Neuroinformatics Insights towards Multiple Neurosyphilis Complications
Abstract
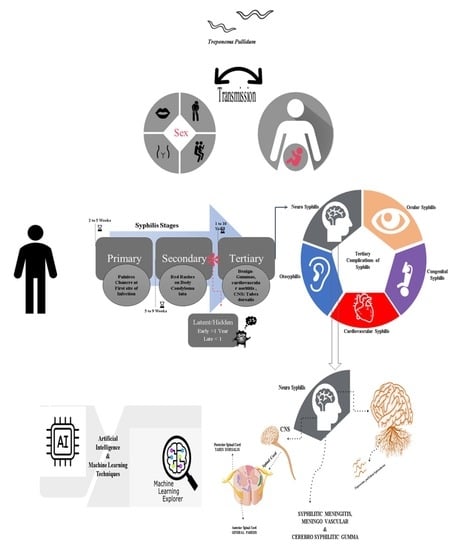
:1. Introduction
2. Treponema pallidum Pathogenesis in Neurological Complications
3. Treponema pallidum Neurological Invasion and Evasion Mechanism
4. Blood–Brain Barrier (BBB) and Central Nervous System (CNS) Crossing by Treponema pallidum towards Neurosyphilis
5. Regulatory T Cell (Treg-Cell) during Neurosyphilis Complications
6. Immunological Changes/Adaptation of Syphilis–HIV Coinfections Associated with Treg-Cell
7. Multiple Neurosyphilitic Maladies
7.1. Neurosyphilitic Meningitis or Syphilitic Meningitis/Meningovascular Syphilis (MVS)
7.2. Syphilitic Myelitis (SM)
7.3. Cerebral Syphilitic Gumma (CSG)
7.4. Atypical Behavior and Neuropsychiatric Symptoms in NS
| Complication | Disorders | Case Report/References |
|---|---|---|
| Neurosyphilitic Patients | Attention Deficit Disorder | [101] |
| Anger/Violent Behavior | [102,103,104] | |
| Anxiety | [102] | |
| Bipolar Disorder | [105] | |
| Behavioral/Neuropsychiatric Changes | ||
| Complex Condition | [106] | |
| Drug/Alcohol | [107,108,109] | |
| Dissociative Disorder | [110] | |
| Hearing Disorder | [111,112,113] | |
| Hormonal Disabilities | [114,115] | |
| Memory Loss and Dementia | [116,117,118] | |
| Psychotic Mania and Hypomania | [105,119] | |
| Panic Disorder | ||
| Personality Disorder | [104] | |
| Post-Traumatic Disorder | ||
| Sleep Disorder/Insomnia | [19,120] | |
| Suicidal Thoughts | [102] | |
| Traumatic Brain Injury | ||
| Trigeminal Nerve Dysfunction | [121] | |
| Weight Loss | [122] |
| Types of Neurosyphilis | Age/Sex | Symptoms | Treatment/Recovery | References |
|---|---|---|---|---|
| NS Meningitis, MVS, SM | 31/M | Paresis of upper extremities, Predominantly in the right arm Intense holocranial headache | Crystalline Sodium Penicillin | [79] |
| 28/M | Low CRP (10 mg/L, reference value: <8 mg/L) with HIV-positive | Benzyl-penicillin | [123] | |
| 43/M | Frontal headache, fever, nausea, vomiting, HIV-positive with tuberculous meningitis | Antiretroviral therapy | [82] | |
| 49/M | Recurrent strokes in the left middle cerebral artery territory; dysphasia, higher cognitive deficits, motor deficits, and subsequent infarcts in the right middle cerebral and anterior cerebral artery territories manifest with seizures and behavioral and social problems | Injection procaine penicillin, 1.8–2.4 million units intramuscularly; Probenecid, 500 mg orally | [124] | |
| 24/F | Severe and persistent headache, migraine headache, significant dizziness, vertigo | Benzathine Penicillin G intramuscularly, 1.6 million units | [80] | |
| 43/F | Rash of legs, numbness, and weakness in the bilateral feet; lesions in the cervical and thoracic cord | Penicillin G intravenous, 24 million units | [86] | |
| 19 patients included M and F | Sensory disturbance, paraparesis, urinary retention | Penicillin | [1] | |
| 29/F | Progressive bilateral lower extremities’ numbness and weakness | Penicillin G, 4 million units; dexamethasone, 5 mg | [87] | |
| 63/M | Progressive lumbago, weakness of both lower extremities, bilateral lower-limb weakness with motor power of 4–5, lower-limb hyporeflexia | Ceftriaxone, Methylprednisolone | [125] | |
| CSG | 62/M | Speech disturbance, medical history of hypertension | The clinical diagnosis was a glioma; patients admitted for the surgery | [126] |
| 52/F | Headache with intensity from very mild to severe attacks and dizziness; presence of a metastatic tumor | Water-soluble penicillin-G administered intravenously | [91] | |
| 44/M Bisexual | General fatigue and rash, HIV-positive; later showed headache, nausea, and vomiting; brain mass lesion detected in the right temporal lobe through MRI | Oral amoxicillin; later ceftriaxone intravenous, 2 g | [127] | |
| 45/M | Severe headache, left-sided weakness MRI identified a small lesion near to sagittal sinus in the right frontal lobe; surgery was performed | Intravenous penicillin, 2.5 million units; intramuscular injections of benzathine penicillin, 2.4 million units | [90] | |
| 59/F | Dysarthria showed a mass in the brain; after surgery, fever and rash were reported with infiltration on the chest | Ceftriaxone | [128] | |
| 50/F | The MRI and CT scan identified headaches and speech disturbances (mixed aphasia), left parietal injury, and later left temporal recurrence; the last relapse of the tumor lesion in the left temporal region was identified with MRI | Intravenous benzathine penicillin | [92] | |
| 6 Patients between 32–61/4M-2F | All 6 patients exhibited 10 lesions, nine of which were located in the cerebral hemisphere, primarily in the grey matter identified by MRI neuroimaging; surgery was performed | High dose of penicillin after surgery | [129] | |
| 52/F | MRI identified intermittent headache lasting for 5 months, vomiting, history of hypertension and hyperlipidemia, multiple nodules with evident perilesional edema in the right temporal lobe; severe edema in the brain tissue of the right temporal lobe was also observed; surgery was performed | Penicillin treatment, 18 million units | [89] | |
| 58/M | Extradural cervical spinal syphilitic gumma; the epidural lesion was removed via a posterior approach; brain MRI revealed a cerebro-meningeal syphilitic gumma | An antibiotic regime based on aqueous penicillin G | [18] | |
| 66/M | MRI identified affective disorder, hypomnesia, convulsion, cerebral swelling, hyperintensity in the cortex/subcortex, and multiple lacunar cerebral infarctions. The presence of a pial arteriovenous fistula was also detected by CT angiography | Diazepam was used for convulsion and antibiotic therapy | [130] | |
| 46/M | Numbness of bilateral lower limbs, lower back pain, irregular defecation, homogeneous peripheral enhancement, and the intramedullary nodule was identified at the T7 level with extensive thoracic cord edema; MRI syphilitic gumma was considered | Penicillin G | [131] | |
| 47/M | History of diabetes mellitus, the patient had generalized seizures, multiple brain tumors were identified through MRI, and multiple cerebral syphilitic gummas were diagnosed | High dose of penicillin | [132] |
8. Advantages of Bioinformatics (BI), Computational Neuroscience (CN), and Neuroinformatics (NI) in Neuroscience
