Abstract
Background: South Africa recently adopted HIV self-test technology (HIVST) to improve HIV testing and encourage earlier treatment initiation in working populations with a low uptake of conventional testing approaches. This study investigates the impact of HIVST on testing outcomes, focusing on both frequent and infrequent working-class testers. The paper also examines the spillover effect of HIVST on antiretroviral (ART) treatment initiation. To identify these effects, the author focused on South Africa and exploited the HIVST distribution data of 6259 beneficiaries of HIVST. Methods: The author used a two-stage least-squared model to quantify the impact of the HIVST on these vulnerable working populations. Results: The results show that HIVST fosters a 27.6% higher testing uptake in infrequently testing workers compared to frequently testing workers, and that the uptake of HIVST is 11.5% higher in rural regions than in urban settings, as well as 14.5% more prominent in infrequent male testers than infrequent female testers. Notably, the positive effects of HIVST are also confirmed by the presence of positive spillover effects in workers screening positive for HIV. The paper documents a 7.6% increase in ART initiation in infrequent testers. Conclusions: There is a case for adopting this technology to improve the uptake of HIV testing and ART initiation as the country seeks to attain the UNAIDS 95–95–95 targets by 2030.
1. Introduction
In 2014, the United Nations (UN) Programme on HIV/AIDS (UNAIDS) announced UNAIDS 95–95–95 targets to end the AIDS epidemic by 2030 [1]. Based on this publication, it was clear that low HIV testing may undermine the global efforts to tackle HIV by 2030, especially in crucial vulnerable African populations [2,3].
Hence, there was a need to find innovative strategies to improve HIV testing, especially in populations with a low uptake of conventional testing approaches [4]. In 2016, the World Health Organisation (WHO) responded to the concerns of the low testing rate by publishing the first global guidelines on HIV self-testing, in which HIV self-testing was recommended as an additional method besides the current conventional testing approaches [5]. It was suggested that HIV self-testing technology has superior specificity [6,7], and that it may improve HIV testing in vulnerable populations.
Currently, the South African health system is battling low rates of HIV testing, especially in the working population. As a result, in 2017, South Africa adopted the HIV Self-Test Technology (HIVST) to improve HIV testing uptake in the working population group. The working class in South Africa has a low appetite for HIV testing [8]. Hence, the workplace distribution of HIVST was implemented—aimed at improving testing uptake—and ART initiation across ten different industries in urban (Gauteng) and predominantly rural provinces (Mpumalanga and North West), with a particular focus on those employees who had never tested before or were infrequent testers, meaning their latest test was done more than 12 months ago.
This technology was implemented based on evidence that the lack of confidentiality is one of the major contributing factors that limited testing uptake in workers [8,9]. Thus, it was recommended for South Africa to change the HIV testing algorithm [10] by targeting the working class and offering them HIV self-testing kits. Some suggested that HIVST may increase the demand for testing in the working populations [11]. In turn, this would enhance the UN 95-95-95 targets.
However, other scholars are sceptical of this view and argue that HIVST does not improve ART uptake [12]. In Lesotho, for example, HIVST distribution failed to improve testing in the vulnerable adolescent population [13]. Therefore, the idea that HIVST is a robust technology to advance the UN 95-95-95 targets may not be valid.
Despite the recent adoption of HIVST in South Africa and the growing debate around this technology, there has not yet been a study done to analyse the impact of HIVST on testing outcomes in the South African workplace environment. No study has explored both the regional and gender differences of HIVST effects.
Furthermore, no study has analysed the spillover effects of HIVST on ART initiations, focusing on workplace distribution. All these exercises are essential in informing policymakers about the robustness of HIVST in driving improvements of HIV epidemiology in the workplace environment. The current study seeks to address these gaps, and it explores the impact of HIVST on the testing uptake of infrequent testers and the spillover effects on ART initiation.
The study focused on two population groups—frequent and infrequent testers within the working-class population—and sought to answer the following questions: (1) What impact does HIVST exert on the testing uptake of infrequent testers? (2) Are there gender and regional differences in the impact of HIVST? (3) Is HIVST likely to influence ART initiation positively? The author believes that providing answers to these interrelated questions unlocks the understanding of the potential legacy of HIVST in advancing health goals in the workplace environment.
