, K.; Leerapun, A.; Pinyopornpanish, K.; Chattipakorn, N. Effects of Metformin on Hepatic Steatosis in Adults with Nonalcoholic Fatty Liver Disease and Diabetes: Insights from the Cellular to Patient Levels. Gut Liver. 2021, 15, 827–840. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Day, C.P. Pathogenesis of steatohepatitis. Best. Pract. Res. Clin. Gastroenterol. 2002, 16, 663–678. [Google Scholar] [CrossRef] [PubMed]
- Marchesini, G.; Bianchi, G.; Tomassetti, S.; Zoli, M.; Melchionda, N. Metformin in non-alcoholic steatohepatitis. Lancet 2001, 358, 893–894. [Google Scholar] [CrossRef] [PubMed]
- Haukeland, J.W.; Konopski, Z.; Eggesbø, H.B.; von Volkmann, H.L.; Raschpichler, G.; Bjøro, K.; Haaland, T.; Løberg, E.M.; Birkeland, K. Metformin in patients with non-alcoholic fatty liver disease: A randomized, controlled trial. Scand. J. Gastroenterol. 2009, 44, 853–860. [Google Scholar] [CrossRef] [PubMed]
- A Garinis, G.; Fruci, B.; Mazza, A.; De Siena, M.; Abenavoli, S.; Gulletta, E.; Ventura, V.; Greco, M.; Abenavoli, L.; Belfiore, A. Metformin versus dietary treatment in nonalcoholic hepatic steatosis: A randomized study. Int. J. Obes. 2010, 34, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Hookman, P.; Barkin, J.S. Current biochemical studies of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis suggest a new therapeutic approach. Am. J. Gastroenterol. 2003, 98, 2093–2097. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Z.; Yang, S.Q.; Chuckaree, C.; Kuhajda, F.; Ronnet, G.; Diehl, A.M. Metformin reverses fatty liver disease in obese, leptin-deficient mice. Nat. Med. 2000, 6, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.-L.; Xu, H.; Li, H.; Zhao, Y.; Hu, X.; Zhao, J.; Guo, X.; Guo, T.; Botchlett, R.; Qi, T.; et al. Metformin Ameliorates Hepatic Steatosis and Inflammation without Altering Adipose Phenotype in Diet-Induced Obesity. PLoS ONE 2014, 9, e91111. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bieber, L.L. Carnitine. Annu. Rev. Biochem. 1988, 57, 261–283. [Google Scholar] [CrossRef] [PubMed]