9. Application of Computational Neuroscience (CN) and Neuroinformatics (NI) and Their Benefits in Brain Complications
10. Computational Neuroscience and Neuroinformatics in Neurosyphilis Complications
10.1. Artificial Intelligence (AI)-, Machine Learning (ML)-, and Deep Learning (DL)-Based Techniques for Neurological Maladies
10.1.1. Computational Models and BBB Permeability Detection in NS
10.1.2. Computational/In Silico Approaches Based on AI for NS Meningitis or SM
10.1.3. Computational/In Silico Modeling for CSG
| Different Neurological Complications | In Silico Model, Systems/Techniques | Motive/Brain Complications | Reference |
|---|---|---|---|
| Meningitis | Fuzzy expert system | Bacterial and aseptic meningitis | [163] |
| Fuzzy cognitive map with TOPSIS | Assessment of meningitis ratio in adults | ||
| Based on decision trees | Meningitis diagnosis | [167] | |
| Based on machine learning algorithms | Prediction of meningitis outbreaks in the Nigerian population | [161] | |
| Based on the genetic algorithm and decision tree | Distinguishing between bacterial and viral meningitis | [173] | |
| Mathematical model | Meningococcal meningitis | [174] | |
| Mathematical modeling | Bacterial meningitis transmission dynamics with control measures | [175] | |
| Cancer/Gumma/Granuloma | Mining prognosis index based on AI and ML | To identify the optimum prognosis index for brain metastases | [170] |
| Deep learning | Lung cancer histopathology images | [172] | |
| Atypical Behavior | Bayesian model | To diagnose psychiatric disorders | |
| Artificial neural networks using cerebral perfusion SPECT data | To identify Alzheimer’s | [176] | |
| Dynamical bifurcation model based on learned expectation and asymmetry | Bipolar disorders | [177] | |
| Deep neural networks | Anxiety | [178] | |
| Linear discriminant analysis based on ML | Depression | [179] | |
| Random forest | Healthy aging | [180] | |
| Through ML text analysis | Cognitive distortions | [181] | |
| In silico modeling based on support vector machine | Stress | [182] | |
| Multicenter ML | Schizophrenia | [183] |
10.1.4. In Silico Model and Techniques for Atypical Behavior and Neuropsychiatric Symptoms in NS
11. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yuan, J.-L.; Wang, W.-X.; Hu, W.-L. Clinical features of syphilitic myelitis with longitudinally extensive myelopathy on spinal magnetic resonance imaging. World J. Clin. Cases 2019, 7, 1282–1290. [Google Scholar] [CrossRef] [PubMed]
- Kojima, N.; Klausner, J.D. An Update on the Global Epidemiology of Syphilis. Curr. Epidemiol. Rep. 2018, 5, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Keuning, M.W.; Kamp, G.A.; Schonenberg-Meinema, D.; Dorigo-Zetsma, J.W.; van Zuiden, J.M.; Pajkrt, D. Congenital syphilis, the great imitator—case report and review. Lancet Infect. Dis. 2020, 20, e173–e179. [Google Scholar] [CrossRef]
- Spiteri, G.; Unemo, M.; Mårdh, O.; Amato-Gauci, A.J. The resurgence of syphilis in high-income countries in the 2000s: A focus on Europe. Epidemiol. Infect. 2019, 147, e143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, T.; Arima, Y.; Yamagishi, T.; Nishiki, S.; Kanai, M.; Ishikane, M.; Matsui, T.; Sunagawa, T.; Ohnishi, M.; Oishi, K. Rapid Increase in Reports of Syphilis Associated With Men Who Have Sex With Women and Women Who Have Sex With Men, Japan, 2012 to 2016. Sex. Transm. Dis. 2018, 45, 139–143. [Google Scholar] [CrossRef] [Green Version]
- The Lancet. Congenital syphilis in the USA. Lancet 2018, 392, 1168. [Google Scholar] [CrossRef]
- Hussain, S.A.; Vaidya, R. Congenital Syphilis. Clin. Dermatol. 2021, 2, 143–161. Available online: https://www.ncbi.nlm.nih.gov/books/NBK537087/ (accessed on 13 September 2021).
- WHO. WHO Publishes New Estimates on Congenital Syphilis, (n.d.). Available online: https://www.who.int/news/item/26-02-2019-who-publishes-new-estimates-on-congenital-syphilis (accessed on 13 March 2021).
- Jaiswal, A.K.; Tiwari, S.; Jamal, S.B.; de Castro Oliveira, L.; Alves, L.G.; Azevedo, V.; Ghosh, P.; Oliveira, C.J.F.; Soares, S.C. The pan-genome of Treponema pallidum reveals differences in genome plasticity between subspecies related to venereal and non-venereal syphilis. BMC Genom. 2020, 21, 33. [Google Scholar] [CrossRef]
- Landry, T.; Smyczek, P.; Cooper, R.; Gratrix, J.; Bertholet, L.; Read, R.; Romanowski, B.; Singh, A.E. Retrospective review of tertiary and neurosyphilis cases in Alberta, 1973–2017. BMJ Open 2019, 9, e025995. [Google Scholar] [CrossRef] [Green Version]
- Aral, S.O.; Over, M.; Manhart, L.; Holmes, K.K. Sexually Transmitted Infections, Disease Control Priorities in Developing Countries. 2006. Available online: https://www.ncbi.nlm.nih.gov/books/NBK11734/ (accessed on 13 September 2021).
- Buitrago-Garcia, D.; Martí-Carvajal, A.J.; Jimenez, A.; Conterno, L.O.; Pardo, R. Antibiotic therapy for adults with neurosyphilis. Cochrane Database Syst. Rev. 2019, 5, CD011399. [Google Scholar] [CrossRef]
- Peeling, R.W.; Mabey, D.; Kamb, M.L.; Chen, X.-S.; Radolf, J.D.; Benzaken, A.S. Syphilis. Nat. Rev. Dis. Primers 2017, 3, 17073. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Li, G.; Fang, J.; Liu, H.; Yang, B.; Xu, Y. Spinal Intramedullary Syphilitic Gumma: An Unusual Presentation of Neurosyphilis. World Neurosurg. 2016, 95, 622.e17–622.e23. [Google Scholar] [CrossRef]
- Ropper, A.H. Neurosyphilis. N. Engl. J. Med. 2019, 381, 1358–1363. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, H.; Koralnik, I.J.; Marra, C.M. Neurosyphilis. Semin. Neurol. 2019, 39, 448–455. [Google Scholar] [CrossRef]
- Tuddenham, S.; Ghanem, K.G. Neurosyphilis: Knowledge Gaps and Controversies. Sex. Transm. Dis. 2018, 45, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Mejdoubi, A.; Khoulali, M.; Raouzi, N.; Nasri, S.; Mebrouk, Y.; Oulali, N.; Moufid, F. Neurosyphilis revealed by compressive cervical spine syphilitic gumma: A case report. Spinal Cord Ser. Cases 2020, 6, 56. [Google Scholar] [CrossRef]
- Ghanem, K.G. Review: Neurosyphilis: A Historical Perspective and Review. CNS Neurosci. Ther. 2010, 16, e157–e168. [Google Scholar] [CrossRef]
- Li, W.; Jiang, M.; Xu, D.; Kou, C.; Zhang, L.; Gao, J.; Qin, K.; Wu, W.; Zhang, X. Clinical and Laboratory Characteristics of Symptomatic and Asymptomatic Neurosyphilis in HIV-Negative Patients: A Retrospective Study of 264 Cases. BioMed Res. Int. 2019, 2019, 2426313. [Google Scholar] [CrossRef] [Green Version]