2. Material and Methods
2.1. Workplace HIVST Distribution and Data Collection
The workplace distribution of HIVST was predominantly conducted in male-dominated sectors such as manufacturing, mining, construction, security, petroleum, and agriculture by the HIVST implementation partners in Mpumalanga, Gauteng, and North West provinces from the year 2017–2020. Two types of workplaces were included: (a) larger companies that did not have formalized HIV testing programs or had a significantly low HIV testing uptake. These companies were contacted before the distribution event for sensitization; (b) smaller workplaces, such as petrol stations or construction sites, without prior arrangement with management [14].
Before distribution took place, the distribution agents demonstrated how the HIVST kit works to a group of employees using video or physical demonstration. After the demonstration, HIVST kits were distributed to consenting clients. Kits were distributed to employees in a brown paper bag with the intervention logo. The bag contained:
- an oral-fluid-based self-testing kit,
- the instruction leaflet,
- a referral card with instructions for further action in the event of a positive screening result,
- a contact number for a representative of the organization distributing the kits.
Kits were distributed to all employees interested in self-screening; about 15% of employees were also offered a kit to take home and offer to their partners. Those testing positive using HIVST were advised to contact the nearby health facility for confirmatory testing, which included the usual counselling session. A list of clinics in the vicinity of the company was provided separately.
Clients who chose to take up HIVST were given an option of self-testing in one of the small tents on-site or taking the kit home for their private use. Confirmatory testing conducted by a professional provider was offered on-site for workers screening positive on-site. Data were also collected on-site, capturing the previous HIV testing history of the worker, age, distribution date, employment sector, gender, region, and the number of kits collected. The primary outcome of this data collection strategy was to discover the population groups with the highest testing appetite for using the HIVST. The secondary outcome was to explore the spillover effect of HIVST distribution on antiretroviral (ART) treatment initiation.
Primary recipients of HIVST consenting to telephonic follow-up calls provided their cell phone numbers during distribution. These workers were followed up telephonically with up to three calls at approximately 2-week, 4-week, and 6-week time intervals post-distribution. During the call, a trained linkage officer administered a standardized questionnaire in order to record kit usage, the HIVST result, and whether recipients had attended confirmatory testing and initiated ART, in the case of a positive result.
This paper analyses the outcomes of 6259 workers who confirmed to have used the HIVST kits (See Figure 1). Furthermore, the paper also explores the spillover effects of HIVST onto ART initiation by using the probability that infrequently testing workers screening positive with HIVST will opt for ART initiation as an outcome. The author excluded clients who had not confirmed HIVST usage. Figure 1 shows the schematic flow of the workplace distribution model.
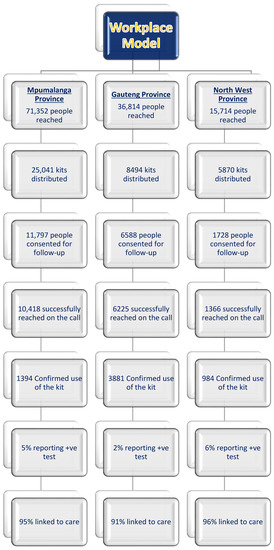
Figure 1.
Workplace model—HIVST distribution flow.
2.2. Statistical Analysis
In order to study the impact of the HIVST technology on testing outcomes, the author defined treatment and control groups based on previous testing history with conventional testing methods using rapid tests. Workers who tested for HIV 3 months before opting for HIVST were defined as frequent testers. Workers who have never tested for HIV in their lifetime, or whose last test was 12 months before opting for HIVST, were defined as infrequent testers. The author considered infrequent testers as the treatment group and frequent testers as the control group because this group has frequently been testing, even without HIVST availability. Thus, the author uses the variations in the testing history to identify the impacts of HIVST on the testing uptake of infrequently testing workers and spillover effects on ART initiation.
The author has reasons to believe these probability estimations may overestimate or underestimate the effects of the HIVST on testing and ART outcomes. Hence, the author opted for a two-stage least-squared (2SLS) model to control for possible biases, instead of the usual ordinary least-squared (OLS) model. Furthermore, several health-policy studies in South Africa have utilized the 2SLS model as a reliable econometric technique to advance health policy analyses [15,16,17,18].