- Vaz, F.M.; Wanders, R.J. Carnitine biosynthesis in mammals. Biochem. J. 2002, 361 Pt. 3, 417–429. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dambrova, M.; Liepinsh, E.; Kalvinsh, I. Mildronate: Cardioprotective action through carnitine-lowering effect. Trends Cardiovasc. Med. 2002, 12, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Koves, T.R.; Ussher, J.R.; Noland, R.C.; Slentz, D.; Mosedale, M.; Ilkayeva, O.; Bain, J.; Stevens, R.; Dyck, J.R.; Newgard, C.B.; et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008, 7, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Mihalik, S.J.; Goodpaster, B.H.; Kelley, D.E.; Chace, D.H.; Vockley, J.; Toledo, F.G.S.; Delany, J.P. Increased levels of plasma acylcarnitines in obesity and type 2 diabetes and identification of a marker of glucolipotoxicity. Obesity 2010, 18, 1695–1700. [Google Scholar] [CrossRef]
- Bjørndal, B.; Burri, L.; Wergedahl, H.; Svardal, A.; Bohov, P.; Berge, R.K. Dietary supplementation of herring roe and milt enhances hepatic fatty acid catabolism in female mice transgenic for hTNFalpha. Eur. J. Nutr. 2012, 51, 741–753. [Google Scholar] [CrossRef] [PubMed]
- Klusa, V.; Beitnere, U.; Pupure, J.; Isajevs, S.; Rumaks, J.; Svirskis, S.; Dzirkale, Z.; Kalvinsh, I. Mildronate and its neuroregulatory mechanisms: Targeting the mitochondria, neuroinflammation, and protein expression. Medicina 2013, 49, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Liepinsh, E.; Vilskersts, R.; Skapare, E.; Svalbe, B.; Kuka, J.; Cirule, H.; Pugovics, O.; Kalvinsh, I.; Dambrova, M. Mildronate