- Houston, S.; Cameron, C.E. Treponema pallidum Dissemination; Facilitating Immune Evasion and Bacterial Persistence. In The Pathogenic Spirochetes: Strategies for Evasion of Host Immunity and Persistence; Springer: Boston, MA, USA, 2012; pp. 3–18. ISBN 9781461454045. [Google Scholar] [CrossRef]
- Costiniuk, C.T.; MacPherson, P.A. Neurocognitive and psychiatric changes as the initial presentation of neurosyphilis. CMAJ 2013, 185, 499–503. [Google Scholar] [CrossRef] [Green Version]
- Linne, M.-L. Neuroinformatics and Computational Modelling as Complementary Tools for Neurotoxicology Studies. Basic Clin. Pharm. Toxicol. 2018, 123 (Suppl. S5), 56–61. [Google Scholar] [CrossRef]
- Nayak, L.; Dasgupta, A.; Das, R.; Ghosh, K.; De, R.K. Computational neuroscience and neuroinformatics: Recent progress and resources. J. Biosci. 2018, 43, 1037–1054. [Google Scholar] [CrossRef] [PubMed]
- Hook, E.W. Syphilis. Lancet 2017, 389, 1550–1557. [Google Scholar] [CrossRef]
- Izard, J.; Renken, C.; Hsieh, C.E.; Desrosiers, D.C.; Dunham-Ems, S.; la Vake, C.; Gebhardt, L.L.; Limberger, R.J.; Cox, D.L.; Marko, M.; et al. Cryo-electron tomography elucidates the molecular architecture of Treponema pallidum, the syphilis spirochete. J. Bacteriol. 2009, 191, 7566–7580. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Howell, J.K.; Bradley, S.D.; Zheng, Y.; Zhou, Z.H.; Norris, S.J. Cellular Architecture of Treponema pallidum: Novel Flagellum, Periplasmic Cone, and Cell Envelope as Revealed by Cryo Electron Tomography. J. Mol. Biol. 2010, 403, 546–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlson, J.A.; Dabiri, G.; Cribier, B.; Sell, S. The immunopathobiology of syphilis: The manifestations and course of syphilis are determined by the level of delayed-type hypersensitivity. Am. J. Dermatopathol. 2011, 33, 433–460. [Google Scholar] [CrossRef]
- MacCallum, W.G. A Text-Book of Pathology; WB Saunders Company: Philadelphia, PA, USA, 1924. [Google Scholar]
- Tavora, F.; Burke, A. Review of isolated ascending aortitis: Differential diagnosis, including syphilitic, Takayasu’s and giant cell aortitis. Pathology 2006, 38, 302–308. [Google Scholar] [CrossRef]
- Kofman, O. The changing pattern of neurosyphilis. Can. Med. Assoc. J. 1956, 74, 807–812. Available online: https://pubmed.ncbi.nlm.nih.gov/13316674/ (accessed on 13 September 2021).
- Timmermans, M.; Carr, J. Neurosyphilis in the modern era. J. Neurol. Neurosurg. Psychiatry 2004, 75, 1727–1730. [Google Scholar] [CrossRef] [Green Version]
- Hooshmand, H.; Escobar, M.R.; Kopf, S.W. Neurosyphilis: A study of 241 patients. JAMA 1972, 219, 726–729. [Google Scholar] [CrossRef]
- Lukehart, S.A.; Hook, E.W.; Baker-Zander, S.A.; Collier, A.C.; Critchlow, C.W.; Handsfield, H.H. Invasion of the central nervous system by Treponema pallidum: Implications for diagnosis and treatment. Ann. Intern. Med. 1988, 109, 855–862. [Google Scholar] [CrossRef]
- Domantay-Apostol, G.P.; Handog, E.B.; Gabriel, M.T.G. Syphilis: The international challenge of the great imitator. Dermatol. Clin. 2008, 26, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Peeling, R.W.; Hook, E.W. The pathogenesis of syphilis: The Great Mimicker, revisited. J. Pathol. 2006, 208, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhou, X.; Cai, J.; Zhao, F.; Cao, T.; Ning, L.; Luo, C.; Xiao, X.; Liu, S. Recombinant Treponema pallidum protein Tp0768 promotes proinflammatory cytokine secretion of macrophages through ER stress and ROS/NF-κB pathway. Appl. Microbiol. Biotechnol. 2021, 105, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Huang, X.; Shi, M.; Luo, L.; Tao, C. Diagnostic role of CXCL13 and CSF serology in patients with neurosyphilis. Sex. Transm. Infect. 2021, 97, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Embers, M.E. The pathogenic spirochetes: Strategies for evasion of host immunity and persistence. In The Pathogenic Spirochetes: Strategies for Evasion of Host Immunity and Persistence; Springer Science & Business Media: Berlin, Germany, 2013; pp. 1–265. [Google Scholar] [CrossRef]
- Barichello, T.; Generoso, J.S.; Milioli, G.; Elias, S.G.; Teixeira, A.L. Pathophysiology of bacterial infection of the central nervous system and its putative role in the pathogenesis of behavioral changes. Rev. Bras. Psiquiatr. 2013, 35, 81–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christodoulides, A.; Boyadjian, A.; Kelesidis, T. Spirochetal Lipoproteins and Immune Evasion. Front. Immunol. 2017, 8, 364. [Google Scholar] [CrossRef] [Green Version]
- Cumberland, M.C.; Turner, T.B. The rate of multiplication of Treponema pallidum in normal and immune rabbits. Am. J. Syph. Gonorrhea Vener. Dis. 1949, 33, 201–212. [Google Scholar]
- Raiziss, G.W.; Severac, M. Rapidity with which spirochaeta pallida invades the blood stream. Arch. Dermatol. Syphilol. 1937, 35, 1101–1109. [Google Scholar] [CrossRef]
- Riviere, G.R.; Thomas, D.D.; Cobb, C.M. In vitro model of Treponema pallidum invasiveness. Infect. Immun. 1989, 57, 2267–2271. [Google Scholar] [CrossRef] [Green Version]
- Thomas, D.D.; Navab, M.; Haake, D.A.; Fogelman, A.M.; Miller, J.N.; Lovett, M.A. Treponema pallidum invades intercellular junctions of endothelial cell monolayers. Proc. Natl. Acad. Sci. USA 1988, 85, 3608–3612. [Google Scholar] [CrossRef] [Green Version]
- Thomas, D.D.; Fogelman, A.M.; Miller, J.N.; Lovett, M.A. Interactions of Treponema pallidum with endothelial cell monolayers. Eur. J. Epidemiol. 1989, 5, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Salazar, J.C.; Hazlett, K.R.; Radolf, J.D. The immune response to infection with Treponema pallidum, the stealth pathogen. Microbes Infect. 2002, 4, 1133–1140. [Google Scholar] [CrossRef]
- LaFond, R.E.; Lukehart, S.A. Biological basis for syphilis. Clin. Microbiol. Rev. 2006, 19, 29–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radolf, J.D.; Deka, R.K.; Anand, A.; Šmajs, D.; Norgard, M.V.; Yang, X.F. Treponema pallidum, the syphilis spirochete: Making a living as a stealth pathogen. Nat. Rev. Microbiol. 2016, 14, 744–759. [Google Scholar] [CrossRef] [Green Version]