The two-stage least-squared (2SLS) model that was estimated is as follows:
In the first equation, “Y” is the HIV outcome for individual “i” in each group “a” (defined as frequent or infrequent testers), and “HIVST” is the predicted receipt of the HIVST technology.
The regression includes age fixed effects (FE) (which capture age differences in our sample), industry fixed effects (including all industries that had workplace HIVST distributions under WRHI), regional fixed effects (including all municipality regions where HIVST kits were distributed), calendar-month fixed effects (which capture the different months of HIV self-testing distribution), a dummy for intensity (which captures the distribution intensity in individuals who received more than one kit), and a dummy for female (for the regressions in which the author estimates effects for both males and females).
The first regression stage also controls the age, industry, region, month, intensity, and gender biases. Thus, the age dummy variable controls any trend across different age groups. Age can influence testing uptake, and younger cohorts are at higher risk for not testing than older cohorts [19]. Hence, the author opted to control these biases using the age control dummy variable. The industry dummy variable control is used for HIV difference across South African industries. It is documented that specific workplaces, for example mining workplaces, have comprehensive HIV testing services, while other industries lack them [8]. Hence, the author opted to control these industry biases using the industry dummy variable. The region dummy variable controls the regional biases of the program. For example, there is a higher demand for HIV services in rural regions than in urban settings, due to a lack of resources [20]. Therefore, controlling for these regional biases is ideal. The month dummy variable controls the seasonal differences in HIV testing. The demand for HIV testing is lower in the winter compared to summer in South Africa. The author opted to control for these seasonality biases. The intensity dummy variable controls the distribution biases in the subjects that receive more than one kit. Testing uptake may be high, driven by the excitement of the newly adopted testing technology—the intensity dummy variable controls for these biases. The gender dummy variable controls the gender biases of HIV testing. For example, men are known to have a lower appetite for HIV testing than women [21]. Hence, the gender control dummy variable mitigates these gender biases.
In the second equation (which corresponds to the first stage regression), participation in the HIVST distribution program is estimated as a function of the treatment dummy variable, which identifies infrequently testing individuals who confirmed that they had used an HIVST kit.
In all model estimations, one needs two assumptions to be fulfilled: first, the instrument has to be relevant in explaining the probability of HIV outcomes, which will be corroborated by the F-test of the first stage equation; and second, the exclusion restriction needs to hold; that is, the instrument should not influence the primary outcome directly through any channel other than the treatment effect of HIVST distribution.
In the current case, this assumption means that differences in testing and ART outcomes between the treated and control groups can only be due to HIVST technology. Since the paper included month and intensity fixed effects, the author is capturing any improvement in testing and ART uptake attributed to the HIVST technology, without the program’s seasonality and distribution intensity biases.
For example, there is no reason to believe that the treatment group should have a higher testing uptake and ART initiation than the control group when observed in the same regions and industries. Furthermore, no other event in the South African health system explains any difference in testing outcomes that would affect only the treatment group, but not the control group. For this reason, the author is confident that the exclusion restriction is satisfied in this case, meaning these changes can only be explained by HIVST technology distribution.
3. Results
3.1. Descriptive Analysis
Table 1 presents the average percentage breakdown of testing and ART uptake in the treatment and control groups. This analysis captures 6259 individuals who confirmed the HIVST kit usage. No spoiled kits were reported from the sample of 6259 individuals.

Table 1.
Summary statistics—workplace HIVST distribution model.
Table 1 shows that 55% of infrequent testers (treated cohorts) opted to test for HIV using the self-test technology compared to the 45% who belong to the frequent testing population (control cohorts). Moreover, 56% of infrequent male testers opted to test for HIV using the self-test technology compared to the 44% of men who belong to the frequent testing population. About 54% of infrequent female testers opted to test for HIV using the self-test technology compared to the 46% of females who belong to the frequent testing population. Likewise, 52% of infrequent urban testers opted to test for HIV using the self-test technology compared to the 48% of urban clients who belong to the frequent testing group. Additionally, 56% of infrequent rural testers opted to test for HIV using this technology compared to 44% of rural clients who belong to the frequent testing cohort. Lastly, 61% of infrequent testers opted to initiate ART after testing using the self-test technology compared to the 39% who belong to the frequent testing population.