decreases carnitine availability and up-regulates glucose uptake and related gene expression in the mouse heart. Life Sci. 2008, 83, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Spaniol, M.; Brooks, H.; Auer, L.; Zimmermann, A.; Solioz, M.; Stieger, B.; Krähenbühl, S. Development and characterization of an animal model of carnitine deficiency. Eur. J. Biochem. 2001, 268, 1876–1887. [Google Scholar] [CrossRef] [PubMed]
- Degrace, P.; Demizieux, L.; Du, Z.-Y.; Gresti, J.; Caverot, L.; Djaouti, L.; Jourdan, T.; Moindrot, B.; Guilland, J.-C.; Hocquette, J.-F.; et al. Regulation of lipid flux between liver and adipose tissue during transient hepatic steatosis in carnitine-depleted rats. J. Biol. Chem. 2007, 282, 20816–20826. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.-Y.; Ma, T.; Liaset, B.; Keenan, A.H.; Araujo, P.; Lock, E.-J.; Demizieux, L.; Degrace, P.; Frøyland, L.; Kristiansen, K.; et al. Dietary eicosapentaenoic acid supplementation accentuates hepatic triglyceride accumulation in mice with impaired fatty acid oxidation capacity. Biochim. Biophys. Acta 2013, 1831, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Simkhovich, B.Z.; Shutenko, Z.V.; Meirēna, D.V.; Khagi, K.B.; Mežapuķe, R.J.; Molodchina, T.N.; Kalvlņš, I.J.; Lukevics, E. 3-(2,2,2-Trimethylhydrazinium)propionate (THP)—A novel gamma-butyrobetaine hydroxylase inhibitor with cardioprotective properties. Biochem. Pharmacol. 1988, 37, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Berge, R.K.; Flatmark, T.; Osmundsen, H. Enhancement of long-chain acyl-CoA hydrolase activity in peroxisomes and mitochondria of rat liver by peroxisomal proliferators. Eur. J. Biochem. 1984, 141, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Bremer, J. The effect of fasting on the activity of liver carnitine palmitoyltransferase and its inhibition by malonyl-coa. Biochim. Biophys. Acta 1981, 665, 628–631. [Google Scholar] [CrossRef] [PubMed]