- Fraser, C.M.; Norris, S.J.; Weinstock, G.M.; White, O.; Sutton, G.G.; Dodson, R.; Gwinn, M.; Hickey, E.K.; Clayton, R.; Ketchum, K.A.; et al. Complete genome sequence of Treponema pallidum, the syphilis spirochete. Science 1998, 281, 375–388. [Google Scholar] [CrossRef]
- Cruz, A.R.; Ramirez, L.G.; Zuluaga, A.V.; Pillay, A.; Abreu, C.; Valencia, C.A.; la Vake, C.; Cervantes, J.L.; Dunham-Ems, S.; Cartun, R.; et al. Immune Evasion and Recognition of the Syphilis Spirochete in Blood and Skin of Secondary Syphilis Patients: Two Immunologically Distinct Compartments. PLoS Negl. Trop. Dis. 2012, 6, e1717. [Google Scholar] [CrossRef] [PubMed]
- Pulzova, L.; Bhide, M.R.; Andrej, K. Pathogen translocation across the blood-brain barrier. FEMS Immunol. Med. Microbiol. 2009, 57, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S. Mechanisms of microbial traversal of the blood–brain barrier. Nat. Rev. Microbiol. 2008, 6, 625–634. [Google Scholar] [CrossRef] [Green Version]
- Coureuil, M.; Lecuyer, H.; Bourdoulous, S.; Nassif, X. A journey into the brain: Insight into how bacterial pathogens cross blood-brain barriers. Nat. Rev. Microbiol. 2017, 15, 149–159. [Google Scholar] [CrossRef]
- Daneman, R.; Prat, A. The Blood–Brain Barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef] [Green Version]
- Katt, M.E.; Linville, R.M.; Mayo, L.N.; Xu, Z.S.; Searson, P.C. Functional brain-specific microvessels from iPSC-derived human brain microvascular endothelial cells: The role of matrix composition on monolayer formation. Fluids Barriers CNS 2018, 15, 7. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.D.; Winkler, E.A.; Singh, I.; Sagare, A.P.; Deane, R.; Wu, Z.; Holtzman, D.M.; Betsholtz, C.; Armulik, A.; Sallstrom, J.; et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature 2012, 485, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Mucke, L.; Eddleston, M. Astrocytes in infectious and immune-mediated diseases of the central nervous system. FASEB J. 1993, 7, 1226–1232. [Google Scholar] [CrossRef] [Green Version]
- Tkáčová, Z.; Káňová, E.; Jiménez-Munguía, I.; Čomor, Ľ.; Širochmanová, I.; Bhide, K.; Bhide, M. Crossing the Blood-Brain Barrier by Neuroinvasive Pathogens. Folia Vet. 2018, 62, 44–51. [Google Scholar] [CrossRef] [Green Version]
- Church, B.; Wall, E.; Webb, J.R.; Cameron, C.E. Interaction of Treponema pallidum, the syphilis spirochete, with human platelets. PLoS ONE 2019, 14, e0210902. [Google Scholar] [CrossRef] [Green Version]
- Forrester, J.V.; McMenamin, P.G.; Dando, S.J. CNS infection and immune privilege. Nat. Rev. Neurosci. 2018, 19, 655–671. [Google Scholar] [CrossRef]
- Cameron, C.E. Identification of a Treponema pallidum laminin-binding protein. Infect. Immun. 2003, 71, 2525–2533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houston, S.; Hof, R.; Francescutti, T.; Hawkes, A.; Boulanger, M.J.; Cameron, C.E. Bifunctional role of the Treponema pallidum extracellular matrix binding adhesin Tp0751. Infect. Immun. 2011, 79, 1386–1398. [Google Scholar] [CrossRef] [Green Version]
- Lithgow, K.V.; Church, B.; Gomez, A.; Tsao, E.; Houston, S.; Swayne, L.A.; Cameron, C.E. Identification of the Neuroinvasive Pathogen Host Target, LamR, as an Endothelial Receptor for the Treponema pallidum Adhesin Tp0751. MSphere 2020, 5, e00195-20. [Google Scholar] [CrossRef] [Green Version]
- Sell, S.; Salman, J.; Norris, S.J. Reinfection of chancre-immune rabbits with Treponema pallidum. I. Light and immunofluorescence studies. Am. J. Pathol. 1985, 118, 248–255. [Google Scholar]
- Edmondson, D.G.; Hu, B.; Norris, S.J. Long-Term In Vitro Culture of the Syphilis Spirochete Treponema pallidum subsp. Pallidum. MBio 2018, 9, e01153-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pulzová, L.; Mlynárčik, P.; Bencúrová, E.; Bhide, M. It Takes Two to Tango: Protein-Protein Interactions in the Translocation of Pathogens Across. In The Blood-Brain Barrier: New Research, 1st ed.; Montenegro, P.A., Juárez, S.M., Eds.; Nova Science Publishers: New York, NY, USA, 2012; pp. 79–116. [Google Scholar]
- Hawley, K.L.; Cruz, A.R.; Benjamin, S.J.; la Vake, C.J.; Cervantes, J.L.; LeDoyt, M.; Ramirez, L.G.; Mandich, D.; Fiel-Gan, M.; Caimano, M.J.; et al. IFNγ Enhances CD64-Potentiated Phagocytosis of Treponema pallidum Opsonized with Human Syphilitic Serum by Human Macrophages. Front. Immunol. 2017, 8, 1227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, B.-F.; Wang, Q.-Q.; Zhang, J.-P.; Hu, W.-L.; Zhang, R.-L. Treponema pallidum induces the activation of endothelial cells via macrophage-derived exosomes. Arch. Dermatol. Res. 2019, 311, 121–130. [Google Scholar] [CrossRef]
- Duffy, S.S.; Keating, B.A.; Perera, C.J.; Moalem-Taylor, G. The role of regulatory T cells in nervous system pathologies. J. Neurosci. Res. 2018, 96, 951–968. [Google Scholar] [CrossRef] [PubMed]
- Dowling, M.R.; Kan, A.; Heinzel, S.; Marchingo, J.M.; Hodgkin, P.D.; Hawkins, E.D. Regulatory T Cells Suppress Effector T Cell Proliferation by Limiting Division Destiny. Front. Immunol. 2018, 96, 2461. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Cheng, Y.; Wang, Y.; Wang, C.; Lu, H.; Guan, Z.; Huang, J.; Gong, W.; Shi, M.; Ni, L.; et al. Aberrant Humoral Immune Responses in Neurosyphilis: CXCL13/CXCR5 Play a Pivotal Role for B-Cell Recruitment to the Cerebrospinal Fluid. J. Infect. Dis. 2017, 216, 534–544. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Zhu, L.; Gao, Z.; Guan, Z.; Lu, H.; Shi, M.; Gao, Y.; Xu, H.; Yang, X.F.; Zhou, P. Increased Interleukin-17 in Peripheral Blood and Cerebrospinal Fluid of Neurosyphilis Patients. PLoS Negl. Trop. Dis. 2014, 8, e3004. [Google Scholar] [CrossRef]
- Li, K.; Wang, C.; Lu, H.; Gu, X.; Guan, Z.; Zhou, P. Regulatory T Cells in Peripheral Blood and Cerebrospinal Fluid of Syphilis Patients with and without Neurological Involvement. PLoS Negl. Trop. Dis. 2013, 7, e2528. [Google Scholar] [CrossRef] [Green Version]
- Santos, A.M.G.; Júnior, V.R.D.S.; Melo, F.L.D.; Aquino, A.E.C.D.A.; Ramos, M.O.A.; Araújo, L.M.; de Lira, C.R.; Sobral, P.M.; Figueiroa, F.; de Melo, H.R.L.; et al. Prevalence and risk factors of syphilis and human immunodeficiency virus co-infection at a university hospital in Brazil. Rev. Soc. Bras. Med. Trop. 2018, 51, 813–818. [Google Scholar] [CrossRef]