3.2. Results of Two-Stage Least-Squared Model
When analysing the results of the 2SLS estimations, in Table 2, the F-statistic of the first stage regression is very large, pointing towards the strong validity of the cohort instrument. One can also observe that the cohort variable is a significant proxy for HIVST technology and a determinant in improving testing uptake. More specifically, the author observed that the probability of HIVST uptake increases by 25.5 percentage points in the infrequent testing sub-population. As the mean for test uptake is 92.3 in our sample of HIVST users, the HIVST increases the probability of infrequent testers opting for testing by 27.6% more than for frequent testers.

Table 2.
The 2SLS estimation of the impact of HIVST on the testing uptake of workers.
The paper next examines whether HIVST also positively impacted HIV testing in rural and urban settings. Table 3 reports the results for the probability of HIV testing in both settings. The author noted that HIVST distribution increased testing uptake more in rural than in urban settings. More specifically, HIVST increases testing uptake by 31.5 percentage points, which implies an improvement of 33.3% in testing uptake for infrequent testing clients. HIVST increases testing uptake by 19.6 percentage points in the urban settings, which implies an improvement of 21.8% in testing uptake for infrequent testing clients.

Table 3.
The 2SLS estimation of the impact of HIVST on the testing uptake in rural and urban workplaces.
The author now focuses on whether the positive testing outcomes are similar for both genders. In order to explore such possible differences, the author repeated the same regressions for males only and then for females only. In Table 4, one can see that HIVST improves testing uptake more in the male population than in the female population. More specifically, HIVST increases testing uptake by 34.6% in males compared to 20.1% recorded in the female population group. These results reflect the South African working environment, with men dominating the working space.

Table 4.
The 2SLS estimation of the impact of HIVST on testing uptake in males and females.
Lastly, the author analyses the potential existence of positive spillover effects of the HIVST program on ART initiation, as having access to HIVST can encourage the uptake of facility-based confirmatory testing and ART in infrequently testing clients with a positive HIVST screening result. Table 5 shows that the HIVST distribution program increases infrequently testing clients’ probability of opting for ART. More specifically, ART initiation increased by 4.5 percentage points. This implies an effect of 7.6% of treated infrequently testing clients. The impact is significant and suggests that HIVST increases testing uptake and increases the probability of ART initiation—one of the main aims of introducing novel HIV testing methods.

Table 5.
The 2SLS estimation of the impact of HIVST on ART uptake of infrequent testers.
3.3. Robustness Checks
In this section, the author provides robust checks to reinforce the validity of the current findings. For comparison purposes, Table 6 and Table 7 show the results of the OLS regressions for the primary testing outcomes. The variable of interest is now the survey variable that identifies recipients of HIVST. The author includes age, industry, region, month, intensity, and gender fixed effects. As explained above, the author has reasons to believe that this is not a randomly assigned program. The OLS estimation may be overestimating or underestimating the effects of the HIVST on testing uptake and ART initiation. Although the author is already controlling for proxies of behavioural bias (age, gender, and distribution intensity), there can still be other variables that determine HIVST program participation that are unobserved and that may directly affect the testing outcomes and ART initiations of the workers—such as access to the necessary information and proximity to healthcare facilities, etc. Indeed, the results in Table 6 and Table 7 are substantially larger in magnitude than the baseline results of the 2SLS models presented in Table 3 and Table 4. These two tables (Table 6 and Table 7) show the inflated biases of the OLS model. Hence, the author opted for the 2SLS model.

Table 6.
OLS estimation of the impact of HIVST on testing uptake in rural and urban workplaces.

Table 7.
OLS estimation of the impact of HIVST on testing uptake in males and females.
4. Discussion
Testing for HIV is widely seen as a crucial part of attaining the UNAIDS 95-95-95 targets aimed at reducing HIV incidence and deaths by 2030 [22]. The distribution of HIVST kits targeting populations with low uptake of conventional testing in the workplace can enhance testing uptake in previously undertested populations. This paper examined the effects of HIVST on HIV testing uptake using a large longitudinal sample of the South African working population.
The estimation shows that workplace HIVST distribution significantly affects the testing outcomes of previously infrequently tested workers. This is an important finding considering the large amount of existing evidence in the literature that shows the positive long-term health outcomes of regular testing for HIV [23,24,25].