- Vik, R.; Bjørndal, B.; Bohov, P.; Brattelid, T.; Svardal, A.; Nygård, O.K.; Nordrehaug, J.E.; Skorve, J.; Berge, R.K. Hypolipidemic effect of dietary water-soluble protein extract from chicken: Impact on genes regulating hepatic lipid and bile acid metabolism. Eur. J. Nutr. 2015, 54, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Vigerust, N.F.; Cacabelos, D.; Burri, L.; Berge, K.; Wergedahl, H.; Christensen, B.; Portero-Otin, M.; Viste, A.; Pamplona, R.; Berge, R.K.; et al. Fish oil and 3-thia fatty acid have additive effects on lipid metabolism but antagonistic effects on oxidative damage when fed to rats for 50 weeks. J. Nutr. Biochem. 2012, 23, 1384–1393. [Google Scholar] [CrossRef] [PubMed]
- Berge, R.K.; Bjørndal, B.; Strand, E.; Bohov, P.; Lindquist, C.; Nordrehaug, J.E.; Svardal, A.; Skorve, J.; Nygård, O. Tetradecylthiopropionic acid induces hepatic mitochondrial dysfunction and steatosis, accompanied by increased plasma homocysteine in mice. Lipids Health Dis. 2016, 15, 24. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Berge, R.; Tronstad, K.; Berge, K.; Rost, T.; Wergedahl, H.; Gudbrandsen, O.; Skorve, J. The metabolic syndrome and the hepatic fatty acid drainage hypothesis. Biochimie 2005, 87, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, H.; Geerts, C.; Furtos, A.; Waters, P.; Cyr, D.; Wang, S.; Mitchell, G.A. The multiple facets of acetyl-CoA metabolism: Energetics, biosynthesis, regulation, acylation and inborn errors. Mol. Genet. Metab. 2023, 138, 106966. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.E.; Evans, A.M. Carnitine and acylcarnitines: Pharmacokinetic, pharmacological and clinical aspects. Clin. Pharmacokinet. 2012, 51, 553–572. [Google Scholar] [CrossRef] [PubMed]