- Guo, N.; Liu, L.; Yang, X.; Song, T.; Li, G.; Li, L.; Jiang, T.; Gao, Y.; Zhang, T.; Su, B.; et al. Immunological Changes in Monocyte Subsets and Their Association With Foxp3+ Regulatory T Cells in HIV-1-Infected Individuals with Syphilis: A Brief Research Report. Front. Immunol. 2019, 10, 714. [Google Scholar] [CrossRef]
- Solomon, H.; Moraes, A.N.; Williams, D.B.; Fotso, A.S.; Duong, Y.T.; Ndongmo, C.B.; Voetsch, A.C.; Patel, H.; Lupoli, K.; McAuley, J.B.; et al. Prevalence and correlates of active syphilis and HIV co-Infection among sexually active persons aged 15–59 years in Zambia: Results from the Zambia Population-based HIV Impact Assessment (ZAMPHIA) 2016. PLoS ONE 2020, 15, e0236501. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, C.; Osbak, K.K.; Crucitti, T.; Kestens, L. The immunological response to syphilis differs by HIV status; a prospective observational cohort study. BMC Infect. Dis. 2017, 17, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barragán, E.P.; Hernández, E.U.; Orozco, B.P.; González, M.S. Meningovascular neurosyphilis with basilar artery thrombosis in HIV patient. J. Infect. Public Health 2018, 11, 439–441. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, S.; Burrascano, J. Neurosyphilis: An Unresolved Case of Meningitis. Case Rep. Infect. Dis. 2015, 2015, 634259. [Google Scholar] [CrossRef]
- Pastuszczak, M.; Zeman, J.; Jaworek, A.K.; Wojas-Pelc, A. Cerebrospinal Fluid Abnormalities in HIV-Negative Patients with Secondary and Early Latent Syphilis and Serum VDRL ≥ 1:32. Indian J. Dermatol. 2013, 58, 325. [Google Scholar] [CrossRef]
- Zamora, J.A.G.; Espinoza, L.A.; Nwanyanwu, R.N. Neurosyphilis with Concomitant Cryptococcal and Tuberculous Meningitis in a Patient with AIDS: Report of a Unique Case. Case Rep. Infect. Dis. 2017, 2017, 4103858. [Google Scholar] [CrossRef] [Green Version]
- Ha, T.; Tadi, P.; Dubensky, L. Neurosyphilis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Thibodeau, R.; Goel, A.; Jafroodifar, A.; Klumpp, M.; Mirchia, K.; Swarnkar, A. Cerebral syphilitic gumma presenting with intracranial gumma and pathologic vertebrae fractures. Radiol. Case Rep. 2021, 16, 916–922. [Google Scholar] [CrossRef]
- Nitrini, R.; de Paiva, A.R.B.; Takada, L.T.; Brucki, S.M.D. Did you rule out neurosyphilis? Dement. Neuropsychol. 2010, 4, 338–345. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Wu, W. Neurosyphilis presenting with myelitis-case series and literature review. J. Infect. Chemother. 2020, 26, 296–299. [Google Scholar] [CrossRef]
- Dong, H.; Liu, Z.; Duan, Y.; Li, D.; Qiu, Z.; Liu, Y.; Huang, J.; Wang, C. Syphilitic meningomyelitis misdiagnosed as spinal cord tumor: Case and review. J. Spinal Cord Med. 2019, 44, 789–793. [Google Scholar] [CrossRef]
- Daumas, A.; Coiffard, B.; Chartier, C.; Amara, A.B.; Alingrin, J.; Villani, P.; Mege, J.-L. Defective Granuloma Formation in Elderly Infected Patients. Front. Cell. Infect. Microbiol. 2020, 10, 189. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Liu, J.; Zhang, W.; Xu, Z.; Hou, H. The Application of MR Spectroscopy and MR Perfusion in Cerebral Syphilitic Gumma: A Case Report. Front. Neurosci. 2020, 14, 544802. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.; Huang, K.; Jiang, T.; Zhou, G.; Wu, T. Cerebral syphilitic gumma masquerading as cerebral metastatic tumors: Case report. Neurosurg. Focus 2019, 47, E15. [Google Scholar] [CrossRef] [PubMed]
- Ying, S.; Li-Min, L. Cerebral syphilis mimicking metastatic tumors: Report and review of the literature. Neurol. India 2018, 66, 1170. [Google Scholar] [CrossRef] [PubMed]
- Barahona, E.A.; Olvera, J.L.N.; Liquidano, M.A.E.; Cobos, A.M.; Arroyo, Á.D.R.; Apo, E.G. Left temporal cerebral syphilitic gumma: Case report and literature review. Rev. Médica Del Hosp. Gen. México 2017, 80, 119–124. [Google Scholar] [CrossRef]
- Beauchemin, P.; Laforce, R. Neurocognitive Changes in Tertiary Neurosyphilis: A Retrospective Chart Review. Can. J. Neurol. Sci. 2014, 41, 452–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crozatti, L.L.; de Brito, M.H.; Lopes, B.N.A.; de Campos, F.P.F. Atypical behavioral and psychiatric symptoms: Neurosyphilis should always be considered. Autops. Case Rep. 2015, 5, 34–47. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, H.; Hou, L.; Zhong, X.; Chen, X.; Li, L.; Wu, Z.; Zheng, D.; Zhang, Y.; Tan, Y.; et al. Clinical and neuropsychological characteristics of general paresis misdiagnosed as primary psychiatric disease. BMC Psychiatry 2016, 16, 230. [Google Scholar] [CrossRef] [Green Version]
- Swain, K. ‘Extraordinarily arduous and fraught with danger’: Syphilis, Salvarsan, and general paresis of the insane. Lancet Psychiatry 2018, 5, 702–703. [Google Scholar] [CrossRef]
- Sharma, S.R.; Hussain, M.; Roy, D. General Paresis of Insane: A Forgotten Entity. Neurol. India 2020, 68, 487. [Google Scholar] [CrossRef]
- Read, P.J.; Donovan, B. Clinical aspects of adult syphilis. Intern. Med. J. 2012, 42, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Antunes, A.P.; Dias, M.C.; Verdelho, A. Neuropsychiatric Symptoms in Reversible Dementias; Springer International Publishing: Basel, Switzerland, 2017; pp. 93–139. [Google Scholar] [CrossRef]
- Mehrabian, S.; Raycheva, M.; Traykova, M.; Stankova, T.; Penev, L.; Grigorova, O.; Traykov, L. Neurosyphilis with dementia and bilateral hippocampal atrophy on brain magnetic resonance imaging. BMC Neurol. 2012, 12, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.H.; Hsu, J.W.; Huang, K.L.; Bai, Y.M.; Ko, N.Y.; Su, T.P.; Li, C.T.; Lin, W.C.; Tsai, S.J.; Pan, T.L.; et al. Sexually Transmitted Infection Among Adolescents and Young Adults With Attention-Deficit/Hyperactivity Disorder: A Nationwide Longitudinal Study. J. Am. Acad. Child Adolesc. Psychiatry 2018, 57, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Saini, H.S.; Sayre, M.; Saini, I.; Elsharkawy, N. Neurosyphilis Presenting as Intermittent Explosive Disorder and Acute Psychosis. Cureus 2019, 11, e6337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Bruijn, S.; Kenyon, C.; Léonard, N.; Vlieghe, E. The big imitator strikes again: A case report of neurosyphilis in a patient with newly diagnosed HIV. Acta Clin. 2017, 72, 372–374. [Google Scholar] [CrossRef] [PubMed]
- Prynn, J.; Hussain, A.; Winnett, A. Diagnosing neurosyphilis: A case of confusion. BMJ Case Rep. 2016, 2016, bcr2016216582. [Google Scholar] [CrossRef] [PubMed]