Regarding gender differences, the paper noted that HIVST effects are more substantial in males than in females. These results are encouraging, considering that men generally have a lower testing rate than females [26].
Moreover, when one analyses differences in the impact of HIVST for rural and urban settings, one can see that HIVST increases testing uptake to a more considerable extent in rural regions. Once again, these results are exciting considering that rural populations are often characterised by low HIV testing due to limited resources [27]. Therefore, this technology is key to the testing services for these vulnerable communities.
Finally, the author also analyses the existence of spillover effects of HIVST to ART initiation. More specifically, the paper finds substantial increases in the probability of ART initiation in those workers with a history of infrequent testing who tested positive, similar to the evidence reported in Malawi [28,29]. Thus, the potential spillover effects of HIVST on ART can be substantial, especially when the distribution focuses on men in the rural workplace environment. The current results are significant from a policy point of view, as they point out the essential positive effects of HIVST technology in the working populations with low uptake of conventional testing. The lessons derived from this paper can assist in the decision-making process of other developing countries seeking to adopt this technology for their workplace distribution model.
Limitations
The author recognizes that additional aspects are not captured by the binary dummy variables, which could potentially induce bias in the current estimation. For example, the workplace data does not provide information on test kits spoiled during distribution. Thus, the author interprets the current results as providing evidence of changes in the testing uptake of the treated group, while not independently confirming the existing possibility of spoiled kits due to client errors in using HIVST. However, the author believes this aspect will not significantly influence the current results, since all clients received demonstrations and training on using HIVST. Lastly, our telephonic follow-up discussion probed potential stigmatizing information, such as the status of the testing worker and if the worker has already initiated the ART. There is an existing possibility that the ART data does not reflect the total of all cases enrolled on ART. A minority of workers were uncomfortable disclosing this information.
5. Conclusions
The South African government has realized that HIVST distribution targeting the vulnerable working class can enhance the testing uptake and ART initiation—thus breaking the historical legacy of low testing appetite in this population segment. This paper has examined the effect of HIVST technology on testing outcomes for both the frequent and infrequent populations using a large longitudinal sample of the South African working class.
The estimation shows that HIVST technology significantly improves testing uptake and ART initiation in the infrequent testing worker population. This work may help other countries to diversify the HIV testing policy by strengthening the case for HIV self-test technology as a much-needed investment for the UNAIDS 95–95–95 targets to end the AIDS epidemic by 2030.
Funding
This analysis was funded by the Bill & Melinda Gates Foundation through the grant “Enhancing the Evidence Base of HIV Self-Testing for Young Men” (BMGF OPP1189095) given to Ezintsha, a division of Wits RHI, and HE2RO.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Research Ethics Committees of the University of the Witwatersrand (ref. M180379) on the 11 May 2018.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the clients to publish this paper.
Data Availability Statement
Data for the study is available from WitsRHI upon reasonable request.
Acknowledgments
The author acknowledges the role played by the staff members of Wits RHI, Reaction, and HE2RO in enabling the data collection for this study.
Conflicts of Interest
The author declares no conflict of interest.
References
- Lima, V.D.; St-Jean, M.; Rozada, I.; Shoveller, J.A.; Nosyk, B.; Hogg, R.S.; Sereda, P.; Barrios, R.; Montaner, J.S.G. Progress towards the United Nations 90-90-90 and 95-95-95 targets: The experience in British Columbia, Canada. J. Int. AIDS Soc. 2017, 20, e25011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, J.; Sullivan, P.S.; Curran, J.W. Progress in the HIV epidemic: Identifying goals and measuring success. PLoS Med. 2019, 16, e1002729. [Google Scholar] [CrossRef] [PubMed]
- Barr, D.; Garnett, G.P.; Mayer, K.H.; Morrison, M. Key populations are the future of the African HIV/AIDS pandemic. J. Int. AIDS Soc. 2021, 24, e25750. [Google Scholar] [CrossRef] [PubMed]
- Hatzold, K.; Gudukeya, S.; Mutseta, M.N.; Chilongosi, R.; Nalubamba, M.; Nkhoma, C.; Munkombwe, H.; Munjoma, M.; Mkandawire, P.; Mabhunu, V.; et al. HIV self-testing: Breaking the barriers to uptake of testing among men and adolescents in sub-Saharan Africa, experiences from STAR demonstration projects in Malawi, Zambia and Zimbabwe. J. Int. AIDS Soc. 2019, 22, e25244. [Google Scholar] [CrossRef]
- WHO. Guidelines on HIV Self-Testing and Partner Notification. WHO Publication. 2016. Available online: https://www.who.int/hiv/pub/self-testing/hiv-self-testing-guidelines/en/ (accessed on 9 August 2022).