- Sanchis-Gomar, F.; Garcia-Gimenez, J.L.; Gomez-Cabrera, M.C.; Pallardo, F.V. Mitochondrial biogenesis in health and disease. Molecular and therapeutic approaches. Curr. Pharm. Des. 2014, 20, 5619–5633. [Google Scholar] [CrossRef] [PubMed]
- Santana, K.N.d.O.; Lelis, D.F.; Mendes, K.L.; Lula, J.F.; Paraíso, A.F.; Andrade, J.M.O.; Feltenberger, J.D.; Cota, J.; da Costa, D.V.; de Paula, A.M.B.; et al. Metformin Reduces Lipogenesis Markers in Obese Mice Fed a Low-Carbohydrate and High-Fat Diet. Lipids 2016, 51, 1375–1384. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Xu, L.; Ye, J.; Li, D.; Wang, W.; Li, X.; Wu, L.; Wang, H.; Guan, F.; Li, P. Cidea promotes hepatic steatosis by sensing dietary fatty acids. Hepatology 2012, 56, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Orlicky, D.J.; Roede, J.R.; Bales, E.; Greenwood, C.; Greenberg, A.; Petersen, D.; McManaman, J.L. Chronic ethanol consumption in mice alters hepatocyte lipid droplet properties. Alcohol. Clin. Exp. Res. 2011, 35, 1020–1033. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lin, M.-J.; Dai, W.; Scott, M.J.; Li, R.; Zhang, Y.-Q.; Yang, Y.; Chen, L.-Z.; Huang, X.-S. Metformin improves nonalcoholic fatty liver disease in obese mice via down-regulation of apolipoprotein A5 as part of the AMPK/LXRalpha signaling pathway. Oncotarget 2017, 8, 108802–108809. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hao, L.; Li, S.; Chen, G.; Hu, X. Regulation of UCP2 in nonalcoholic fatty liver disease: From mechanisms to natural product. Chem. Biol. Drug Des. 2024, 103, e14461. [Google Scholar] [CrossRef]