- Seo, E.H.; Yang, H.J.; Kim, S.H.; Park, J.H.; Yoon, H.-J. Psychotic mania as the solitary manifestation of neurosyphilis. Ann. Gen. Psychiatry 2018, 17, 24. [Google Scholar] [CrossRef] [Green Version]
- Neetu, R.; Sukhani, P.K.; Dubey, R. Neurosyphilis—A Forgotten Disease: Case Reports with Ten Years Follow-Up and Review of Literature. Neurol. India 2020, 68, 889–893. [Google Scholar] [CrossRef]
- Nguyen, A.; Berngard, S.C.; Lopez, J.P.; Jenkins, T.C. A Case of Ocular Syphilis in a 36-Year-Old HIV-Positive Male. Case Rep. Infect. Dis. 2014, 2014, 352047. [Google Scholar] [CrossRef]
- Muylaert, B.; Almeidinha, Y.; Borelli, N.; Esteves, E.; Oliveira, A.R.; Cestari, M.; Garbelini, L.; Eid, R.; Michalany, A.; De Oliveira Filho, J. Malignant syphilis and neurosyphilis in an immunocompetent patient. J. Am. Acad. Dermatol. 2016, 74, AB152. [Google Scholar] [CrossRef]
- Su, S.; Mao, L.; Zhao, J.; Chen, L.; Jing, J.; Cheng, F.; Zhang, L. Epidemics of HIV, HCV and syphilis infection among synthetic drugs only users, heroin-only users and poly-drug users in Southwest China. Sci. Rep. 2018, 8, 6615. [Google Scholar] [CrossRef] [PubMed]
- Boyle, A.; Zafar, R.; Riley, V.; Lindesay, J. Neurosyphilis presenting with dissociative symptoms. J. Neurol. Neurosurg. Psychiatry. 1995, 59, 452–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borges, C.R.; de Almeida, S.M.; Sue, K.; Koslyk, J.L.A.; Sato, M.T.; Shiokawa, N.; Teive, H.A.G. Neurosyphilis and ocular syphilis clinical and cerebrospinal fluid characteristics: A case series. Arq. De Neuro-Psiquiatria. 2018, 76, 373–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arain, Z.; Abbas, Y.; Adams, A. Pediatric otosyphilis—An unusual cause of conductive hearing loss. Radiol. Case Rep. 2020, 15, 65–70. [Google Scholar] [CrossRef]
- De Goffau, M.J.; Doelman, J.C.; van Rijswijk, J.B. Unilateral sudden hearing loss due to otosyphilis. Clin. Pract. 2011, 1, 296–298. [Google Scholar] [CrossRef]
- Eren, F.; Aygül, R.; Ekmekci, H.; Öztürk, Ş. Neurosyphilis Presenting with Ptosis and Diplopia as the First Complaints: Case Report. Turk. J. Neurol. 2018, 24, 330–333. [Google Scholar] [CrossRef]
- Pozzobon, T.; Facchinello, N.; Bossi, F.; Capitani, N.; Benagiano, M.; di Benedetto, G.; Zennaro, C.; West, N.; Codolo, G.; Bernardini, M.; et al. Treponema pallidum (syphilis) antigen TpF1 induces angiogenesis through the activation of the IL-8 pathway. Sci. Rep. 2016, 6, 18785. [Google Scholar] [CrossRef] [Green Version]
- Akinci, E.; Oncu, F.; Topcular, B. Neurosyphilis in Psychiatric Settings: Three Case Reports. Turk. J. Psychiatry 2016, 28, 61–66. [Google Scholar] [CrossRef] [Green Version]
- Tatar, Z.B.; Cansiz, A.; Köksal, A.; Kurt, E. A Case of Neurosyphilis Presenting with Dementia and Psychiatric Symptoms. J. Neuropsychiatry Clin. Neurosci. 2014, 26, E39–E40. [Google Scholar] [CrossRef]
- Blažeković, A.; Ozretić, D.; Habek, M.; Bilić, E.; Borovečki, F. Neurosyphilis: The shape of a rising threat. Int. J. Infect. Dis. 2018, 76, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.F.; Wang, L.Y.; Chiang, J.H.; Shen, Y.C. Bipolar Disorder Is Associated With an Increased Risk of Sexually Transmitted Infections: A Nationwide Population-based Cohort Study. Sex. Transm. Dis. 2018, 45, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-R.; Zhang, H.-L.; Huang, S.-J.; Zeng, Y.-L.; Xi, Y.; Guo, X.-J.; Liu, G.-L.; Tong, M.-L.; Zheng, W.-H.; Liu, L.-L.; et al. Psychiatric Manifestations as Primary Symptom of Neurosyphilis Among HIV-Negative Patients. J. Neuropsychiatry Clin. Neurosci. 2014, 26, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, L.J.D.; Marchiori, E.; Bahia, P.R.V. Neurosyphilis manifesting as trigeminal nerve dysfunction. Rev. Soc. Bras. Med. Trop. 2018, 51, 404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bezalely, S.; Jacob, G.; Flusser, G.; Ablin, J. Syphilis: An unusual manifestation? Case Rep. 2014, 2014, bcr2014204871. [Google Scholar] [CrossRef] [Green Version]
- Wagemakers, A.; Hepp, D.; Killestein, J.; Peferoen, L.; Ang, W. Acute syphilitic meningitis in an HIV-infected patient. IDCases 2018, 13, e00423. [Google Scholar] [CrossRef]
- Munshi, S.; Raghunathan, S.K.; Lindeman, I.; Shetty, A.K. Meningovascular syphilis causing recurrent stroke and diagnostic difficulties: A scourge from the past. BMJ Case Rep. 2018, 2018, bcr2018225255. [Google Scholar] [CrossRef]
- He, D.; Jiang, B. Syphilitic myelitis: Magnetic resonance imaging features. Neurol. India 2014, 62, 89–91. [Google Scholar] [CrossRef]
- Xi, Y.; Liang, Z.; Zhang, S.; Chu, M.; Lu, Y. MRI of neurosyphilis presenting as brain tumor: A case report. Radiol. Infect. Dis. 2015, 2, 197–200. [Google Scholar] [CrossRef] [Green Version]
- Koizumi, Y.; Watabe, T.; Ota, Y.; Nakayama, S.I.; Asai, N.; Hagihara, M.; Yuka, Y.; Hiroyuki, S.; Toyonori, T.; Masakazu, T. Cerebral Syphilitic Gumma Can Arise Within Months of Reinfection: A Case of Histologically Proven Treponema pallidum Strain Type 14b/f Infection With Human Immunodeficiency Virus Positivity. Sex. Transm. Dis. 2018, 45, e1–e4. [Google Scholar] [CrossRef]
- Yoon, Y.K.; Kim, M.J.; Chae, Y.S.; Kang, S.-H. Cerebral Syphilitic Gumma Mimicking a Brain Tumor in the Relapse of Secondary Syphilis in a Human Immunodeficiency Virus-Negative Patient. J Korean Neurosurg. Soc. 2013, 53, 197–200. [Google Scholar] [CrossRef]
- Li, C.; Wang, S.; Tang, G.; Liu, L.; Chen, G. Neuroimaging findings of cerebral syphilitic gumma. Exp. Ther. Med. 2019, 18, 4185–4192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.; Han, F. Neurosyphilis complicated with pial arteriovenous fistula: A rare case report. Medicine 2019, 98, e17770. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Xu, Z.; Hou, H. Diagnosis and Treatment of Spinal Syphilitic Gumma: A Case Report. Front. Neurol. 2020, 10, 1352. [Google Scholar] [CrossRef]
- Sasaki, R.; Tanaka, N.; Okazaki, T.; Yonezawa, T. Multiple cerebral syphilitic gummas mimicking brain tumor in a non-HIV-infected patient: A case report. J. Infect. Chemother. 2019, 25, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Luscombe, N.M.; Greenbaum, D.; Gerstein, M. What is bioinformatics? An introduction and overview. Yearb. Med. Inform. 2018, 10, 83–100. [Google Scholar] [CrossRef] [Green Version]