- Verrall, A.J.; Seah, V.; Lye, D.C.; Leo, Y.S.; Archuleta, S. High specificity of OraQuick® rapid HIV-1/2 antibody testing during dengue infection. J. Clin. Virol. 2020, 131, 104584. [Google Scholar] [CrossRef]
- Siedner, M.J.; Baisley, K.; Koole, O.; Mpofana, I.; Ording-Jespersen, G.; Matthews, P.; Herbst, K.; Smit, T.; Pillay, D. Does antiretroviral therapy use affects the accuracy of HIV rapid diagnostics assays? Experience from a demographic health and surveillance site in rural South Africa. Diagn. Microbiol. Infect. Dis. 2020, 9, 115031. [Google Scholar] [CrossRef]
- Weihs, M.; Meyer-Weitz, A. Barriers to workplace HIV testing in South Africa: A systematic review of the literature. AIDS Care 2016, 28, 495–499. [Google Scholar] [CrossRef]
- Mwisongo, A.; Mohlabane, N.; Tutshana, B.; Peltzer, K. Barriers and facilitators associated with HIV testing uptake in South African health facilities offering HIV Counselling and Testing. Health SA Gesondheid 2016, 21, 86–95. [Google Scholar]
- Mashishi, B.; Makhathini, Z.; Adu-Gyamfi, C. The evolving HIV epidemic and its impact on the HIV testing algorithm: Is it time to change the HIV testing algorithm in South Africa? J. Clin. Virol. 2021, 144, 104990. [Google Scholar] [CrossRef]
- Kelvin, E.A.; George, G.; Kinyanjui, S.; Mwai, E.; Romo, M.L.; Oruko, F.; Odhiambo, J.O.; Nyaga, E.N.; Mantell, J.E.; Govender, K. Announcing the availability of oral HIV self-test kits via text message to increase HIV testing among hard-to-reach truckers in Kenya: A randomized controlled trial. BMC Public Health 2019, 19, 7. [Google Scholar] [CrossRef] [Green Version]
- Shahmanesh, M.; Mthiyane, T.N.; Herbsst, C.; Neuman, M.; Adeagbo, O.; Mee, P.; Chimbindi, N.; Smit, T.; Okesola, N.; Harling, G.; et al. Effect of peer-distributed HIV self-test kits on demand for biomedical HIV prevention in rural KwaZulu-Natal, South Africa: A three-armed cluster-randomised trial comparing social networks versus direct delivery. BMJ Glob. Health 2021, 6, e004574. [Google Scholar] [CrossRef] [PubMed]
- Amstutz, A.; Kopo, M.; Lejone, T.I.; Khesa, L.; Kao, M.; Muhairwe, J.; Glass, T.R.; Labhardt, N.D. “If it is left, it becomes easy for me to get tested”: Use of oral self-tests and community health workers to maximize the potential of home-based HIV testing among adolescents in Lesotho. J. Int. AIDS Soc. 2020, 23, e25563. [Google Scholar] [CrossRef]
- Matsimela, K.; Sande, L.A.; Mostert, C.; Majam, M.; Phiri, J.; Zishiri, V.; Madondo, C.; Khama, S.; Chidarikire, T.; d’Elbée, M.; et al. The cost and intermediary cost-effectiveness of oral HIV self-test kit distribution across 11 distribution models in South Africa. BMJ Glob. Health 2021, 6, e005019. [Google Scholar] [CrossRef] [PubMed]
- Mostert, C.; Vall, C. Long run educational and spill-over effects of cash transfer. Evidence from South Africa. Econ. Hum. Biol. 2020, 36, 100817. [Google Scholar] [CrossRef] [PubMed]
- Mostert, C.M. The impact of national health promotion policy on stillbirth and maternal mortality in South Africa. Public Health 2021, 198, 118–122. [Google Scholar] [CrossRef]
- Mostert, C. The impact of the medical aid schemes on health outcomes of the South African population in the post-apartheid era. J. Community Med. Public Health Care 2021, 8, 92. [Google Scholar] [CrossRef]