- Buczyńska, A.; Sidorkiewicz, I.; Krętowski, A.J.; Adamska, A. Examining the clinical relevance of metformin as an antioxidant intervention. Front. Pharmacol. 2024, 15, 1330797. [Google Scholar] [CrossRef]
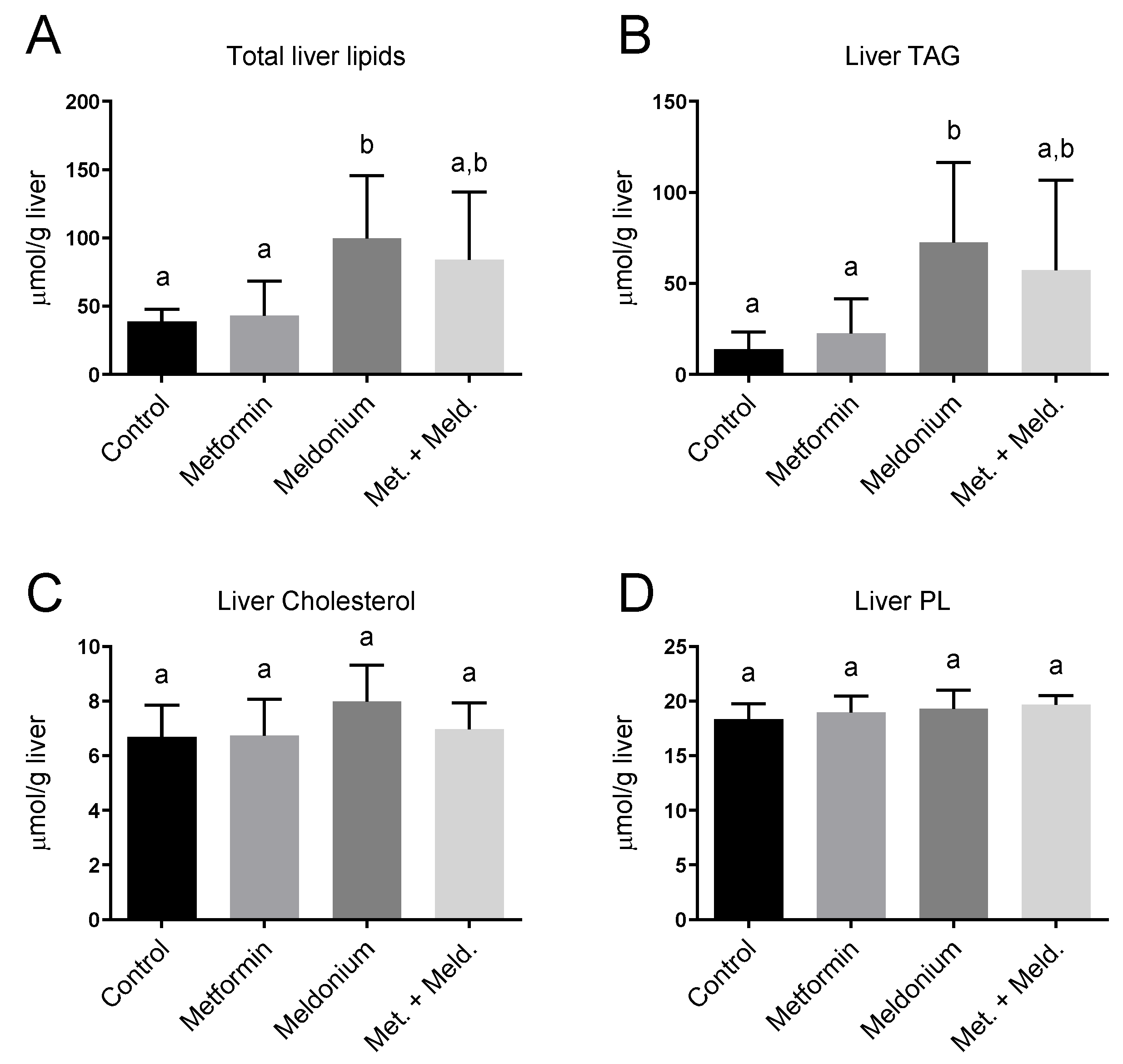
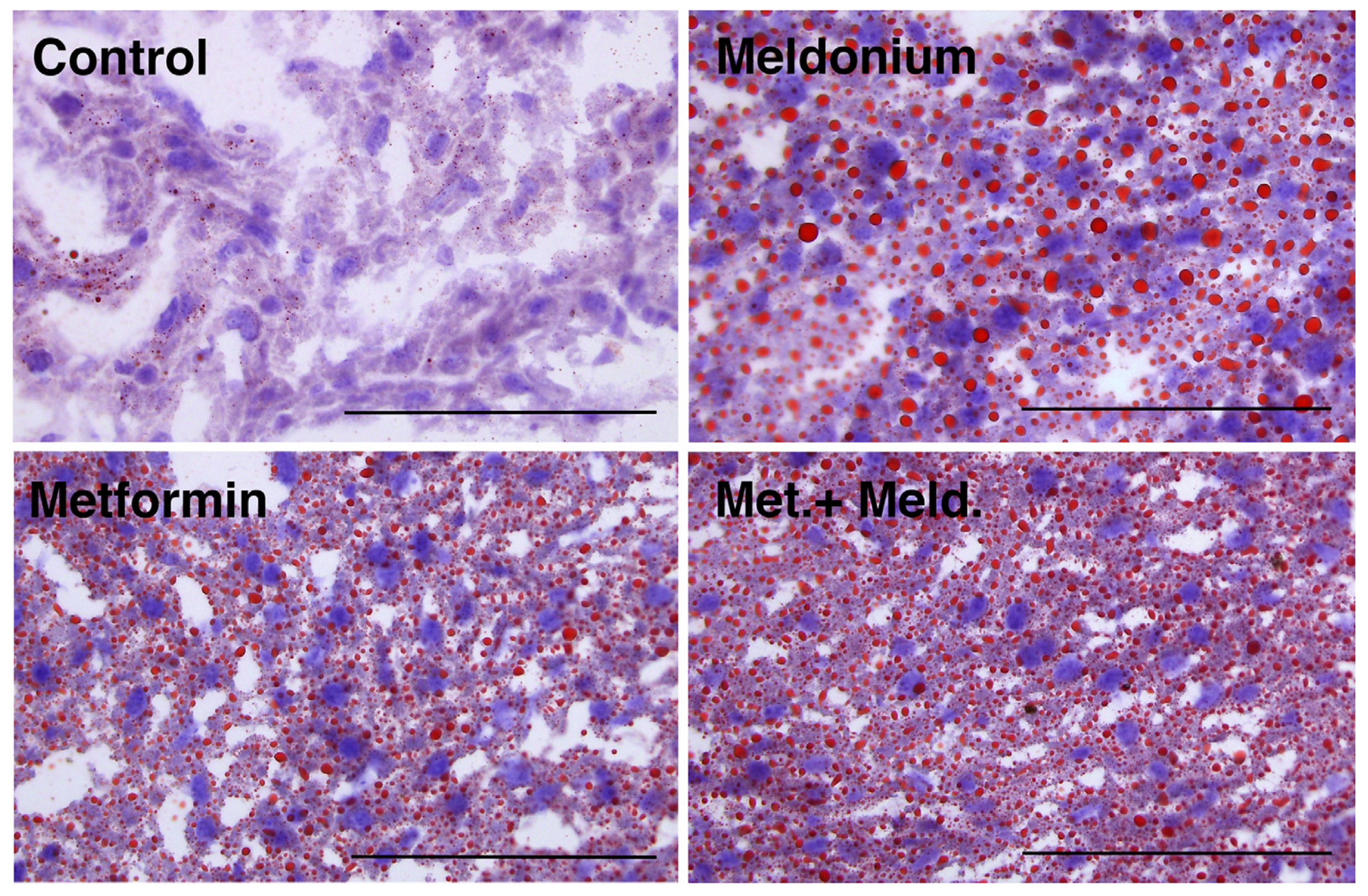


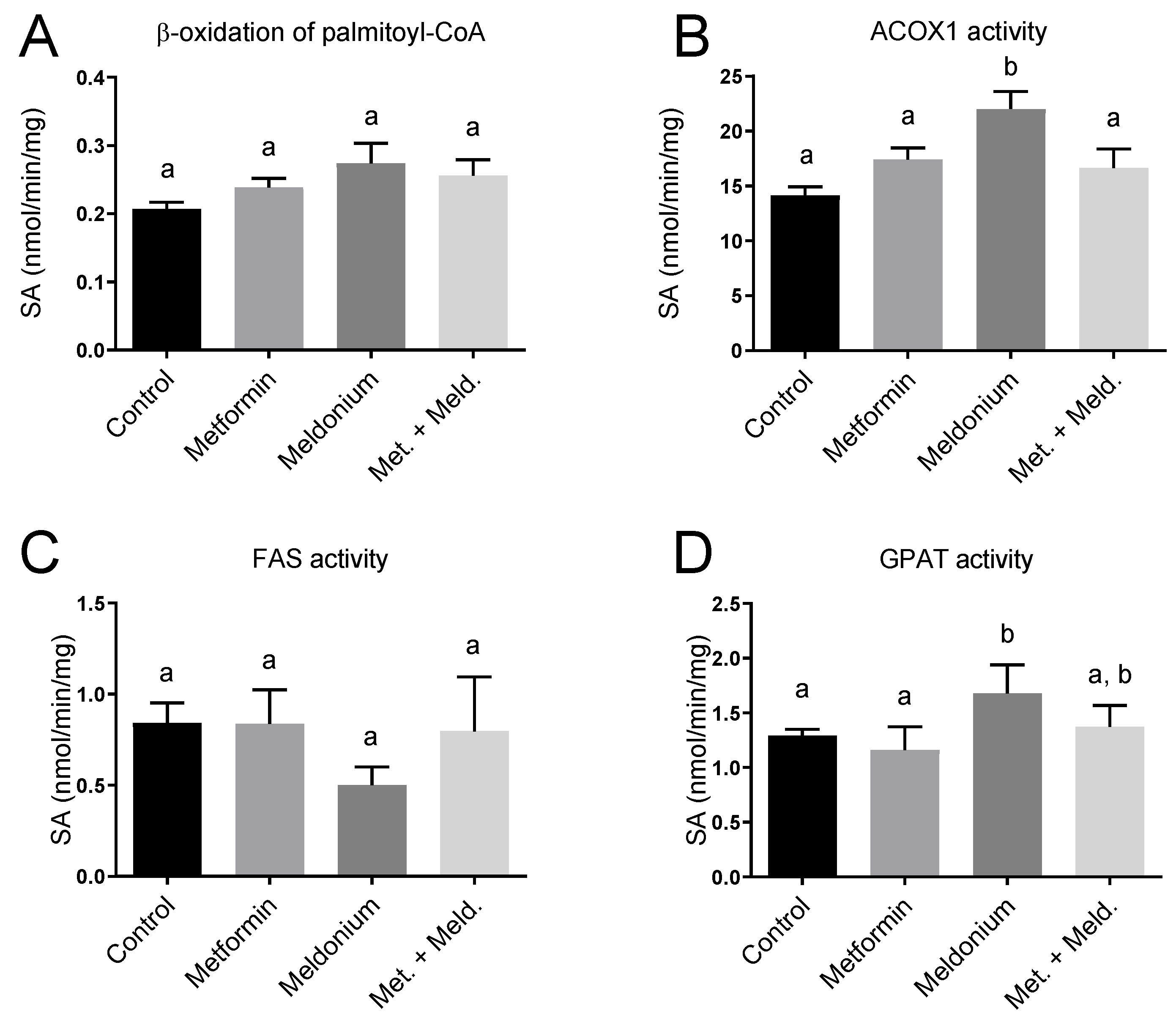
| Parameter | Control | Metformin | Meldonium | Met. + Meld. |
|---|---|---|---|---|
| Start weight (g) | 29.1 ± 2.3 a | 30.7 ± 2.1 a | 29.9 ± 1.8 a | 30.2 ± 3.3 a |
| End weight (g) | 33.1 ± 2.3 a | 33.8 ± 1.9 a | 32.6 ± 2.1 a | 30.7 ± 3.7 a |
| Weight gain (g) | 4.1 ± 1.8 a | 3.1 ± 1.4 a | 2.7 ± 0.9 a | 0.5 ± 2.5 b |
| Visceral fat depot (g) 2 | 0.75 ± 0.18 a | 0.75 ± 0.34 a | 0.63 ± 0.28 a | 0.53 ± 0.27 b |
| Subcutaneous fat depot (g) | 0.51 ± 0.25 a | 0.64 ± 0.29 a | 0.45 ± 0.24 a | 0.45 ± 0.16 a |
| Hepatic index (liver (g)/body weight (g) | 4.71 ± 0.51 a | 4.97 ± 0.43 a | 5.14 ± 0.74 a | 5.17 ± 0.48 a |
| Feed intake (g/mouse/week) | 29.7 ± 3.2 a | 29.7 ± 2.9 a | 27.5 ± 4.4 a | 28.6 ± 4.3 a |
| Gene Symbol | Gene Name | Control | Metformin | Meldonium | Met. + Meld. |
|---|---|---|---|---|---|
| Cpt2 | Carnitine palmitoyltransferase 2 | 1.00 ± 0.31 a | 1.28 ± 0.76 a | 1.15 ± 0.38 a | 1.50 ± 0.40 a |
| Cpt1a | Carnitine palmitoyltransferase 1a | 1.00 ± 0.24 a | 1.75 ± 0.63 a | 1.46 ± 0.68 a | 2.82 ± 0.93 b |
| Hmgcs2 | HMG-CoA synthase 2 | 1.00 ± 0.28 a | 1.58 ± 0.67 a,b | 1.73 ± 0.63 a,b | 1.95 ± 0.61 b |
| Acox1 | Acyl-CoA oxidase 1 | 1.00 ± 0.38 a | 1.82 ± 0.69 a,b | 1.28 ± 0.58 a,b | 2.15 ± 0.72 b |
| Ppara | Peroxisome proliferator-activated receptor alpha | 1.00 ± 0.36 a | 1.51 ± 0.57 b | 1.02 ± 0.51 a | 1.46 ± 0.43 a,b |
| Pparg | Peroxisome proliferator-activated receptor gamma | 1.00 ± 0.40 a | 1.73 ± 0.72 a | 1.14 ± 0.74 a | 1.40 ± 0.74 a |
| Ppargca1 | PPAR gamma coactivator 1 | 1.00 ± 0.29 a | 1.01 ± 0.47 a | 1.24 ± 0.62 a,b | 1.92 ± 0.8 b |