- Kasabov, N. Springer Handbook of Bio-/Neuroinformatics; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Bilotta, M.; Tradigo, G.; Veltri, P. Bioinformatics data models, representation and storage. Encycl. Bioinform. Comput. Biol. ABC Bioinform. 2018, 1, 110–116. [Google Scholar] [CrossRef]
- Morse, T.M. Neuroinformatics: From bioinformatics to databasing the brain. Bioinform. Biol. Insights 2008, 2, BBI.S540. [Google Scholar] [CrossRef] [Green Version]
- Nazipova, N.N.; Isaev, E.A.E.; Kornilov, V.V.; Pervukhin, D.V.E.; Morozova, A.A.E.; Gorbunov, A.A.; Ustinin, M.N. Big Data in Bioinformatics. Mat. Biolog. Bioinform. 2017, 12, 102–119. [Google Scholar] [CrossRef]
- Jaiswal, A.K.; Tiwari, S.; Tavares, G.C.; da Silva, W.M.; Oliveira, L.D.; Ibraim, I.C.; Guimarães, L.C.; Gomide, A.C.P.; Jamal, S.B.; Pantoja, Y.; et al. Pan-omics focused to Crick’s central dogma. In Pan-Genomics: Applications, Challenges, and Future Prospects; Elsevier: Amsterdam, Netherlands, 2020. [Google Scholar] [CrossRef]
- Kiernan, M.C. A fine neuroscience vintage. J. Neurol. Neurosurg. Psychiatry 2015, 86, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Érdi, P. Teaching computational neuroscience. Cogn. Neurodyn. 2015, 9, 479–485. [Google Scholar] [CrossRef] [Green Version]
- Wood, H. A rapid e-volution. Nat. Rev. Neurol. 2011, 7, 415. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, G.M.; Mirsky, J.S.; Healy, M.D.; Singer, M.S.; Skoufos, E.; Hines, M.S.; Nadkarni, P.M.; Miller, P.L. The Human Brain Project: Neuroinformatics tools for integrating, searching and modeling multidisciplinary neuroscience data. Trends Neurosci. 1998, 21, 460–468. [Google Scholar] [CrossRef]
- Polavaram, S.; Ascoli, G. Neuroinformatics. Scholarpedia 2015, 10. [Google Scholar] [CrossRef]
- Trappenberg, T. Foundations of Computational Neuroscience, 2nd ed; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Thomas, J.I. Current Status of Consciousness Research from the Neuroscience Perspective. Acta Sci. Neurol. 2019, 2, 38–44. [Google Scholar]
- Jangid, A.; Chaudhary, L.; Sharma, K. Computational Neuroscience and Its Applications: A Review. Intell. Energy Manag. Technol. 2021, 1, 159–169. [Google Scholar] [CrossRef]
- Marra, C.M. Other central nervous system infections: Cytomegalovirus, Mycobacterium tuberculosis, and Treponema pallidum. Handb. Clin. Neurol. 2018, 152, 151–166. [Google Scholar] [CrossRef]
- Singh, A.E. Ocular and neurosyphilis: Epidemiology and approach to management. Curr. Opin. Infect. Dis. 2020, 33, 66–72. [Google Scholar] [CrossRef]
- Yasaka, K.; Abe, O. Deep learning and artificial intelligence in radiology: Current applications and future directions. PLoS Med. 2018, 15, e1002707. [Google Scholar] [CrossRef] [Green Version]
- Senders, J.T.; Arnaout, O.; Karhade, A.V.; Dasenbrock, H.H.; Gormley, W.B.; Broekman, M.L.; Smith, T.R. Natural and Artificial Intelligence in Neurosurgery: A Systematic Review. Neurosurgery 2018, 83, 181–192. [Google Scholar] [CrossRef] [Green Version]
- Segato, A.; Marzullo, A.; Calimeri, F.; de Momi, E. Artificial intelligence for brain diseases: A systematic review. APL Bioeng. 2020, 4, 041503. [Google Scholar] [CrossRef]
- Agrebi, S.; Larbi, A. Use of artificial intelligence in infectious diseases. Artif. Intell. Precis. Health 2020, 415–438. [Google Scholar] [CrossRef]
- Valliani, A.A.-A.; Ranti, D.; Oermann, E.K. Deep Learning and Neurology: A Systematic Review. Neurol. Ther. 2019, 8, 351–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alsenan, S.; Al-Turaiki, I.; Hafez, A. A Recurrent Neural Network model to predict blood–brain barrier permeability. Comput. Biol. Chem. 2020, 89, 107377. [Google Scholar] [CrossRef]
- Sivandzade, F.; Cucullo, L. In-vitro blood–brain barrier modeling: A review of modern and fast-advancing technologies. J. Cereb. Blood Flow Metab. 2018, 38, 1667–1681. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Ge, H.; Su, X.; Wang, R.; Zeng, J.; Miao, J. High HbA1c level is correlated with blood-brain barrier disruption in syphilis patients. Neurol. Sci. 2019, 41, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Helms, H.C.; Abbott, N.J.; Burek, M.; Cecchelli, R.; Couraud, P.-O.; Deli, M.A.; Förster, C.; Galla, H.J.; Romero, I.A.; Shusta, E.V.; et al. In vitro models of the blood–brain barrier: An overview of commonly used brain endothelial cell culture models and guidelines for their use. J. Cereb. Blood Flow Metab. 2016, 36, 862–890. [Google Scholar] [CrossRef]
- Saber, R.; Rihana, S.; Mhanna, R. In silico and in vitro Blood-Brain Barrier models for early stage drug discovery. In Proceedings of the International Conference on Advances in Biomedical Engineering, ICABME, Tripoli, Lebanon, 17–19 October 2019. [Google Scholar] [CrossRef]
- Chai, Q.; He, W.Q.; Zhou, M.; Lu, H.; Fu, Z.F. Enhancement of blood-brain barrier permeability and reduction of tight junction protein expression are modulated by chemokines/cytokines induced by rabies virus infection. J. Virol. 2014, 88, 4698–4710. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.; Divakaran, R.; Konda, L.S.K.; Kristam, R. A classification model for blood brain barrier penetration. J. Mol. Graph. Model. 2020, 96, 107516. [Google Scholar] [CrossRef]
- Tian, X.; Xu, Q.; Wang, Y. Prediction of Meningitis Outbreaks in Nigeria Using Machine Learning Algorithms. In Proceedings of the 2019 2nd Artificial Intelligence and Cloud Computing Conference, Kobe, Japan, 21–23 December 2019. [Google Scholar] [CrossRef]
- Merline, W. Risk Factors of Meningitis in Adults-An Analysis Using Fuzzy Cognitive Map with TOPSIS. Int. J. Sci. Innov. Math. Res. 2014, 2, 418–425. Available online: www.arcjournals.org (accessed on 15 September 2021).
- Langarizadeh, M.; Khajehpour, E.; Khajehpour, H.; Farokhnia, M.; Eftekhari, M. A Fuzzy Expert System for Distinguishing between Bacterial and Aseptic Meningitis. Iran. J. Med. Phys. 2015, 12, 1–6. [Google Scholar] [CrossRef]
- Oye, N.D.; Thomas, L.L. Fuzzy Model for Diagnosis of Bacterial Meningitis. Int. J. Comput. Appl. Technol. Res. 2019, 8, 33–51. [Google Scholar] [CrossRef]
- Abubakar, A.M.; Abdulsalam, K.A.; Adebisi, J.A. Application of Artificial Neural Network for Diagnosis of Cerebrospinal Meningitis. J. Eng. Res. 2019, 24, 12–25. Available online: http://jer.unilag.edu.ng/article/view/575 (accessed on 15 September 2021).