- Mostert, C.M. The impact of the school feeding programme on the education and health outcomes of South African children. Child. Youth Serv. Rev. 2021, 126, 106029. [Google Scholar] [CrossRef]
- Ajayi, A.I.; Awopegba, O.E.; Adeagbo, O.A.; Ushie, B.A. Low coverage of HIV testing among adolescents and young adults in Nigeria: Implication for achieving the UNAIDS first 95. PLoS ONE 2020, 15, e0233368. [Google Scholar] [CrossRef]
- Julien, A.; Anthierens, S.; Van Rie, A.; West, R.; Maritze, M.; Twine, R.; Kahn, K.; Lippman, S.A.; Pettifor, A.; Leslie, H.H. Health Care Providers’ Challenges to High-Quality HIV Care and Antiretroviral Treatment Retention in Rural South Africa. Qual. Health Res. 2021, 31, 722–735. [Google Scholar] [CrossRef]
- Hlongwa, M.; Mashamba-Thompson, T.; Makhunga, S.; Hlongwana, K. Barriers to HIV testing uptake among men in sub-Saharan Africa: A scoping review. Afr. J. AIDS Res. 2020, 19, 13–23. [Google Scholar] [CrossRef] [Green Version]
- Hale, B.; Harbertson, J.; Kolou, M.; Sevalie, S.; Cole, R.; Parwon, Z.J.; Merkel, M.O.; Triplett, D.; Wankie, C.; Adams, M.; et al. Reconfirming HIV serostatus in three West African Military ART clinics. J. Clin. Virol. 2021, 141, 104898. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.; Xue, Y.; Gao, J.; Zhu, Q.; Liu, J.; Jiang, Y.; Jin, C. Fifteen years of the proficiency testing program for HIV-1 viral load testing laboratories in China, 2005–2019. J. Clin. Virol. 2021, 142, 104911. [Google Scholar] [CrossRef]
- Hecht, J.; Sanchez, T.; Sullivan, P.S.; DiNenno, E.A.; Cramer, N.; Delaney, K.P. Increasing Access to HIV Testing Through Direct-to-Consumer HIV Self-Test Distribution—United States, March 31, 2020–March 30, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1322–1325. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.; Hahn, E.; Rao, A.; Mwinnyaa, G.; Black, J.; Maharaj, R.; Mvandaba, N.; Nyanisa, Y.; Quinn, T.C.; Hansoti, B. The impact of HIV knowledge and attitudes on HIV testing acceptance among patients in an emergency department in the Eastern Cape, South Africa. BMC Public Health 2020, 20, 1066. [Google Scholar] [CrossRef] [PubMed]
- Fields, E.L.; Gayles, T.A. Considerations for Addressing Low HIV Testing Rates among Adolescent Men Who Have Sex with Men. Pediatrics 2020, 145, e20193996. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Pan, X.; Yang, J.; Ma, Q.; Jiang, J.; Wang, W.; Qiu, J.; Zou, Y.; Wang, P.; Zhao, D.; et al. HIV risk behavior and HIV testing among rural and urban men who have sex with men in Zhejiang Province, China: A respondent-driven sampling study. PLoS ONE 2020, 15, e0231026. [Google Scholar] [CrossRef]
- Indravudh, P.P.; Fielding, K.; Kumwenda, M.K.; Nzawa, R.; Chilongosi, R.; Desmond, N.; Nyirenda, R.; Neuman, M.; Johnson, C.C.; Baggaley, R.; et al. Effect of community-led delivery of HIV self-testing on HIV testing and antiretroviral therapy initiation in Malawi: A cluster-randomised trial. PLoS Med. 2020, 18, e1003608. [Google Scholar] [CrossRef]
- MacPherson, P.; Lalloo, D.; Webb, E.; Maheswaran, H.; Choko, A.T.; Makombe, S.D.; Butterworth, A.E.; Van Oosterhout, J.J.; Desmond, N.; Thindwa, D.; et al. Effect of Optional Home Initiation of HIV Care Following HIV Self-testing on Antiretroviral Therapy Initiation Among Adults in Malawi. JAMA 2014, 312, 372–379. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).