| Ucp2 | Uncoupling protein 2 | 1.00 ± 0.35 a,b | 1.36 ± 0.48 a,b | 0.99 ± 0.53 a | 1.85 ± 0.85 b |
| Cd36 | CD36 molecule | 1.00 ± 0.42 a | 1.39 ± 0.66 a,b | 1.99 ± 0.98 b | 2.36 ± 1.00 b |
| Components | g/kg |
|---|---|
| Carbohydrates (total) | 662.9 |
| Cornstarch | 387.6 |
| Dyetrose | 128.9 |
| Sucrose | 97.6 |
| Cellulose Fiber | 48.8 |
| Protein (total) | 219.4 |
| Casein 1 | 219.4 |
| Fat (total) | 68.3 |
| Soy Bean Oil | 19.5 |
| Lard | 48.8 |
| Micronutrients (total) | 49.3 |
| AIN-93G-MX min. mix | 34.2 |
| AIN-93G-VX vit. mix | 9.8 |
| L-Cysteine | 2.9 |
| Choline bitartrate | 2.4 |
| tert-Butylhydroquinone | 0.014 |
| Drugs 2 | |
| Meldonium | 3.1 |
| Metformin | 1.7 |
| Meldonium + Metformin | 3.1 + 1.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bjørndal, B.; Le, T.-M.T.; Strand, E.; Madsen, L.; Berge, R.K. Metformin Lowers Plasma Triacylglycerol Levels in Mice with Impaired Carnitine Biosynthesis and Fatty Liver. SynBio 2024, 2, 240-253. https://doi.org/10.3390/synbio2030014
Bjørndal B, Le T-MT, Strand E, Madsen L, Berge RK. Metformin Lowers Plasma Triacylglycerol Levels in Mice with Impaired Carnitine Biosynthesis and Fatty Liver. SynBio. 2024; 2(3):240-253. https://doi.org/10.3390/synbio2030014
Chicago/Turabian StyleBjørndal, Bodil, Tra-My Thi Le, Elin Strand, Lise Madsen, and Rolf K. Berge. 2024. "Metformin Lowers Plasma Triacylglycerol Levels in Mice with Impaired Carnitine Biosynthesis and Fatty Liver" SynBio 2, no. 3: 240-253. https://doi.org/10.3390/synbio2030014
APA StyleBjørndal, B., Le, T.-M. T., Strand, E., Madsen, L., & Berge, R. K. (2024). Metformin Lowers Plasma Triacylglycerol Levels in Mice with Impaired Carnitine Biosynthesis and Fatty Liver. SynBio, 2(3), 240-253. https://doi.org/10.3390/synbio2030014