- Zaccari, K.; Marujo, E.C. Machine Learning for Aiding Meningitis Diagnosis in Pediatric Patients. Int. J. Med. Health Sci. 2019, 13, 411–419. [Google Scholar] [CrossRef]
- Lelis, V.M.; Guzman, E.; Belmonte, M.V. Non-invasive meningitis diagnosis using decision trees. IEEE Access 2020, 8, 18394–18407. [Google Scholar] [CrossRef]
- Alile, S.; Bello, M. A Machine Learning Approach for Diagnosing Meningococcal Meningitis. Int. J. Sci. Res. Comput. Sci. Eng. 2020, 8, 13–25. [Google Scholar]
- Huang, S.; Yang, J.; Fong, S.; Zhao, Q. Artificial intelligence in cancer diagnosis and prognosis: Opportunities and challenges. Cancer Lett. 2020, 471, 61–71. [Google Scholar] [CrossRef]
- Huang, S.; Yang, J.; Fong, S.; Zhao, Q. Mining Prognosis Index of Brain Metastases Using Artificial Intelligence. Cancers 2019, 11, 1140. [Google Scholar] [CrossRef] [Green Version]
- Marino, S.; Cilfone, N.A.; Mattila, J.T.; Linderman, J.J.; Flynn, J.L.; Kirschner, D.E. Macrophage polarization drives granuloma outcome during Mycobacterium tuberculosis infection. Infect. Immun. 2015, 83, 324–338. [Google Scholar] [CrossRef] [Green Version]
- Coudray, N.; Ocampo, P.S.; Sakellaropoulos, T.; Narula, N.; Snuderl, M.; Fenyö, D.; Moreira, A.L.; Razavian, N.; Tsirigos, A. Classification and mutation prediction from non–small cell lung cancer histopathology images using deep learning. Nat. Med. 2018, 24, 1559–1567. [Google Scholar] [CrossRef]
- D’Angelo, G.; Pilla, R.; Tascini, C.; Rampone, S. A proposal for distinguishing between bacterial and viral meningitis using genetic programming and decision trees. Soft Comput. 2019, 23, 11775–11791. [Google Scholar] [CrossRef]
- Martínez, M.J.F.; Merino, E.G.; Sánchez, E.G.; Sánchez, J.E.G.; del Rey, A.M.; Sánchez, G.R. A Mathematical Model to Study the Meningococcal Meningitis. Procedia Comput. Sci. 2013, 18, 2492–2495. [Google Scholar] [CrossRef] [Green Version]
- Asamoah, J.K.K.; Nyabadza, F.; Seidu, B.; Chand, M.; Dutta, H. Mathematical Modelling of Bacterial Meningitis Transmission Dynamics with Control Measures. Comput. Math. Methods Med. 2018, 2018, 2657461. [Google Scholar] [CrossRef] [Green Version]
- Świetlik, D.; Białowąs, J. Application of Artificial Neural Networks to Identify Alzheimer’s Disease Using Cerebral Perfusion SPECT Data. Int. J. Environ. Res. Public Health 2019, 16, 1303. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.-S.; Chou, T. A Dynamical Bifurcation Model of Bipolar Disorder Based on Learned Expectation and Asymmetry in Mood Sensitivity. Comput. Psychiatry 2018, 2, 205. [Google Scholar] [CrossRef]
- Tran, T.; Kavuluru, R. Predicting mental conditions based on “history of present illness” in psychiatric notes with deep neural networks. J. Biomed. Inform. 2017, 75S, S138–S148. [Google Scholar] [CrossRef]
- Khondoker, M.; Dobson, R.; Skirrow, C.; Simmons, A.; Stahl, D. A comparison of machine learning methods for classification using simulation with multiple real data examples from mental health studies. Stat. Methods Med. Res. 2016, 25, 1804–1823. [Google Scholar] [CrossRef] [Green Version]
- Caballero, F.F.; Soulis, G.; Engchuan, W.; Sánchez-Niubó, A.; Arndt, H.; Ayuso-Mateos, J.L.; Haro, J.M.; Chatterji, S.; Panagiotakos, D.B. Advanced analytical methodologies for measuring healthy ageing and its determinants, using factor analysis and machine learning techniques: The ATHLOS project. Sci. Rep. 2017, 7, 43955. [Google Scholar] [CrossRef] [Green Version]
- Simms, T.; Ramstedt, C.; Rich, M.; Richards, M.; Martinez, T.; Giraud-Carrier, C. Detecting Cognitive Distortions Through Machine Learning Text Analytics. In Proceedings of the 2017 IEEE International Conference on Healthcare Informatics, ICHI 2017, Park City, UT, USA, 23–26 August 2017; pp. 508–512. [Google Scholar] [CrossRef]
- SahaKoustuv, D. ChoudhuryMunmun, Modeling Stress with Social Media Around Incidents of Gun Violence on College Campuses. Proc. ACM Hum. Comput. Interact. 2017, 1, 1–27. [Google Scholar] [CrossRef]
- Dluhoš, P.; Schwarz, D.; Cahn, W.; van Haren, N.; Kahn, R.; Španiel, F.; Horáček, J.; Kašpárek, T.; Schnack, H. Multi-center machine learning in imaging psychiatry: A meta-model approach. Neuroimage 2017, 155, 10–24. [Google Scholar] [CrossRef]
- di Liu, G.; Li, Y.C.; Zhang, W.; Zhang, L. A Brief Review of Artificial Intelligence Applications and Algorithms for Psychiatric Disorders. Engineering 2020, 6, 462–467. [Google Scholar] [CrossRef]
- Nigri, E.; Ziviani, N.; Cappabianco, F.; Antunes, A.; Veloso, A. Explainable Deep CNNs for MRI-Based Diagnosis of Alzheimer’s Disease. In Proceedings of the International Joint Conference on Neural Networks, Glasgow, UK, 19–24 July 2020. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaiswal, A.K.; Jamal, S.B.; Gabriel Rodrigues Gomes, L.; Profeta, R.; Sales-Campos, H.; Oliveira, C.J.F.; Figueira Aburjaile, F.; Tiwari, S.; Barh, D.; Silva, M.V.d.; et al. Neuroinformatics Insights towards Multiple Neurosyphilis Complications. Venereology 2022, 1, 135-160. https://doi.org/10.3390/venereology1010010
Jaiswal AK, Jamal SB, Gabriel Rodrigues Gomes L, Profeta R, Sales-Campos H, Oliveira CJF, Figueira Aburjaile F, Tiwari S, Barh D, Silva MVd, et al. Neuroinformatics Insights towards Multiple Neurosyphilis Complications. Venereology. 2022; 1(1):135-160. https://doi.org/10.3390/venereology1010010
Chicago/Turabian StyleJaiswal, Arun Kumar, Syed Babar Jamal, Lucas Gabriel Rodrigues Gomes, Rodrigo Profeta, Helioswilton Sales-Campos, Carlo Jose Freire Oliveira, Flávia Figueira Aburjaile, Sandeep Tiwari, Debmalya Barh, Marcos Vinicius da Silva, and et al. 2022. "Neuroinformatics Insights towards Multiple Neurosyphilis Complications" Venereology 1, no. 1: 135-160. https://doi.org/10.3390/venereology1010010